Impact of GLP-1RA on the Risk of Adverse Liver Outcomes Among Patients With Alcohol-Associated Liver Disease and Type 2 Diabetes
Funding: The authors received no specific funding for this work.
This paper has been accepted as an oral presentation in plenary session of the AASLD meeting to be held in San Diego, California, from 15 to 19 November.
ABSTRACT
Background and Aims
We sought to characterise the impact of GLP-1RA on adverse liver outcomes (ALO) among patients with alcohol-associated liver disease (ALD) and Type 2 diabetes mellitus (T2DM).
Methods
Patients with T2DM newly diagnosed with ALD between 2013 and 2020 were identified using IBM MarketScan database and were categorised by GLP-1RA exposure. Overlap propensity score weighting (OPSW) followed by Poisson regression models was used to analyse adjusted risk of ALO, a composite endpoint defined by first occurrence of hepatic decompensation (HD), portal hypertension (PH), hepatocellular carcinoma (HCC) or liver transplantation (LT) relative to GLP-1RA.
Results
Among 14 730 patients, most individuals were male (n = 9752, 66.2%) with median age of 57 (IQR 52–61) years; 2.2% (n = 317) of patients had GLP-1RA exposure. Overall, 32.0% (n = 4717) of patients experienced HD, 15.9% (n = 2345) had PH, 3.8% (n = 563) developed HCC, while 2.5% (n = 374) underwent transplantation. Non-GLP-1RA patients had higher incidence of HD (32.2% vs. 22.4%) and HCC (3.9% vs. 0.3%) versus patients taking GLP-1RA (both p < 0.001); in contrast, there was no difference in incidence of PH (14.5% vs. 16.0%) and LT (1.3% vs. 2.6%) (both p > 0.05). After OPSW, overall incidence of ALO was lower in GLP-1RA cohort (GLP-1RA: 12.0%, 95%CI 9.0–16.0 vs. non-GLP-1RA: 21.0%, 95%CI 20.0–22.0) with an absolute incidence risk reduction of 9.0% (95%CI 3.0%–15.0%) associated with GLP-1RA. GLP-1RA was most strongly associated with lower likelihood of HD with reduced adjusted incidence rate of 0.56 (95%CI 0.36–0.86) relative to non-GLP-1RA individuals.
Conclusions
GLP-1RA may have a hepatoprotective impact among patients with ALD and T2DM.
Abbreviations
-
- AIDS
-
- acquired immunodeficiency syndrome
-
- ALD
-
- alcohol-associated liver disease
-
- ALO
-
- adverse liver outcomes
-
- CCI
-
- Charlson Comorbidity Index
-
- COBRA
-
- Consolidated Omnibus Budget Reconciliation Act
-
- Diff
-
- difference
-
- DPP-4
-
- dipeptidyl peptidase-4 inhibitors
-
- GLP-1RA
-
- glucagon-like peptide-1 receptor agonists
-
- HD
-
- hepatic decompensation
-
- HCC
-
- hepatocellular carcinoma
-
- HMO
-
- health maintenance organisations
-
- ICD
-
- international classification of diseases
-
- IQR
-
- interquartile range
-
- LT
-
- liver transplantation
-
- MASH
-
- metabolic dysfunction-associated steatohepatitis
-
- OPSW
-
- overlap propensity score weighting
-
- PH
-
- portal hypertension
-
- RAAS
-
- renin–angiotensin–aldosterone axis
-
- SBP
-
- spontaneous bacterial peritonitis
-
- SD
-
- standard deviation
-
- STROBE
-
- strengthening the reporting of observational studies in epidemiology
-
- T2DM
-
- type 2 diabetes mellitus
-
- US
-
- United States
Summary
- We sought to study impact of GLP-1RA on adverse liver outcomes among patients with alcohol-associated liver disease (ALD) and T2DM.
- GLP-1RA can potentially mitigate the risk of adverse liver outcomes, particularly decompensation, among patients with ALD.
- Randomised controlled trials are needed to prove the role of GLP-1RA among patients with ALD and T2DM.
- Healthcare organisations and policymakers should focus on improving access to this broad-spectrum class of drugs for patients with diabetes.
1 Introduction
Alcohol-associated liver disease (ALD) is a frequent complication of excessive alcohol intake that can encompass a spectrum of conditions from steatosis, acute inflammation and cirrhosis that may lead to hepatocellular carcinoma (HCC) [1, 2]. Over the past two decades, half a million individuals have died from alcohol-related causes with more than 60% of these deaths attributed to liver disease alone [3]. During the COVID-19 pandemic, online alcohol sales surged by more than 200%, accompanied by an approximately 40% rise in alcohol consumption, which further contributed to a spike in alcohol-related health disorders [4, 5]. The burden of liver disease is even greater among patients with diabetes with potentially higher risk of hepatic complications [6]. In fact, patients with diabetes and concurrent diseases such as metabolic dysfunction-associated steatohepatitis (MASH) and chronic hepatitis have a twofold higher risk of decompensation [7]. As such, there has been increased interest in examining the relationship of ALD with diabetes, as well as characterising optimal management approaches in clinical practice.
The metabolism of ethanol to acetaldehyde and eventually to acetate can cause a redox shift by impairing the NADH/NAD ratio [8]. This shift can disrupt metabolic pathways such as gluconeogenesis, glycogenolysis and the β-oxidation of fatty acids, potentially leading to ALD [9]. Owing to the shared pathogenesis of altered metabolism of glucose as well as lipids, a strong reciprocal relationship exists between diabetes and alcohol-related liver pathologies [10, 11]. Additionally, incretins such as glucagon-like peptide-1 are commonly implicated in food regulation with glycaemic control and may share neuronal pathways related to alcohol-mediated behaviours [12]. A relatively newer class of antiglycaemic drugs, including glucagon-like peptide-1 receptor agonists (GLP-1RAs), targets the incretin pathways and has become popular for managing diabetes [10, 13]. Previous studies have demonstrated that this class of medications can reduce aminotransferase levels among patients with metabolic-associated fatty liver disease, suggesting benefits beyond diabetes management [13]. These benefits may be due to associated weight reduction, decreased inflammatory markers and reduced lipotoxicity [10, 14]. Furthermore, Quddos et al. reported that semaglutide and tirzepatide are linked to decreased alcohol consumption among patients with obesity [12].
To date, the clinical evidence of the effectiveness of GLP-1RA medications among patients with ALD remains ill-defined. Patients with severe ALD such as cirrhosis are often excluded from randomised controlled trials due to safety concerns, underscoring a need for an observational study [15]. Therefore, the objective of the current study was to characterise the impact of GLP-1RAs on adverse liver outcomes (ALO) among patients with ALD and Type 2 diabetes mellitus (T2DM).
2 Methods
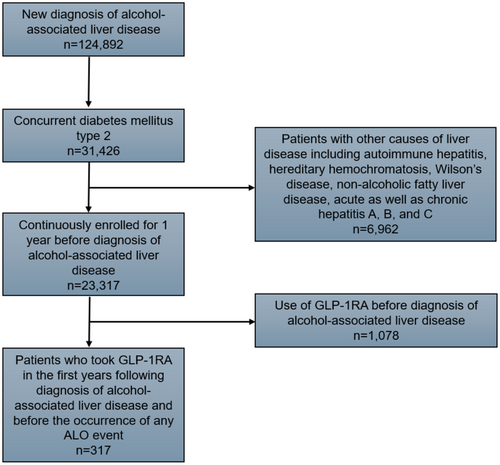
This retrospective cohort study utilised the IBM MarketScan database, a comprehensive national dataset containing longitudinal individual-level information on privately insured patients across inpatient, outpatient and prescription drug services [16]. The database has been previously validated to study epidemiological and clinical outcomes [17, 18]. The International Classification of Diseases, ninth and tenth editions (ICD-9/10) codes, identified patients under 65 years of age with T2DM, who had at least one inpatient and two outpatient diagnoses of ALD from 2013 to 2020 (Table S1) [19]. The index date of diagnosis was defined as the first record of ALD following 12 months of continuous enrolment in an insurance plan. Similarly, patients with prior exposure to GLP-1RA before the index diagnosis of ALD and individuals with other liver conditions were excluded (Figure 1). The national drug codes were employed to query the pharmaceutical records to identify patients who had at least one claim for GLP-1RAs therapy (semaglutide, albiglutide, liraglutide, dulaglutide, lixisenatide or exenatide) within a year after the index diagnosis of ALD. The remaining cohort undergoing routine care was characterised as the non-GLP-1RA group. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. The current study was deemed exempt from approval by the Institutional Review Board at Ohio State University since the IBM Marketscan database meets the criteria for limited-use data.
2.1 Variables and Exposure
Variables of interest included patient age, sex, Charlson Comorbidity Index (CCI), claim-based frailty index, employment status, benefit plan type, US census region (northeast, northcentral, south and west) and baseline concurrent medical conditions (Table 1). The CCI was calculated after excluding mild and moderate-to-severe liver disease along with diabetes without chronic complications and was categorised as previously defined [20-22]. The claim-based frailty index is a measure of deficit accumulation frailty within the prior 12 months, estimated from the administrative dataset using Current Procedural Terminology Codes, Healthcare Common Procedure Coding System Codes and ICD codes [23]. Patients were grouped into three categories based on their employment status: retired, actively employed and ‘Other’. The ‘Other’ category included dependents, individuals with long-term disabilities and Consolidated Omnibus Budget Reconciliation Act (COBRA) continuers. Additionally, ICD-9/10 codes identified any concurrent baseline medical conditions related to the cardiovascular, central nervous and gastrointestinal systems (Table 2).
| Unweighted | Weighteda | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient characteristics | Total N = 14 730 | GLP-1RA N = 317 (2.2%) | Non-GLP-1RA N = 14 413 (97.8%) | Standard diff.b | p | GLP-1RA (%)c | Non-GLP-1RA (%)c | Standard diff.c | p |
| Age, years | |||||||||
| Mean (SD) | 55.3 ± 6.3 | 54.7 ± 6.6 | 55.8 ± 7.3 | 15.8 | 0.005 | 54.8 ± 6.1 | 54.7 ± 1.0 | −0.022 | 0.100 |
| Median (IQR) | 57 (52–61) | 56 (51–60) | 58 (52–61) | −1.0 | 0.005 | 56 (51–60) | 56 (51–60) | −0.022 | 0.100 |
| Sex | |||||||||
| Male | 9752 (66.2%) | 158 (49.8) | 9594 (66.6) | 34.6 | < 0.001 | 49.8 | 50.8 | 0.020 | 0.810 |
| Female | 4978 (33.8%) | 159 (50.2) | 4819 (33.4) | 34.6 | < 0.001 | 50.2 | 49.2 | −0.020 | 0.810 |
| Region | |||||||||
| Northeast | 2839 (19.3%) | 51 (16.1) | 2788 (19.3) | 8.4 | 0.001 | 16.1 | 16.7 | −0.016 | 0.260 |
| North Central | 2757 (18.7%) | 71 (22.4) | 2686 (18.6) | 9.4 | 0.001 | 22.4 | 18.9 | 0.087 | 0.260 |
| South | 6738 (45.7%) | 166 (52.4) | 6572 (45.6) | 13.6 | 0.001 | 50.4 | 50.1 | 0.006 | 0.260 |
| West | 2294 (15.6%) | 29 (9.1) | 2265 (15.7) | 20.1 | 0.001 | 11.1 | 14 | −0.088 | 0.260 |
| Type of health insurance | |||||||||
| PPO | 8224 (55.8%) | 173 (54.6) | 8051 (55.9) | 2.6 | 0.038 | 54.6 | 53.6 | 0.020 | 0.470 |
| HMO | 1916 (13.0%) | 30 (9.5) | 1886 (13.1) | 11.4 | 0.038 | 10.5 | 13.5 | −0.092 | 0.470 |
| Comprehensive | 802 (5.4%) | 14 (4.4) | 788 (5.5) | 5.1 | 0.038 | 4.4 | 5.1 | −0.033 | 0.470 |
| POS | 969 (6.6%) | 20 (6.3) | 949 (6.6) | 1.2 | 0.038 | 6.3 | 6.8 | −0.020 | 0.470 |
| Otherd | 2819 (19.1%) | 80 (25.2) | 2739 (19.0) | 15.0 | 0.038 | 24.2 | 21 | 0.077 | 0.470 |
| Employment status | |||||||||
| Other | 7769 (52.7%) | 199 (62.8) | 7570 (52.5) | 21.0 | 0.001 | 62.8 | 58.9 | 0.080 | 0.310 |
| Full/part-time | 2336 (15.9%) | 45 (14.2) | 2291 (15.9) | 4.8 | 0.001 | 12.2 | 12.7 | −0.015 | 0.310 |
| Retired | 4625 (31.4%) | 73 (23.0) | 4552 (31.6) | 19.4 | 0.001 | 25.0 | 28.4 | −0.077 | 0.310 |
| CCI | |||||||||
| 0 | 8639 (58.6%) | 191 (60.3%) | 8448 (58.6%) | 7.5 | 0.183 | 60.3 | 60 | 0.006 | 0.800 |
| 1–2 | 3595 (24.4%) | 84 (26.5%) | 3511 (24.4%) | 10.5 | 0.183 | 26.5 | 24.8 | 0.033 | 0.800 |
| 3–4 | 1290 (8.8%) | 27 (8.5%) | 1263 (8.8) | 2.5 | 0.183 | 8.5 | 8.3 | 0.015 | 0.800 |
| 5+ | 1206 (8.2%) | 15 (4.7%) | 1191 (8.3%) | 14.6 | 0.183 | 4.7 | 6.8 | −0.090 | 0.800 |
| Claim-based frailty index | |||||||||
| Nonfrail | 6993 (47.5%) | 134 (42.3) | 6859 (47.6) | 10.7 | 0.030 | 42.9 | 46.6 | −0.074 | 0.310 |
| Prefrail | 7021 (47.7%) | 173 (54.6) | 6848 (47.5) | 14.2 | 0.030 | 53.8 | 48.9 | 0.098 | 0.310 |
| Frail | 716 (4.9%) | 10 (3.2) | 706 (4.9) | 8.6 | 0.030 | 3.3 | 4.5 | −0.062 | 0.310 |
- Abbreviations: CCI, Charlson Comorbidity Index; diff, difference; GLP-1RA, glucagon-like peptide-1 receptor agonist; IQR, interquartile range; SD, standard deviation.
- a Pseudosample was created using the overlap weighting technique, ensuring that GLP-1RA initiation is independent of other clinicodemographic characteristics of the patients.
- b Absolute difference in means/proportions divided by pooled SD. The absolute value greater than 0.10 represented an imbalance between two study groups; smaller values indicate better balance.
- c Overlap weighted proportions and standardised differences were calculated using all patient demographics and medical history.
- d Including, COBRA, long-term disability, surviving spouse/dependent or other/unknown.
| Unweighted | Weighteda | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient characteristics | Total N = 14 730 | GLP-1RA N = 317 (2.2%) | Non-GLP-1RA N = 14 413 (97.8%) | Standard diff.b | p | GLP-1RA (%)c | Non-GLP-1RA (%)c | Standard diff.c | p |
| Myocardial infarction | 328 (2.2%) | 2 (0.6%) | 326 (2.3%) | 14.3 | 0.052 | 1.6 | 2.1 | −0.037 | 0.110 |
| Congestive heart failure | 1220 (8.3%) | 14 (4.4%) | 1206 (8.4%) | 16.4 | 0.012 | 5.4 | 7.7 | −0.093 | 0.090 |
| Peripheral vascular disease | 628 (4.3%) | 8 (2.5%) | 620 (4.3%) | 9.9 | 0.121 | 2.5 | 3.6 | −0.064 | 0.430 |
| Cerebrovascular disease | 475 (3.2%) | 8 (2.5%) | 467 (3.2%) | 4.2 | 0.475 | 2.5 | 3.2 | −0.042 | 0.640 |
| Dementia | 36 (0.2%) | 0 (0.0%) | 36 (0.2%) | 418.7 | 0.373 | 0 | 0.3 | −0.078 | 0.350 |
| Hemiplagia or paraplegia | 73 (0.5%) | 1 (0.3%) | 72 (0.5%) | 3.2 | 0.644 | 0.3 | 0.5 | −0.032 | 0.740 |
| Diabetes with complications | 2585 (17.5%) | 68 (21.5%) | 2517 (17.5%) | 10.1 | 0.065 | 21.5 | 18.8 | 0.067 | 0.420 |
| Hypertension | 9917 (67.3%) | 219 (69.1%) | 9698 (67.3%) | 3.9 | 0.499 | 69.1 | 68.2 | 0.019 | 0.810 |
| Obesity | 1259 (8.5%) | 52 (16.4%) | 1207 (8.4%) | 24.5 | < 0.001 | 16.4 | 15.6 | 0.022 | 0.790 |
| Dyslipidaemia | 8435 (57.3%) | 198 (62.5%) | 8237 (57.1%) | 11.0 | 0.059 | 62.5 | 59.1 | 0.070 | 0.400 |
| Renal disease | 1344 (9.1%) | 18 (5.7%) | 1326 (9.2%) | 13.4 | 0.031 | 5.7 | 7.6 | −0.076 | 0.350 |
| Chronic pulmonary disease | 1429 (9.7%) | 34 (10.7%) | 1395 (9.7%) | 3.3 | 0.533 | 10.7 | 9.7 | 0.033 | 0.690 |
| Peptic ulcer disease | 217 (1.5%) | 4 (1.3%) | 213 (1.5%) | 1.7 | 0.752 | 1.3 | 1.2 | 0.009 | 0.970 |
| Rheumatic disease | 287 (1.9%) | 11 (3.5%) | 276 (1.9%) | 9.9 | 0.048 | 3.5 | 2.3 | 0.072 | 0.380 |
| AIDS/HIV | 107 (0.7%) | 1 (0.3%) | 106 (0.7%) | 5.7 | 0.384 | 0.3 | 0.7 | −0.057 | 0.550 |
| No malignancy | 13 163 (89.4%) | 295 (93.1%) | 12 868 (89.3%) | 13.4 | 0.031 | 6.9 | 8.3 | −0.053 | 0.520 |
| Metastatic solid tumour | 399 (2.7%) | 4 (1.3%) | 395 (2.7%) | 10.0 | 0.109 | 1.3 | 2.0 | −0.055 | 0.490 |
| Combination drugs | 238 (1.6%) | 12 (3.8) | 426 (3.0) | 4.4 | 0.390 | 3.8 | 3.1 | 0.038 | 0.640 |
| Metformin | 422 (2.9%) | 17 (5.4) | 405 (2.8) | 13.1 | 0.007 | 5.4 | 4.9 | 0.023 | 0.790 |
| SGLT-2 | 251 (1.7%) | 16 (5.0) | 235 (1.6) | 19.1 | < 0.001 | 5.0 | 4.4 | 0.028 | 0.690 |
| DPP-4 | 764 (5.2%) | 48 (15.1) | 716 (5.0) | 34.1 | < 0.001 | 15.1 | 14.0 | 0.031 | 0.690 |
| Sulphonylureas | 414 (2.8%) | 11 (3.5) | 403 (2.8) | 4.0 | 0.473 | 3.5 | 2.1 | 0.085 | 0.300 |
| Thiazolidinedione | 108 (0.7%) | 3 (0.9) | 105 (0.7) | 2.2 | 0.653 | 0.9 | 0.9 | 0.000d | 0.920 |
| Statins | 324 (2.2%) | 8 (2.5) | 316 (2.2) | 2.0 | 0.691 | 2.5 | 2.0 | 0.034 | 0.660 |
| Hepatic decompensation | 4717 (32.0%) | 71 (22.4%) | 4646 (32.2%) | 22.1 | < 0.001 | 22.4 | 22.9 | −0.012 | 0.890 |
| Ascites | 2820 (19.1%) | 27 (8.5%) | 2793 (19.4%) | 31.9 | < 0.001 | 8.5 | 8.9 | −0.014 | 0.850 |
| Hepatic encephalopathy | 2290 (15.5%) | 40 (12.6%) | 2250 (15.6%) | 8.6 | 0.146 | 12.6 | 12.7 | −0.003 | 0.960 |
| Hepatorenal syndrome | 150 (1.0%) | 0 (0.0%) | 150 (1.0%) | 398.2 | 0.068 | 0 | 0.5 | −0.100 | 0.190 |
| SBP | 366 (2.5%) | 4 (1.3%) | 362 (2.5%) | 8.8 | 0.157 | 1.3 | 1.7 | −0.033 | 0.670 |
| Variceal bleeding | 700 (4.8%) | 12 (3.8%) | 688 (4.8%) | 4.9 | 0.413 | 3.8 | 3.9 | −0.005 | 0.960 |
| Hepatocellular carcinoma | 563 (3.8%) | 1 (0.3) | 562 (3.9%) | 25.3 | 0.001 | 0.3 | 0.3 | 0.000d | 0.960 |
| Liver transplant | 374 (2.5%) | 4 (1.3%) | 370 (2.6%) | 9.4 | 0.144 | 1.3 | 1.2 | 0.009 | 0.980 |
| Portal Hypertension | 2345 (15.9%) | 46 (14.5%) | 2299 (16.0%) | 4.2 | 0.488 | 14.5 | 12.6 | 0.056 | 0.500 |
- Abbreviations: AIDS, acquired immunodeficiency syndrome; DPP-4, dipeptidyl peptidase-4 inhibitors; GLP-1RA, glucagon-like peptide-1 receptor agonist; SBP, spontaneous bacterial peritonitis; SGLT-2, sodium–glucose cotransporter-2.
- a Pseudosample was created using the overlap weighting technique, ensuring that GLP-1RA initiation is independent of other clinicodemographic characteristics of the patients.
- b Absolute difference in means/proportions divided by pooled SD. The absolute value greater than 0.10 represented an imbalance between two study groups; smaller values indicate better balance.
- c Overlap weighted proportions and standardised differences calculated by using all patient demographics and medical history.
- d Overlapping weighting resulted in an exact balance for this variable.
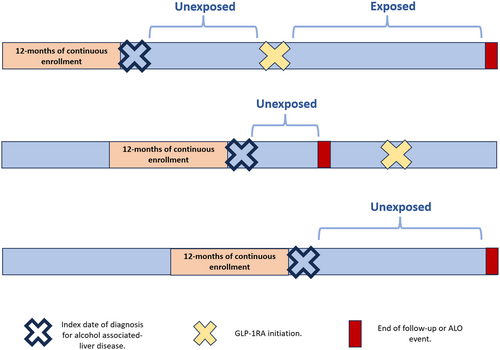
The primary exposure variable was the initiation of GLP-1RAs, treated as a time-varying factor to accommodate the duration between the index diagnosis of ALD and the start date of GLP-1RA therapy. Accordingly, patients were categorised into the exposure group if GLP-1RAs were initiated before (1) the occurrence of an ALO event, (2) the end of enrolment in the insurance plan or (3) the conclusion of the study period. Conversely, patients who commenced GLP-1RA therapy after said events were classified as unexposed (Figure 2).
2.2 Outcome of Interest
The primary outcome of interest was ALO. ALO was defined as a composite measure of the first occurrence of hepatic decompensation, HCC, portal hypertension or liver transplantation [10]. Hepatic decompensation included ascites, hepatorenal syndrome, variceal bleeding, spontaneous bacterial peritonitis (SBP) and hepatic encephalopathy. These outcomes were determined by the record of two outpatient or one inpatient diagnosis identified through ICD-9/10 codes, as defined previously (Table S2) [10, 19]. The secondary outcomes were the cause-specific incidence of each of the four individual components of the composite ALO. Patients with a baseline history of ALO at the time of their initial ALD diagnosis were included in the study, yet were not considered in the outcome analyses. For example, if a patient had no prior history of portal hypertension but did have a history of ascites before the index diagnosis of ALD, any subsequent record of ascites during follow-up was not considered an incident event and was excluded from outcome analyses. However, any record of portal hypertension during follow-up was included in both the primary and secondary composite outcomes, which was consistent with previous methodologies [10].
2.3 Follow-Up Time
To examine primary outcomes, patients were followed from the date of initial diagnosis of ALD until the occurrence of the ALO event. For individuals who did not experience any ALO events, follow-up continued until either their enrolment in the insurance plan ended or until the conclusion of the study period, whichever came first. Similarly, in evaluating the secondary outcome, if a patient developed a single ALO before other events, the initial event was regarded as the primary focus and follow-up was extended only until the incidence of the initial ALO event [10].
2.4 Statistical Analyses
Categorical variables were summarised with frequencies and percentages with comparisons made using either the chi-square or the Fisher exact test (χ2). Continuous variables were expressed as medians with interquartile ranges (IQR) and analysed using either the Student's t-test or the Wilcoxon rank-sum test, as deemed suitable. Overlap propensity score weighting (OPSW) was employed to mitigate the confounding influence of baseline clinicodemographic characteristics on the clinical suitability of GLP-1RAs [24]. OPSW achieves precise balance across covariates included in the propensity score estimation via logistic regression, closely mimicking key aspects of randomised controlled trials such as statistical accuracy and covariate equilibrium [25]. Consequently, these attributes render OPSW superior to alternative weighting techniques such as inverse probability of treatment weighting [24, 25]. The multivariable logistic regression models estimating propensity scores were adjusted for all clinicodemographic characteristics, including their first-order interactions, as covariates (Table 1).
Two methods were employed to assess the effectiveness of OPSW in addressing baseline differences between the GLP-1RAs and non-GLP-1RAs groups. In the initial approach, general overlap-weighted standardised differences were used to compare the two cohorts, with a difference of less than 0.1 indicating a negligible contrast in characteristics [26]. Subsequently, OPSW-based t-tests and χ2 were utilised to analyse the association of patient characteristics in the weighted sample with GLP-1RA exposure. Since the exposure was modelled as a time-varying factor, the cumulative incidence of ALO over a 3-year follow-up period was estimated using the method described by Simon and Makuch [27]. Cause-specific incidence rates for each group were calculated by dividing the number of outcome-specific events by the total person-years of follow-up. In turn, incidence rate ratio and absolute incidence rate differences for all outcomes were calculated in relation to GLP-1RAs status. The OPSW-based Poisson regression models were used to analyse the association of GLP-1RAs with outcomes to obtain adjusted risk estimates for each year of follow-up (at 1 to 3 years). All statistical analyses were performed using SAS 9.4 (SAS Institute); statistical significance was defined as a p-value of less than 0.05.
3 Results
3.1 Baseline Characteristics
Among 14 730 patients, most individuals were male (n = 9752, 66.2%) with a median age of 57 (IQR: 52–61) years. Most patients were enrolled with a preferred provider organisation (n = 8224, 55.8%), followed by health maintenance organisations (HMO) (n = 1916, 13.0%), comprehensive plans (n = 802, 5.4%) and point of service plans (n = 969, 6.6%) with the remainder enrolled in other benefit plans (n = 2819, 19.1%). Over one-half of patients had a CCI score of 0 (n = 8639, 58.6%), while a quarter had a CCI score of 1–2 (n = 3595, 24.4%). There was a roughly equal distribution among nonfrail (n = 6993, 47.5%) and prefrail (n = 7021, 47.7%) patients, with a small subset of individuals who were categorised as frail (n = 716, 4.9%).
Among other baseline clinical characteristics, 8.5% (1259) of patients had obesity, 8.3% (n = 1220) suffered from congestive heart failure and 9.1% (n = 1344) had renal disease. Overall, 32.0% (n = 4717) of patients experienced hepatic decompensation, 15.9% (n = 2345) had features of portal hypertension, 3.8% (n = 563) had an incident development of an HCC and 2.5% (n = 374) underwent liver transplantation. Overall, 2.2% (n = 317) of patients were exposed to GLP-1RA use (Table 1).
3.2 Characteristics Relative to GLP-1RA Exposure
Compared with non-GLP-1RA patients, individuals who were exposed to GLP-1RA were more likely to be female (50.2% vs. 33.4%), younger (56 vs. 58 years) and less likely to be enrolled with an HMO (9.5% vs. 13.1%) or be retirees (23.0% vs. 31.6%) (all p < 0.05). Furthermore, patients who started GLP-1RA had lower likelihood of claim-based frailty (3.2% vs. 4.9%; p = 0.030); however, there was no difference relative to overall comorbidity burden based on CCI (p = 0.183). Additionally, differences were noted among certain key baseline comorbidities whereby patients on GLP-1RA were less likely to have congestive heart failure (4.4% vs. 8.4%; p = 0.012), renal disease (5.7% vs. 9.2%; p = 0.031), yet be more likely to have obesity (16.4% vs. 8.4; p < 0.001) or rheumatic disease (3.5% vs. 1.9%; p = 0.048). Similarly, patients with GLP-1RA initiation had higher concurrent use of other antiglycaemic medications such as metformin (5.4% vs. 2.8%), sodium–glucose cotransporter-2 inhibitors (5.0% vs. 1.6%) and dipeptidyl peptidase-4 inhibitors (15.1 vs. 5.0) (all p < 0.05). Of note, patients taking GLP-1RA had a lower incidence of hepatic decompensation (22.4% vs. 32.2%) and HCC (0.3% vs. 3.0%) compared with individuals not taking GLP-1RA (both p < 0.001); there was no association of GLP-1RA with the incidence of portal hypertension (14.5% vs. 16.0%; p = 0.488) and liver transplantation (1.3% vs. 2.6%; p = 0.144) (Table 2).
3.3 OPSW
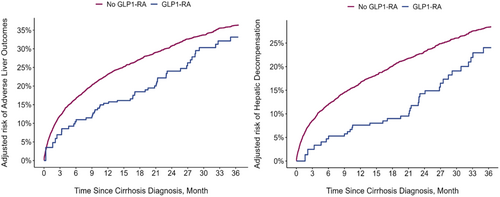
The median follow-up time for all patients was 42.2 (IQR: 17.8–84.0) months (GLP-1RA, 55.0 [IQR: 26.0–91.3] months vs. non-GLP-1RA, 42.0 [IQR: 17.5–83.8] months). On average, patients waited 5.5 months (standard deviation [SD]: ±3.8) after being diagnosed with ALD before starting GLP-1RA. Similarly, the median time from GLP-1RA exposure to the incidence of ALO was 14.0 (IQR: 6.6–27.8) months. After applying OPSW, all the clinicodemographic characteristics of patients who did and did not receive GLP-1RA were well balanced (Table 1). Of note, patients on GLP-1RA had a lower occurrence of composite ALO, with 46 events over 379 person-years versus 75 ALO events over 353 person-years in the unexposed group. The overall incidence of ALO was lower in the GLP-1RA cohort (GLP-1RA: 12.0%, 95%CI 9.0–16.0 vs. non-GLP-1RA: 21.0%, 95%CI 20.0–22.0) with an absolute incidence risk reduction of 9.0% (95%CI 3.0%–15.0%) associated with GLP-1RA (Figure 3a). On further stratification and cause-specific analyses, this association was primarily driven by a reduction in the incidence of hepatic decompensation among the different ALO components. Particularly, there was a 7.0% (95%CI 4.0–8.0) absolute reduction in the incidence of hepatic decompensation with an incidence rate of 8.0% (95%CI 6.0–12.0) in the GLP-1RA group versus 15.0% (95%CI 14.0–16.0) in the non-GLP-1RA group (Figure 3b). In contrast, there were no differences in incidence rates of portal hypertension, HCC and liver transplantation between the two cohorts. On Poisson regression, after accounting for other clinicodemographic characteristics, the adjusted incidence rate of ALO and hepatic decompensation among GLP-1RA initiation group remained lower at 0.57 (95%CI 0.39–0.82) and 0.56 (95%CI 0.36–0.86), respectively (Table 3).
| Outcomes | Number of events | Person-years | Incidence rates per person-years (95%CI) | Absolute incidence rate difference per person-years (95%CI) | Incidence rate ratio (95% CI) | Adjusted incidence rate (95%CI)c |
|---|---|---|---|---|---|---|
| Adverse liver outcomes | ||||||
| No GLP-1RA | 75 | 353 | 0.21 (0.20–0.22) | — | — | Ref. |
| GLP-1RA | 46 | 379 | 0.12 (0.09–0.16) | −0.09 (−0.15 to −0.03) | 0.55 (0.38–0.80) | 0.57 (0.39–0.82)d |
| Hepatic decompensation | ||||||
| No GLP-1RA | 57 | 381 | 0.15 (0.14–0.16) | — | — | Ref. |
| GLP-1RA | 33 | 400 | 0.08 (0.06–0.12) | −0.07 (−0.08 to −0.04) | 0.55 (0.42–0.74) | 0.56 (0.36–0.86)d |
| Hepatocellular carcinoma | ||||||
| No GLP-1RA | 9 | 429 | 0.02 (0.02–0.03) | — | — | Ref. |
| GLP-1RA | 2 | 427 | 0.01 (0.00–0.05) | −0.02 (−0.02 to 0.02) | 0.21 (0.05–1.82) | 0.32 (0.06–1.63) |
| Liver transplant | ||||||
| No GLP-1RA | 4 | 433 | 0.01 (0.008–0.012) | — | — | Ref. |
| GLP-1RA | 1 | 428 | 0.002 (0.001–0.009) | −0.007 (−0.008 to −0.004) | 0.22 (0.11–0.71) | 0.57 (0.60–5.49) |
| Portal Hypertension | ||||||
| No GLP-1RA | 35 | 398 | 0.09 (0.08–0.10) | — | — | Ref. |
| GLP-1RA | 29 | 400 | 0.07 (0.05–0.11) | −0.02 (−0.03 to 0.01) | 0.81 (0.61–1.10) | 0.76 (0.46–1.24) |
- Abbreviations: CI, confidence interval, GLP-1RA: glucagon-like peptide 1 receptor agonists.
- a Glucagon-like peptide-1 receptor agonists initiation status was modelled as a time-dependent covariate.
- b Adverse liver outcomes were defined as (1) hepatic decompensation (ascites, hepatic encephalopathy, hepatorenal syndrome, spontaneous bacterial peritonitis and variceal bleeding), (2) hepatocellular carcinoma, (3) liver transplant and (4) portal hypertension.
- c Adjusted using overlapping weights estimated using age, sex, region of residence, type of health insurance, year of diagnosis, employment status, history of smoking, obesity, dyslipidaemia, hypertension, Charlson Comorbidity Index, claims-based frailty index, metformin, SGLT-2, DPP-4, sulphonylureas, thiazolidinediones and statins.
- d p < 0.05.
3.4 Sensitivity Analyses
After stratifying patients by baseline liver compensation status, there was an equal benefit of GLP-1RA relative to the reduced incidence of overall ALO (baseline compensated ALD: adjusted IR of ALO: 0.63, 95%CI 0.40–0.99; p = 0.043 vs. baseline decompensated ALD: adjusted IR of ALO: 0.42, 95%CI 0.40–0.45; p < 0.001) (Table S3). Two additional sensitivity analyses performed using inverse probability of treatment weighting with redefinitions of the primary and secondary outcomes as events occurring 6 months after the index date demonstrated similar findings.
4 Discussion
Over the past decade, the primary cause of death due to chronic liver disease has shifted from viral hepatitis to fatty liver disease [28]. Consequently, there is a growing emphasis on understanding fatty liver such as ALD and MASH to address the rising liver-related health burden [29, 30]. Despite efforts to regulate alcohol access and reduce alcohol abuse, ALD continues to be one of the leading causes of liver-related illness and mortality [29-31]. In fact, over half of deaths from alcohol-related causes are due to liver disease with the associated mortality nearly doubling each year [3, 28]. Traditionally, medications such as acamprosate and naltrexone are approved for managing alcohol use disorder yet these drugs do not offer any metabolic benefits for concurrent alcohol-induced dysfunction [12, 32]. Of note, GLP-1RAs—which are well recognised in the management of diabetes mellitus through improved glycaemic control—have been suggested to help create an aversion to alcohol consumption [12]. Nonetheless, the impact of GLP-1RA intake on liver outcomes among patients with ALD had not been investigated. Therefore, the current study was important as it defined the association of GLP-1RA initiation with the incidence of ALO among patients with T2DM who were newly diagnosed with ALD. Notably, patients with GLP-1RA initiation after an index diagnosis of ALD had a lower overall incidence of ALO, particularly hepatic decompensation. Interestingly, on a stratified analysis, the benefit of GLP-1RA on composite ALO remained consistent across patients with or without baseline liver compensation.
There is evidence that alcohol-associated fatty liver disease, particularly when accompanied by advanced fibrosis, is an independent predictor of both liver-specific and all-cause mortality [33]. Diabetes, which is disproportionately common among patients with ALD, is another known risk factor that can contribute to liver-associated complications [34]. To this end, antidiabetic drugs such as metformin, thiazolidinediones and GLP-1RA can reverse steatosis and improve liver function [35]. In fact, previous studies have demonstrated that GLP-1RAs, particularly semaglutide, were associated with the resolution of steatohepatitis in patients with T2DM [36, 37]. This effect may result from reduced insulin resistance and weight loss, which in turn can improve overall metabolic function and reduce the liver fat content [36]. These desirable effects may explain findings in the current study, which demonstrated that initiation of GLP-1RA treatment reduced the incidence rate of ALO per person-years by 9% among patients with ALD. Interestingly, after adjusting for confounding variables in multivariable analysis, GLP-1RA remained independently associated with a 43% reduced incidence of composite ALO.
Managing ALO may differ based on different baseline liver compensation or decompensation status [10]. Prior evidence has suggested that GLP-1RA may be superior to other antidiabetic medications in reducing the risk of HCC and hepatic decompensation [38]. Similarly, among patients with T2DM who have been diagnosed with MASH, GLP-1RA has been suggested to be associated with improved liver-related outcomes [10]. In the present study, the beneficial effects of GLP-1RA were consistent across patients with incidence of hepatic decompensation (22.4% vs. 32.2%) and HCC (0.3% vs. 3.9%) diagnosis in the unweighted models of patients newly diagnosed with ALD. Importantly, after OPSW-based analysis, the effects of GLP-1RA on ALO were demonstrated to be primarily mediated through a marked (44%) reduction in the incidence of hepatic decompensation. Given that GLP-1RAs are primarily excreted unchanged through the kidneys, the benefits of the drug may be mainly among patients with compensated liver disease [39]. To address this question, a separate analysis was conducted among patients with baseline decompensation to assess the impact of GLP-1RA initiation on ALO. This analysis demonstrated that patients with already diagnosed liver decompensation experienced the same benefits of taking GLP-1RA as individuals with compensated liver disease. These findings aligned with a previous study in which patients with baseline decompensation associated with MASH had equally favourable effects from initiation of GLP-1RA [10]. There is prior evidence that GLP-1RA has the potential to enhance and possibly reverse diabetic nephropathy by affecting the renin–angiotensin–aldosterone axis (RAAS) [40]. It is worth noting that there is a common underlying mechanism associated with hepatorenal syndrome, and enhanced renal function may be a reason for similar benefits of GLP-1RA among patients with decompensated liver disease [41].
While the benefits may extend beyond managing T2DM alone, utilisation of GLP-1RA remains relatively low [42]. Previously reported usage data ranged from as low as 9% to 15% among patients with diabetes [10, 43]. In the current study, only about 3% of patients initiated GLP-1RA treatment, likely due to the inclusion of patients with T2DM and concurrent ALD. The underuse of GLP-1RA can be multifaceted and may include factors such as therapeutic inertia among clinicians, patients' concerns about adverse effects and the mode of delivery [44, 45]. Additionally, disparities in care have been noted with patients from higher socioeconomic backgrounds being more likely to receive modern antidiabetic treatments, including GLP-1RA [43]. Similarly, rising costs can be another contributing factor to low initiation rates of GLP-1RA [46]. According to a report by Sumarsono et al., GLP-1RAs are the second costliest antiglycaemic medication with a notable 135% cost increase over the last 5 years [46]. Additionally, the Patient Protection and Affordable Care Act is expected to increase the cost burden on patients and private insurers may also shift cost shares [47]. Consequently, rising treatment expenses can place patients at risk of ‘financial toxicity’, which in turn can reduce preference for GLP-1RA [47, 48]. Recently, following increased attention to the wide-ranging benefits of GLP-1RA, the FDA approved this class of drugs for additional indications, including weight management and the secondary prevention of cardiovascular events [42]. Data from the current study suggest that there may be additional benefits of GLP-1RA for patients with liver disease. Therefore, healthcare organisations and policymakers should focus on improving access to this class of drugs for patients with diabetes.
The findings of the current study should be interpreted considering certain limitations. The use of a retrospective administrative database may be prone to inaccurate data input and missing values. The IBM MarketSdcan database included only individuals with employer-sponsored health insurance in the United States, which may not represent other populations, such as uninsured individuals or those covered by government-sponsored health plans like Medicare or Medicaid [16]. Additionally, data collection relied on convenience sampling, which may limit the generalisability of the results. Since ICD and national drug codes were used to select the patient cohort, exposure of interest and outcomes, there was a risk of misclassification bias and residual confounding attributable to potential coding errors [49]. This was an observational study that only reported associations and could not ascertain causation. Nonetheless, the causal inference approach using overlap weighting alleviated confounding by indication [10]. Moreover, GLP-1RA was modelled as a time-varying variable to address immortal time bias. Approximately one-third of patients prescribed GLP-1RA fail to complete treatment [50]. To this point, the current study was unable to ascertain treatment completion rates, potentially limiting the study of the long-term benefits of GLP-1RA therapy. However, the focus of our investigation primarily centred on a 3-year follow-up period, which helped mitigate this limitation. Furthermore, due to the limited number of variables, granular assessment of patient-level factors (e.g., race/ethnicity) or severity of comorbidities that may drive the effects of GLP-1RA could not be assessed. Despite these limitations, the study provides valuable insight into the impact that GLP-1RA may have on patients with concurrent liver disease, which otherwise would be challenging to study in randomised controlled trials.
In conclusion, GLP-1RA may potentially mitigate the risk of ALO, particularly hepatic decompensation, among patients with ALD and concurrent T2DM. As such, these medications may be an effective therapeutic tool among these patients. Future trials are needed to further investigate the role of GLP-1RA among patients with liver disease.
Acknowledgements
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Disclaimer
The authors have nothing to report.
Open Research
Data Availability Statement
The data for this study were obtained from the IBM Marketscan database. There are restrictions to the availability of these data, which is used under licence for this study. Data can be accessed with permission from the IBM Marketscan Commercial Database.




