Impact of PNPLA3 in Lean Individuals and in Cryptogenic Steatotic Liver Disease
Handling Editor: Luca Valenti
Funding: Vincent Wong was supported by the General Research Fund from the Research Grants Council, the Hong Kong SAR Government (14106923).
Yuya Seko and Huapeng Lin are co-first authors.
ABSTRACT
Background
Although metabolic dysfunction-associated steatotic liver disease (MASLD) is strongly associated with obesity, around 20% of individuals with hepatic steatosis may nonetheless have normal body mass index, a condition often referred to as lean nonalcoholic fatty liver disease (NAFLD). Under the new nomenclature and definition of MASLD, lean NAFLD can be further divided into lean MASLD (when there is one or more cardiometabolic risk factors) and cryptogenic steatotic liver disease (when there is no cardiometabolic risk factor).
Results
Current studies suggest that the at-risk PNPLA3 rs738409 variant is more common among individuals with lean NAFLD than their overweight and obese counterparts. However, even in this group, cardiometabolic risk factors are often required for the development of hepatic steatosis and liver injury. In the general population, PNPLA3 gene polymorphism is associated with an increased risk of MASLD, more severe liver histology (i.e., the presence of steatohepatitis and fibrosis) and future development of hepatocellular carcinoma and cirrhotic complications. Emerging data also suggest that individuals carrying the PNPLA3 GG genotype might have a greater reduction in hepatic steatosis and liver enzymes with lifestyle intervention and metabolic treatments, such as glucagon-like peptide-1 receptor agonists.
Conclusion
Studies have not specifically examined the impact of PNPLA3 in lean individuals.
Summary
- Around 20% of individuals with hepatic steatosis are classified as lean metabolic dysfunction-associated steatotic liver disease (MASLD).
- The at-risk PNPLA3 rs738409 variant is more common among individuals with lean MASLD than overweight and obese MASLD.
- On the other hand, emerging data also suggest that individuals carrying the PNPLA3 GG genotype might have a greater reduction in hepatic steatosis and liver enzymes with lifestyle intervention and metabolic treatments.
1 Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as nonalcoholic fatty liver disease (NAFLD) or metabolic-associated fatty liver disease, affects over 30% of the general population and is a rapidly increasing aetiology of cirrhosis and hepatocellular carcinoma (HCC) [1]. Although obesity is a strong risk factor for hepatic steatosis, it is clear from epidemiological studies that a subset of individuals can develop hepatic steatosis despite relatively normal body mass index (BMI), a condition often referred to as lean NAFLD or MASLD. Studies in the past 20 years have characterised the clinical features, risk factors and natural history of this special population. In this review, we summarise the literature on the interaction between PNPLA3 and adiposity in the development and progression of steatotic liver disease (SLD).
2 Lean MASLD and Cryptogenic SLD
- MASLD: At least one cardiometabolic risk factor and no or low alcohol consumption (< 20 g/day in women and < 30 g/day in men)
- MASLD and increased alcohol consumption (MetALD): At least one cardiometabolic risk factor and increased alcohol consumption (20–50 g/day in women and 30–60 g/day in men)
- Alcohol-associated liver disease: Alcohol consumption > 50 g/day in women and > 60 g/day in men, regardless of the presence of cardiometabolic risk factors
- Specific aetiology of SLD: Such as drug-induced steatosis, monogenic diseases and others
- Cryptogenic SLD: No recognised cardiometabolic risk factor and alcohol consumption < 50 g/day in women and < 60 g/day in men
Since the publication of the consensus, numerous studies have confirmed that over 95% of individuals previously diagnosed with NAFLD would fulfil the cardiometabolic criteria of MASLD [3]. However, the same may not be true in the lean population as such individuals would not fulfil the BMI criteria (overweight defined as BMI ≥ 25 kg/m2 or ≥ 23 kg/m2 in the Asian population) in the first place. In the general population, around 90% of lean individuals with NAFLD would have lean MASLD, whereas the remaining 10% would be classified as cryptogenic SLD (personal communication with Dr. Sherlot Song). In this review, please note that almost all studies included individuals based on the lean NAFLD criteria, though we expect the major findings would apply to lean MASLD and cryptogenic SLD as well.
Because MASLD is a new concept, epidemiological data on cryptogenic SLD are scarce. In a systematic review and meta-analysis of 93 studies from 24 countries or areas, 19.2% of individuals with NAFLD were lean [4]. Among people who were lean, the prevalence of NAFLD was 10.6%, whereas the incidence was 23.2 per 1000 person-years. As mentioned above, these figures represent a mixture of people with lean MASLD and cryptogenic SLD.
By definition, individuals with cryptogenic SLD do not have cardiometabolic risk factors, though a significant proportion would develop these risk factors and therefore become MASLD during longitudinal follow-up. Compared with individuals who are overweight or obese, those with lean MASLD also have less severe metabolic diseases in general. They also tend to have less severe steatohepatitis and fibrosis, though MASH and advanced fibrosis have been reported in lean MASLD [5].
The natural history of lean MASLD and cryptogenic SLD remains an area of controversy. While some studies suggest that lean individuals were less likely to develop cirrhotic complications and HCC, others reported a higher rate of hepatic complications during long-term follow-up. In a systematic review and meta-analysis of 10 cohort studies involving 109 151 individuals with NAFLD, compared with individuals who were overweight or obese, those with lean NAFLD had a higher risk of liver-related mortality (relative risk 1.88, 95% confidence interval [CI] 1.02–3.45) [6]. In contrast, there was no difference in all-cause mortality, cardiovascular mortality, hepatic decompensation and HCC between the two groups. This is puzzling as liver-related mortality should be the result of hepatic decompensation and HCC. Besides, if lean MASLD indeed progresses faster, cross-sectional studies should be able to detect more severe histologic features, such as the fibrosis stage in this population, but this has never been demonstrated before. One possible explanation of this paradox is reverse causality, with individuals losing weight as they become sicker through liver disease progression or the development of comorbidities.
3 PNPLA3 in Lean Individuals and Cryptogenic SLD
3.1 Epidemiology
The prevalence of the PNPLA3 genotype in lean SLD varies widely by race and by the type of population studied (Table 1). The different definitions of “lean” in each study must also be taken into account. Among Europeans, no significant difference in the prevalence of the PNPLA3 genotype was reported between lean and non-lean MASLD [7, 8]. Feldman et al. examined the prevalence of PNPLA3 G alleles in a cohort of 187 Austrian participants and reported a higher prevalence of G alleles in lean NAFLD individuals compared to lean controls, but no difference was found between them and obese NAFLD individuals [7]. A large-scale cohort study by Younes et al. also reported that the prevalence of the PNPLA3 genotype was not significantly different between obesity and not-obesity MASLD [8]. However, these studies were hospital-based cohorts, and the results for community-based cohorts are unknown. On the other hand, a study from an Asian community-based cohort reported that the PNPLA3 G allele was more common in lean MASLD than in obese MASLD. Wei et al. investigated the association between the PNPLA3 genotype and disease severity by proton magnetic resonance spectroscopy and vibration-controlled transient elastography in 911 community subjects from Hong Kong [12]. Subjects with the PNPLA3 G allele were significantly higher in non-obese MASLD, with a BMI cutoff of 25 kg/m2 (78.4%), than in obese MASLD (59.8%) (p = 0.001). Another report from Hong Kong by Lin et al. showed that the prevalence of PNPLA3 GG was higher in lean MASLD (30.3%), with a BMI cutoff of 23 kg/m2, than in overweight MASLD (17.9%) and obese MASLD (17.4%) (p = 0.003) [13]. In a hospital-based cohort study of 540 biopsy-proven Japanese individuals by Honda et al. the prevalence of PNPLA3 GG was 47.8% in non-obese MASLD, which was significantly higher than that in obese MASLD (36.5%, p = 0.02) [14]. These results suggest that, at least in Asian MASLD, the prevalence of the PNPLA3 G allele seems to be higher in lean MASLD than in obese MASLD. However, the number of cases in each of these studies is insufficient to prove the association between the gene polymorphisms and pathology in lean MASLD, and final conclusions will require the examination of a large number of cases.
| Author, year | Ethnicity | Cohort | Number of lean MASLD | Definition of lean (kg/m2) | Prevalence of PNPLA3 genotype CC/CG/GG | Findings |
|---|---|---|---|---|---|---|
| Feldman et al., 2017 [7] | Caucasian | Hospital-based 116 MASLD | 55 | BMI < 25 |
22/29/3 in lean MASLD 29/22/10 in obese MASLD (p = 0.759) |
Lean MASLD subjects were similar to obese MASLD regarding their impaired glucose tolerance. Lean MASLD subjects had a higher rate of the PNPLA3 CG/GG variant (p = 0.007) but not obese MASLD (p = 0.464). |
| Younes et al., 2022 [8] | Caucasian (Italy, UK, Spain, Australia) | Hospital-based 1339 biopsy-proven MASLD | 195 | BMI < 25 |
30/52/28 in lean MASLD 223/292/174 in non-lean MASLD (p = 0.569) |
MASH and advanced fibrosis were less common in lean patients. The prevalence of PNPLA3, LRE development and prognosis was not significantly different. |
| Nishioji et al., 2015 [9] | Asian (Japan) | Community-based 824 subjects | 60 | BMI < 23 |
7/33/20 in lean MASLD 10/38/22 in overweight MASLD. 37/71/34 in obese MASLD |
The prevalence of MASLD was more affected by the G allele in lean (OR 3.52; 95%-CI: 1.42–8.71; p = 0.0063) and in overweight (OR 2.60; 95% CI: 1.14–5.91; p = 0.0225) than in obese individuals (not significant). |
| Niriella et al., 2019 [10] | South Asia (Sri Lanka) | Community-based 936 MASLD | 84 | BMI < 23 | 5/37/42 | PNPLA3 GG had an OR of 1.51 (0.99–2.31) for MASLD in lean subjects. |
| Park et al., 2023 [11] | Asian (Korean) | Community-based 6939 subjects | 483 | BMI < 23 | 136/230/117 | PNPLA3 GG or TM6SF2 CT, TT had an OR of 1.867 for lean MASLD. |
| Wei et al., 2015 [12] | Asian (Hong Kong) |
Community-based 911 subjects 262 MASLD |
135 | BMI < 25 |
CC/CG, GG 29/105 in non-obese MASLD 51/76 in obese MASLD (p = 0.001) |
PNPLA3 G had an OR of 4.372 (2.448–7.806) for MASLD in non-obese subjects. IHTG, but not liver stiffness was significantly associated with the PNPLA3 genotype. |
| Lin et al., 2021 [13] | Asian (Hong Kong) |
Community-based 904 subjects. 259 MASLD |
66 | BMI < 23 |
21.2%/48.4%/30.3% in lean MASLD The prevalence of GG was 17.9% in overweight MASLD and 17.4% in obese MASLD (p = 0.003) |
Compared with the CC, the GG was associated with the greatest increase in the risk of MASLD in lean subjects (OR 6.04), compared with overweight (OR 3.43) and obese subjects (OR 2.51). PNPLA3 was strongly associated with IHTG in lean subjects but not in the overweight and obese groups. (p < 0.001). The association between the PNPLA3 rs738409 genotype and ALT level was also highly significant in lean subjects (p = 0.003) but not in the overweight and obese subjects. There was no significant association between the PNPLA3 rs738409 genotype and LSM. |
| Honda et al., 2016 [14] | Asian (Japan) | Hospital-based 540 biopsy-proven MASLD and 1012 controls | 134 | BMI < 25 | GG values were 47.8% in non-obese and 36.5% in obese MASLD (p = 0.02) |
Non-obese MASLD had a higher prevalence of the GG genotype than obese MASLD. In non-obese MASLD, the GG genotype was associated with lobular inflammation, hepatocyte ballooning and NAFLD activity score but not steatosis and fibrosis stage. |
- Abbreviations: ALT = alanine aminotransferase; BMI = body mass index; CI = confidence interval; IHTG = intrahepatic triglyceride content; LSM = liver stiffness measurement; MASLD = metabolic dysfunction-associated steatotic liver disease; NAFLD = nonalcoholic fatty liver disease; OR = odds ratio.
The risk of the PNPLA3 genotype on the development of MASLD by body weight has also been reported in several Asian studies. Nishioji et al. conducted a Japanese community-based study and reported that the PNPLA3 G allele contributed to the development of MASLD with an odds ratio (OR) of 3.52 (95% CI: 1.42–8.71) in the lean population, which was stronger than the OR of 2.60 (95% CI: 1.14–5.91) in the overweight population, and not significant in the obese population [9]. Similarly, Lin et al. reported that compared with the CC genotype, the GG genotype was associated with the greatest increase in the risk of MASLD (OR 6.04, 95% CI: 2.62–13.91) in lean subjects, compared with overweight (OR 3.43, 95% CI: 1.06–11.14) and obese subjects (OR 2.51, 95% CI 0.93–6.78) [13]. Thus, the contribution of PNPLA3 to MASLD appears to weaken as body weight increases and is strongest in lean MASLD in Asian countries. Looking from another angle, the higher prevalence of PNPLA3 gene polymorphism in East Asians and Hispanics may in part explain why SLD develops at a lower BMI in these populations [15].
3.2 Disease Severity
Although many studies have reported that PNPLA3 is involved in the pathogenesis of MASLD, there are limited studies on whether its effects are intensified in lean MASLD. Wei et al. assessed intrahepatic triglycerides (IHTG) and liver stiffness measurements (LSMs) by proton magnetic resonance spectroscopy and vibration-controlled transient elastography. The carriage of the PNPLA3 G allele was significantly associated with IHTG (p < 0.01) but not liver stiffness (p = 0.08) in non-obese subjects [12]. Since only eight individuals developed advanced fibrosis on transient elastography, there was no significant association between the effect of PNPLA3 by body weight on fibrosis progression [12]. Similarly, Lin et al. also evaluated the impact of PNPLA3 in lean individuals [13]. PNPLA3 rs738409 was significantly associated with IHTG in lean subjects (p < 0.001) but not in the overweight and obese groups. Serum level of alanine aminotransferase also significantly increased in a stepwise fashion in the CC, CG and GG groups only in lean subjects (p = 0.003).
Although many observational studies have reported an association between the PNPLA3 genotype and fibrosis progression, no significant correlation has been reported in lean subjects. This may be due to the small number of individuals with lean MASLD and an even smaller number of individuals with fibrosis progression in community-based studies. Younes et al. and Honda et al. investigated the association between liver pathology and the PNPLA3 genotype in lean MASLD [8, 14]. Younes et al. reported that lean subjects with MASLD had significantly less steatosis, less lobular inflammation, less ballooning and less advanced liver fibrosis as compared with the non-lean group [8]. However, there is no comparison of pathological findings by the PNPLA3 genotype in lean MASLD. Honda et al. reported that in non-obese NAFLD, the rs738409 GG genotype was associated with lobular inflammation (p = 0.0055), hepatocyte ballooning (p = 0.031) and NAFLD activity score (p = 0.031), whereas BMI and type 2 diabetes, but not the rs738409 GG genotype, were associated with the severity of histology in obese MASLD [14]. Thus, in lean MASLD, PNPLA3 might contribute to steatosis, but whether it contributes to liver fibrosis remains to be determined. It is also unclear whether the effect of the PNPLA3 genotype on the pathogenesis of MASLD is stronger in lean subjects than in non-lean subjects. Moreover, as far as we have been able to find, no studies evaluating the risk of event occurrence by PNPLA3 in lean MASLD have been reported.
3.3 Clinical Outcomes
Lean individuals with SLD face significant long-term risks, with liver-related and non-liver-related mortality rates of 4.1 and 4.0 per 1000 person-years, respectively [4]. A meta-analysis involving approximately 110 000 SLD individuals indicated that lean individuals have a similar risk of cardiovascular mortality and adverse liver-related events compared to their non-lean counterparts. However, they have a significantly higher risk of liver-related mortality [6]. It remains unclear whether hepatic outcomes in lean SLD individuals are comparable across different PNPLA3 rs738409 variants.
4 PNPLA3 and Response to Treatment
4.1 Lifestyle Intervention
Several studies suggest that dietary patterns, including the intake of sweetened beverages and vegetables, may interact with the PNPLA3 rs738409 variant to influence steatosis severity [16-19]. Nobili et al. reported that high consumption of sweetened beverages increased steatosis severity in Italian children and adolescents with the PNPLA3 rs738409 variant, while low vegetable intake seemed to attenuate this effect [17]. Vilar-Gomez et al. found higher carbohydrate intake in individuals with significant fibrosis, especially among carriers of the PNPLA3 rs738409 G allele [16]. Conversely, several studies, including a randomised controlled trial in Latino youth with obesity, found no significant association between sugar intake and liver outcomes [20]. Sevastianova et al. demonstrated that weight loss through a low-carbohydrate diet effectively reduced liver fat content, particularly in carriers of the rs738409 G allele [18]. They also found that overfeeding with simple carbohydrates increased liver fat and stimulated de novo lipogenesis in overweight subjects, especially in PNPLA3 148 CC carriers; however, these effects were reversible with weight loss [19]. These findings suggest that high carbohydrate intake may exacerbate the reduced capacity of individuals with the PNPLA3 variant to hydrolyze triglycerides in the liver and that weight loss through a low-carbohydrate diet may be particularly beneficial for PNPLA3 148 GG carriers [18].
Additionally, in the study of Shen et al., they found out that the PNPLA3 rs738409 GG genotype conferred a higher risk of SLD, but these individuals were more sensitive to the beneficial effects of lifestyle modification [21]. There was a dose–response relationship between the degree of weight reduction and improvement in hepatic steatosis in both non-obese and obese individuals with SLD, though individuals with normal BMI do not need to lose as much weight to achieve the same degree of response [22].
4.2 Drug Treatment
In a small study of people with biopsy-proven SLD and type 2 diabetes treated with alogliptin, participants with the rs738409 G allele showed a positive correlation between temporal changes in glycated haemoglobin and aminotransferase levels [23]. Additionally, in participants who lost weight, those with the CG and GG genotypes showed greater improvements in total cholesterol and triacylglycerols, with similar improvements in glycated haemoglobin. A 12-week randomised clinical trial investigated the effects of a combination of dapagliflozin and n-3 carboxylic acids on magnetic resonance imaging proton density fat fraction (MRI-PDFF) in people with type 2 diabetes and SLD [24]. Baseline MRI-PDFF was lower in individuals with the PNPLA3 rs738409 CC genotype than in those with the CG and GG genotypes. In response to the combination therapy, the relative PDFF reduction was greater in individuals with the CG and GG genotypes than in those with the CC genotype. In a preclinical study, exendin-4 treatment was more effective in reducing intrahepatic fat and activating lipid oxidation in PNPLA3 148 CC cells compared to 148 GG cells [25]. In individuals with type 2 diabetes, a 24-week exenatide treatment improved liver fat content more significantly in those with the 148 CC genotype than those with the 148 GG genotype. Therefore, they concluded that PNPLA3 I148M may modify the anti-SLD response to exenatide. In a recently published retrospective study, Urias et al. found that among individuals receiving semaglutide, ALT levels decreased more in individuals with PNPLA3 risk alleles despite having similar baseline ALT levels [26].
While the interaction between PNPLA3 and various lifestyle and environmental factors discussed above is intriguing, it is noteworthy that none of the studies specifically examined the interaction in lean individuals. Further studies are needed.
5 Conclusions
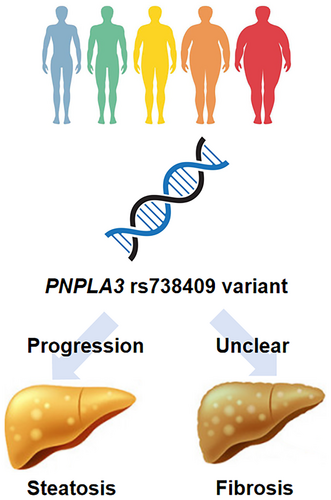
Studies in the past 15 years have highlighted important interactions between host genetics and various metabolic conditions and environmental factors in the pathogenesis of MASLD and MASH. Although the relative impact of PNPLA3 gene polymorphism appears greater in lean individuals than in their overweight and obese counterparts, it is clear that the presence of cardiometabolic risk factors remains crucial in the development of MASLD [27]. Since current studies suggest that the at-risk PNPLA3 G allele not only increases the risk of SLD and severe liver disease in individuals exposed to harmful factors (e.g., alcohol, metabolic dysfunction) but also provides the beneficial effects in individuals exposed to protective factors (e.g., lifestyle intervention, coffee, GLP-1 receptor agonists), it is tempting to propose that the gene polymorphism exaggerates the body's response to metabolic triggers (Figure 1). As data in the lean population remain scarce, further studies are needed to shed light on the role of genetics in diagnosis, prognostication and personalised therapies in this special population (Box 1). In terms of clinical applications, PNPLA3 testing can be useful when a clinician encounters individuals with SLD who are nevertheless lean to determine the genetic contribution to hepatic steatosis. A negative PNPLA3 test result might prompt a further workup for secondary or monogenic causes of SLD. Whether PNPLA3 can be combined with other clinical factors and biomarkers to select individuals for treatment or HCC surveillance will need further data.
BOX 1. Knowledge Gaps in the Application of Genetic Testing in Lean MASLD and Cryptogenic SLD.
Diagnosis
|
Prognostication
|
Personalised therapies
|
- Abbreviations: MASLD = metabolic dysfunction-associated steatotic liver disease; SLD = steatotic liver disease.
Author Contributions
The authors contributed equally to the literature review and drafting of the manuscript. They all approved the final version of the manuscript.
Conflicts of Interest
Vincent Wong served as a consultant or advisory board member for AbbVie, Boehringer Ingelheim, Echosens, Gilead Sciences, Intercept, Inventiva, Novo Nordisk, Pfizer, Sagimet Biosciences, TARGET PharmaSolutions and Visirna and a speaker for Abbott, AbbVie, Echosens, Gilead Sciences, Novo Nordisk and Unilab. He has received a research grant from Gilead Sciences and is a co-founder of Illuminatio Medical Technology.
Statement on Reproducing Data From Other Sources
We did not reproduce data from other sources.
Open Research
Data Availability Statement
This is a review article and does not contain original data.




