Impact of NAFLD on the outcome of patients with chronic hepatitis B in Asia
Funding information: This work was supported by grants from the National Natural Science Foundation of China (No. 81870407 to Lai Wei).
Handling Editor: Luca Valenti
Abstract
Hepatitis B virus (HBV) infection and nonalcoholic fatty liver disease (NAFLD) are two major causes of chronic liver disease (CLD) that can cause liver cirrhosis and hepatocellular carcinoma (HCC). It is a trend to superimpose NAFLD on chronic HBV infection in Asia. This review presents the epidemiology of concurrent NAFLD in chronic hepatitis B (CHB) patients and focuses on the impact of concurrent NAFLD on the outcome of CHB patients in Asia. Although CHB patients tend to have a lower prevalence and incidence of NAFLD than the general population, concurrent NAFLD among CHB patients is still common and has an upward trend over time. Concurrent NAFLD can promote hepatitis B surface antigen (HBsAg) seroclearance and might inhibit HBV replication but exacerbate liver fibrosis. The impacts of concurrent NAFLD on HCC risk, all-cause mortality and antiviral treatment response in CHB patients remain controversial.
Abbreviations
-
- aHR
-
- adjusted hazard ratio
-
- ALT
-
- alanine aminotransaminase
-
- AST
-
- aspartate aminotransferase
-
- BMI
-
- body mass index
-
- CAP
-
- controlled attenuation parameter
-
- CHB
-
- chronic hepatitis B
-
- CHC
-
- chronic hepatitis C
-
- CI
-
- confidence interval
-
- CLD
-
- chronic liver disease
-
- DAAs
-
- direct-acting antivirals
-
- DM
-
- diabetes mellitus
-
- ETR
-
- end-of-treatment response
-
- HBeAg
-
- hepatitis B e antigen
-
- HBsAg
-
- hepatitis B surface antigen
-
- HBV
-
- hepatitis B virus
-
- HCV
-
- hepatitis C virus
-
- HDL
-
- high-density lipoprotein
-
- HOMA-IR
-
- homeostatic model assessment of insulin resistance
-
- LDL
-
- low-density lipoprotein
-
- HCC
-
- hepatocellular carcinoma
-
- HDV
-
- hepatitis D virus
-
- HIV
-
- human immunodeficiency virus
-
- LS
-
- liver stiffness
-
- MAFLD
-
- metabolic dysfunction-associated fatty liver disease
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NAs
-
- nucleos(t)ide analogues
-
- NASH
-
- non-alcoholic steatohepatitis
-
- OR
-
- Odds ratio
-
- PEG-IFN
-
- pegylated interferon
-
- qHBsAg
-
- quantitative HBsAg
-
- SMD
-
- standardized mean difference
-
- SVR
-
- sustained virologic response
Key points
- Concurrent nonalcoholic fatty liver disease (NAFLD) is common and increasing among chronic hepatitis B (CHB) patients in Asia.
- CHB patients tend to have a lower prevalence and incidence of NAFLD than the general population.
- Concurrent NAFLD can promote hepatitis B surface antigen seroclearance and might inhibit hepatitis B virus replication.
- Severe steatosis and steatohepatitis might exacerbate liver fibrosis in CHB patients.
- The impacts of concurrent NAFLD on hepatocellular carcinoma risk, all-cause mortality, and antiviral treatment response in CHB patients remain controversial.
1 INTRODUCTION
Hepatitis B virus (HBV) infection and nonalcoholic fatty liver disease (NAFLD) are two major causes of chronic liver disease (CLD) that can cause liver cirrhosis and hepatocellular carcinoma (HCC).1, 2 Although vaccination has dramatically reduced new HBV infection, WHO estimated 3.5% of the population worldwide (257 million people) were chronically infected with HBV in 2015.3 Most Asian regions are medium to high prevalence areas of HBV infection.4 Nowadays, NAFLD is the most common CLD in the world, with a global prevalence of about 25%.2 Accompanied by the epidemic of obesity and change of lifestyle, the burden of NAFLD in Asia continues to increase and the prevalence is about 29%.5 It is a trend to superimpose NAFLD on chronic HBV infection in Asia. Recently, the international fatty liver expert group proposed a new name as metabolic dysfunction-associated fatty liver disease (MAFLD), which partially overlaps with NAFLD.6 This paper still uses the naming and definition of NAFLD because of most data coming from NAFLD related studies. We will review the epidemiology of concurrent NAFLD in chronic hepatitis B (CHB) patients and focus on the impact of concurrent NAFLD on the outcome of CHB patients in Asia.
| Location | study | Study period | No. of HBV patients | Concurrent NAFLD | Diagnostic method | Factors associated with concurrent NAFLD in CHB patients by multivariable analysis |
|---|---|---|---|---|---|---|
| Hong Kong | Wong et al, 20127 | 2008–2010 | 75 | 10 (13.5%) | 1H-MRS | age |
| Chan et al, 20178 | 2006–2009 | 270 | 107 (39.6%) | Histology | NA | |
| Seto et al, 20189 | 2015–2016 | 1606 | 655 (40.8%) | Transient elastography | NA | |
| India | Rastogi et al, 201010 | NA | 350 | 118 (33.7%) | Histology | Triglyceride |
| Iran | Minakari et al, 200911 | NA | 132 | 56 (42.4%) | Histology | Triglyceride |
| Poortahmasebi et al, 201412 | 2010–2011 | 160 | 71 (44.4%) | Histology | Sex, BMI, cholesterol, fasting glucose | |
| Israel | Peleg et al, 201913 | 2007–2017 | 524 | 241 (46.0%) | Histology or ultrasound | NA |
| Mainland China | Peng et al, 200814 | 2002–2006 | 153 | 41 (26.8%) | Histology | Sex, BMI, ALT |
| Shi et al, 200815 |
2005 2006 2007 |
750 902 263 |
84 (11.2%) 129 (14.3%) 47 (17.9%) |
Histology | BMI, fasting glucose, triglyceride, uric acid, apolipoprotein B | |
| Zheng et al, 201016 | 2005–2009 | 204 | 106 (52.0%) | Histology | Fasting insulin | |
| Wang et al, 201417 |
2002–2009 2010–2011 |
2733 479 |
418 (15.3%) 136 (28.4%) |
Histology | Sex, age, intrahepatic HBsAg-positive staining | |
| Gong et al, 201518 | 2011–2013 | 89 | 31 (34.5%) | Histology | NA | |
| Zhang et al, 201619 | 2011–2015 | 364 | 118 (32.4%) | Histology | Age, BMI, haemoglobin, triglyceride, uric acid | |
| Huang et al, 202020 | 2016–2018 | 2110 | 632 (29.9%) | Ultrasound | NA | |
| Lv et al, 202121 | 2013–2017 | 5680 | 1769 (31.1%) | Transient elastography | BMI, ALT, triglyceride, LDL, platelet | |
| Lin et al, 202122 | 2016–2018 | 4734 | 1275 (26.9%) | Ultrasound | NA | |
| Malaysia | Wong et al, 202023 | 2013–2017 | 614 | 294 (47.9%) | Transient elastography | Malay ethnicity, DM, dyslipidemia, advanced liver fibrosis |
| South Korea | Yun et al, 200924 | 2005–2006 | 86 | 44 (51.2%) | Histology | Triglyceride, HOMA-IR |
| Lee et al, 201925 | 2007–2015 | 321 | 70 (21.8%) | Histology | NA | |
| Taiwan | Lin et al, 200726 | 2004–2005 | 817 | 277 (33.9%) | Ultrasound | NA |
| Wang et al, 200827 | NA | 50 | 28 (56.0%) | Ultrasound | NA | |
| Thailand | Charatcharoenwitthaya et al, 201728 | 2010–2013 | 256 | 98 (38.3%) | Histology | BMI, central obesity, DM, HDL-cholesterol |
| Turkey | Altlparmak et al, 200529 | 1997–2002 | 164 | 64 (39.0%) | Histology | NA |
| Cindoruk et al, 200730 | 2002–2006 | 140 | 48 (34.3%) | Histology | NA | |
| Yilmaz et al, 201531 | NA | 88 | 28 (31.8%) | Histology | HOMA-IR |
- Abbreviations: ALT, alanine aminotransaminase; BMI, body mass index; CHB, chronic hepatitis B; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; DM, diabetes mellitus; LDL, low-density lipoprotein; NA, not available; NAFLD, nonalcoholic fatty liver disease.
2 PREVALENCE AND INCIDENCE OF CONCURRENT NAFLD IN CHB PATIENTS IN ASIA
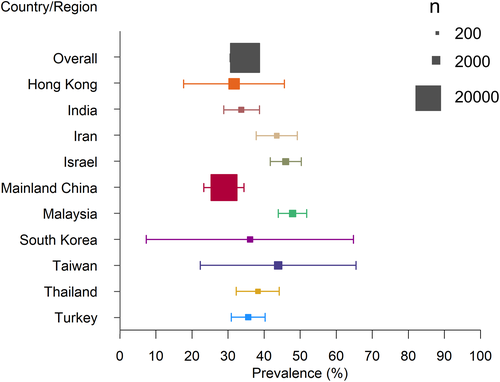
Table 1 showed the studies on the prevalence of concurrent NAFLD in CHB patients in Asian countries or regions. The pooled prevalence of concurrent NAFLD in CHB patients regardless of the diagnostic method was 29.0% (range 13.5%–56.0%). Most studies were from Mainland China (36%) and Hong Kong (12%). Of the countries or regions in which more than two studies had been done, the pooled prevalence of concurrent NAFLD in CHB patients was 39.6% in Hong Kong, 43.5% in Iran, 25.9% in Mainland China, 28.0% in South Korea, 35.2% in Taiwan, and 35.7% in Turkey. Despite the study number, period and diagnostic method, concurrent NAFLD in Asian CHB patients was most prevalent in Malaysia (47.9%) and least prevalent in Mainland China (25.9%). Figure 1 shows the prevalence of concurrent NAFLD among CHB patients in Asian countries or regions. Two studies from Mainland China demonstrated an upward trend in the prevalence of concurrent NAFLD over time.15, 17 We found that the pooled prevalence of concurrent NAFLD in CHB patients increased from 21.0% before 2010 to 31.8% after 2010. All these findings concur with the rising trend of global and Asian prevalence of NAFLD.2, 5 The independent risk factors of concurrent NAFLD in CHB patients mainly included sex,12, 14, 17 age,7, 17, 19 body mass index (BMI),12, 14, 15, 19, 21, 28 disordered glucose metabolism,12, 15, 16, 23, 24, 28, 31 dyslipidemia,10-12, 15, 19, 21, 23, 24, 28 and uric acid,15, 19 which are also risk factors of NAFLD in general population.32 Most cross-sectional studies of CHB patients only did not find HBV markers, including hepatitis B e antigen (HBeAg) and HBV DNA, were independent risk factors of hepatic steatosis. It seems that host metabolic factors but not viral factors are responsible for hepatic steatosis in CHB patients.
Patients with HBV infection seem to have a lower prevalence of NAFLD than the general population.33 A cross-sectional study (n = 1013) from Hong Kong indicated that the prevalence of NAFLD was 13.5% in hepatitis B patients and 28.3% in controls (p = 0.003).7 A large-scale cross-sectional study (n = 33 439) from Taiwan demonstrated that fatty liver was diagnosed in 38.9% of CHB patients and 44.5% of non-HBV subjects (p < 0.001).34 A cross-sectional study (n = 14 452) from Mainland China found that the prevalence of NAFLD was 29.9% in the current HBV infection group, 35.8% in the past HBV infection group, and 31.9% in the non-infection group (p < 0.001).20 After adjusting for demographic and metabolic factors, patients with HBV infection still had a lower risk of NAFLD in these studies.
Some cohort studies provide us with a good opportunity to see whether HBV protects or promotes concurrent NAFLD in CHB patients. A large-scale cohort study (n = 83 339) from South Korea with a mean follow-up of 6.6 years found that the annual incidence of NAFLD development was 40.6 per 1000 person-years in hepatitis B surface antigen (HBsAg)-positive subjects and 43.5 per 1000 person-years in HBsAg-negative subjects (adjusted hazard ratio [aHR] = 0.83, 95% confidence interval [CI]: 0.73–0.94), indicating HBV infection is negatively correlated with the incidence of NAFLD.35 Another cohort study of adult CHB patients (n = 2393) from Mainland China indicated that the incidence rate of NAFLD was 63.89 per 1000 person-years, and detectable HBV DNA levels were negatively associated with the development of NAFLD in type 2 diabetes mellitus (DM) subgroup (HR = 0.37, 95% CI: 0.14–0.98).36
The mechanism of the phenomenon that CHB patients have a lower chance of NAFLD than the general population remains unclear. Adiponectin, a type of adipokine that can improve insulin sensitivity and alleviate hepatic steatosis, was found to be higher in HBV-infected patients and cell models.37, 38 Nevertheless, other basic studies showed HBV, HBsAg, or HBx might lead to metabolic dysregulation and hepatic steatosis in animal and cell models through upregulating hepatic de novo lipogenesis39, 40 and fatty acid-binding protein,41 inhibiting apolipoprotein B secretion42 and fatty acid oxidation.43 These paradoxes need to be clarified in more large-scale prospective cohort studies and appropriate animal models that imitate NAFLD and HBV infection simultaneously.44
3 IMPACTS OF CONCURRENT NAFLD ON THE OUTCOME OF CHB PATIENTS IN ASIA
3.1 Concurrent NAFLD can promote HBsAg seroclearance
HBsAg seroclearance is an important endpoint for CHB patients and is associated with a lower risk of decompensated cirrhosis, HCC, and liver-related death.45, 46 Patients with spontaneous or nucleos(t)ide analogues (NAs)-induced HBsAg seroclearance to have similarly low HCC risk.47 HBsAg seroclearance may occur in about 1% of cases per year.48 After HBsAg seroclearance, coexisting fatty liver is an independent risk factor for HCC.49
HBsAg seroclearance is affected by a variety of host and viral factors, such as quantitative HBsAg (qHBsAg), HBeAg status, and HBV DNA level at baseline.48 Some studies show that HBsAg seroclearance is also affected by concurrent NAFLD. A case-control study by Chu et al. from Taiwan including 54 patients with HBsAg seroclearance and 108 patients with HBsAg persistence demonstrated that moderate (odds ratio [OR] = 3.22, p = 0.02) and severe (OR = 3.87, p = 0.04) hepatic steatosis were significantly associated with spontaneous HBsAg seroclearance, although confounders were not adjusted.50 The authors proposed that liver fatty infiltration might interfere with the cytoplasmic distribution of HBsAg and increase HBsAg seroclearance.50 Another study from this team including 155 patients with HBsAg seroclearance showed that the age of patients with hepatic steatosis to achieve spontaneous HBsAg seroclearance was about 5 years earlier than those without (48.7 ± 8.9 years vs. 53.4 ± 8.9 years, p = 0.001).51 Of note, patients with hepatic steatosis had a significantly higher incidence of abnormal alanine aminotransferase (ALT) (23.2% vs. 0%, p < 0.0001), aspartate aminotransferase (AST) (30.4% vs. 0%, p < 0.0001), and were more likely to progress to cirrhosis (10.1% vs. 3.5%, p = 0.09) than those without, implying disparity between HBsAg seroclearance and long-term prognosis.51 A cohort study of 4376 asymptomatic HBeAg negative HBsAg carriers from Taiwan found a significantly higher incidence of spontaneous HBsAg seroclearance in patients with hepatic steatosis than in those without (p < 0.001).52 Another cohort study from this team including 1386 HBeAg positive CHB patients and 5235 HBeAg negative CHB patients showed that hepatic steatosis was an independent predictor (HR = 1.222, p = 0.022) of spontaneous HBsAg seroclearance.53 A prospective cohort study from Hong Kong reported during a mean follow-up of 5.5 years, 22 of 330 (6.7%) treatment-naïve CHB patients with normal ALT and low viraemia achieved HBsAg seroclearance.54 Patients with hepatic steatosis had lower qHBsAg at baseline (4.89 log10mIU/ml vs. 5.65 log10mIU/ml, p = 0.002) and a higher cumulative probability of HBsAg seroclearance at 36 months (9.9% vs. 3.7%, p = 0.008). Cox regression analysis confirmed that hepatic steatosis was independently associated with HBsAg seroclearance (HR = 3.246, p = 0.013).54
Hepatic steatosis is related not only to the spontaneous HBsAg seroclearance but also to HBsAg seroclearance after treatment. In a study from Mainland China, 23 paediatric CHB subjects received pegylated interferon (PEG-IFN) monotherapy and 57 paediatric CHB subjects received PEG-IFN and NAs combination therapy.55 NAFLD significantly increased HBsAg loss at 96 weeks of antiviral treatment (aHR = 3.245, 95% CI: 1.288–8.176).55
3.2 Concurrent NAFLD might inhibit HBV replication
Many cross-sectional studies did not find a correlation between NAFLD and HBV DNA levels.7, 11, 12, 14, 15, 23, 24, 28-31 But a cross-sectional study of 204 CHB patients from Mainland China showed that mean HBV DNA levels were significantly lower in patients with steatosis than in those without (3.29 log10IU/ml vs. 5.61 log10IU/ml, p = 0.0081).16 Similarly, another cross-sectional study of 3212 patients with chronic HBV infection from Mainland China demonstrated that patients with steatosis had lower percentages of serum HBeAg-positive and detectable HBV DNA, and intrahepatic HBsAg- and HBcAg-positive staining than those without (all p < 0.001).17 A meta-analysis of seven studies and 3067 CHB patients also showed a negative association between HBV viral load and hepatic steatosis (standardized mean difference [SMD] = −74.12, p < 0.001).56 A matched case-control study by Hui et al. from Hong Kong including 1202 CHB patients indicated that patients with steatosis had lower median serum HBV DNA than controls (3.0 log10IU/ml vs. 3.4 log10IU/ml, p < 0.05) in treatment-naïve patients and the inverse relationship still existed after multivariate analysis.57 Median HBV DNA levels decreased with the increase of steatosis severity (no steatosis vs. mild to moderate steatosis vs. severe steatosis = 3.1 log10IU/ml vs. 2.9 log10IU/ml vs. 2.6 log10IU/ml, p = 0.032), suggesting the potential negative effects of NAFLD on viral replication. Of note, the authors simultaneously found severe steatosis was associated with a higher percentage of severe fibrosis, implying a disparity between suppressed HBV DNA and exacerbated fibrosis in CHB and concomitant NAFLD patients.57 The negative association between NAFLD and HBV DNA was also supported by a retrospective study from Israel including 524 treatment-naïve CHB patients.13
Although the above studies showed that hepatic steatosis could promote HBsAg seroclearance and might inhibit HBV replication, we should pay attention to the confounders, such as age, in the interpretation of the conclusion. And as shown by Chu et al.51 and Hui et al.,57 we also need to notice the disparity between HBV virologic changes and long-term prognosis in CHB and concomitant NAFLD patients.
3.3 Concurrent NAFLD might exacerbate liver fibrosis in CHB patients
The severity of fibrosis is an important determinant of prognosis in CHB patients.58 Predictors for liver fibrosis progression in CHB patients include old age, male gender, alcohol consumption, co-infection with HCV, hepatitis D virus (HDV), or human immunodeficiency virus (HIV), elevated ALT, high HBV DNA level, and so on.59 Many previous cross-sectional studies did not find a correlation between hepatic steatosis and fibrosis in CHB patients.11, 14-16, 24, 29, 31 Nonalcoholic steatohepatitis (NASH) is considered the progressive form of NAFLD. In 2017, a study from Thailand including 256 CHB patients with HBV DNA >2000 IU/ml showed that 38% of the entire patients had hepatic steatosis and 18% of the patients with steatosis had steatohepatitis.28 Biopsy-proven hepatic steatosis was not associated with significant fibrosis (OR = 1.31, p = 0.296) and advanced fibrosis (OR = 0.91, p = 0.808), but hepatic steatohepatitis was independently associated with significant fibrosis (OR = 10.0, p = 0.002) and advanced fibrosis (OR = 3.45, p = 0.032) after adjusting for viral factors and metabolic syndrome. It seems that steatohepatitis, but not simple steatosis, plays a role in disease progression in HBV infection.28 In 2018, the aforementioned match case-control study of 1202 CHB patients from Hong Kong used transient elastography to test liver stiffness (LS) and controlled attenuation parameter (CAP).57 Patients with severe steatosis had an increased percentage of severe fibrosis (23.2% vs. 12.6%, P = 0.005) when compared to those with mild or moderate steatosis. It suggests that severe steatosis is associated with increased fibrosis in CHB patients.57 A cross-sectional study from the same team including 1606 CHB patients also showed patients with severe steatosis had an increased percentage of severe fibrosis (21.4% vs. 11.9%, p < 0.001) when compared to those with no, mild or moderate steatosis.9 Further multivariate analysis indicated the positive association between severe steatosis and severe fibrosis existed in both treatment-naïve patients (OR = 3.60, 95% CI: 1.21–10.75) and patients receiving NAs treatment for at least three years (OR = 1.95, 95% CI: 1.06–3.61). This study shows that severe steatosis is an additional metabolic risk factor of CHB-related fibrosis besides obesity, metabolic syndrome, and DM.9 In 2019, a cohort study from the same team including 459 HBeAg negative patients evaluated the evolution of liver fibrosis across a 10-year interval.60 No treatment with NAs (OR = 2.259, 95% CI: 1.032–4.945), metabolic syndrome (OR = 4.379, 95% CI: 1.128–16.999) and hepatic steatosis (OR = 7.799, 95% CI: 2.271–26.776) were associated with fibrosis progression. This study emphasizes the importance of severe steatosis in CHB long-term management, especially for the patients with HBV DNA suppressed by NAs treatment.60 In 2020, the aforementioned prospectively cohort study of 330 treatment-naïve CHB patients with normal ALT and low viraemia from the same team found the proportion of patients with F3/F4 fibrosis increased from 4.2% at baseline to 8.7% at 3 years.54 The patients with persistent severe steatosis had a higher rate of liver fibrosis progression (41.3% vs. 23%, p = 0.05) when compared to those without steatosis. Persistent severe steatosis was independently associated with fibrosis progression (OR = 2.379, p = 0.01) and resolution of severe steatosis was independently associated with fibrosis regression (OR = 4.385, p = 0.003). It reminds us that persistent severe hepatic steatosis increases the risk of fibrosis progression despite virological quiescence.54 In 2020, a retrospective cohort study of 614 CHB patients from Malaysia with a median follow-up of 45 months also used transient elastography to diagnose fibrosis and steatosis.23 Hepatic steatosis was independently associated with advanced fibrosis (OR = 1.956, 95% CI: 1.250–3.060).23 After analysis of the above studies showing the positive association of hepatic steatosis and fibrosis in CHB patients, we find that they have a relatively large sample size and further investigate the relationship of steatohepatitis or severe steatosis with fibrosis.
Obesity, metabolic dysregulation, and DM are included in the diagnosis criteria for the new term “MAFLD”. Literature shows that obesity, metabolic syndrome and DM are important risk factors for liver fibrosis or cirrhosis in CHB patients. In 2008, a prospective study from Taiwan including 2903 male HBsAg-positive subjects with a mean follow-up of 14.7 years showed that obesity was associated with the development of fatty liver (aOR = 9.72, p < 0.001) and cirrhosis (aOR = 2.37, p = 0.005).61 In 2009, a study of 1466 CHB patients from Hong Kong showed that metabolic syndrome was more prevalent in patients with probable cirrhosis than those without cirrhosis (24% vs. 11%, p < 0.001).62 Metabolic syndrome (OR = 1.7, 95% CI: 1.1–2.6) was independently associated with probable cirrhosis after adjusting for anthropometric, biochemical and virological factors.62 Subsequently, the team followed this cohort prospectively and found that coincidental metabolic syndrome (aOR = 2.0, p = 0.015), central obesity (aOR = 2.0, p = 0.05), and low level of high-density lipoprotein cholesterol (aOR = 1.9, p = 0.04) were independent risk factors of fibrosis progression.63 In 2014, another prospective study from the same team including 413 treatment-naïve HBeAg-positive CHB patients showed that advanced liver fibrosis was more prevalent in patients with pre-metabolic and metabolic syndromes than in normal patients (33.0% vs. 53.1% vs. 18.4%, all p < 0.05), supporting that pre-metabolic and metabolic syndromes increase the risk of advanced liver fibrosis.64 A large cohort study of 8237 CHB patients from Taiwan found that the incidence rate of cirrhosis was 1.31 per 10 000 person-years in patients with newly diagnosed DM and 0.28 per 10 000 person-years in those without DM.65 Newly diagnosed DM was an independent risk factor of cirrhosis (aHR = 2.055, p < 0.001) and decompensation (aHR = 1.801, p = 0.005) after adjusting for age, sex, antiviral treatment, HCC, and comorbidity indexes.65
3.4 Impact of concurrent NAFLD on HCC risk in CHB patients remains controversial
HBV infection is the common cause of HCC worldwide, especially in Asian countries, whilst the role of NAFLD on HCC is usually underestimated.66 Some studies have shown that NAFLD increases the risk of HCC in CHB patients. In 2017, a retrospective cohort study from Hong Kong showed that biopsy-proven concurrent fatty liver was found in 107 (39.6%) of 270 CHB patients without significant alcohol intake at baseline and 9 (81.8%) of 11 incident HCC patients at a median follow-up of 79.9 months.8 Concurrent fatty liver (aHR = 7.3, p = 0.013) was independently associated with HCC development in addition to age, cirrhosis, and APOC3 rs2854116 TC/CC genotype.8 In 2019, another retrospective cohort study from South Korea showed that biopsy-proven fatty liver was found in 70 (21.8%) of 321 CHB patients without excessive alcohol intake at baseline and 8 (47.1%) of 17 incident HCC patients at a median follow-up of 5.3 years.25 The 5-year cumulative incidences of HCC among patients with and without fatty liver were 8.2% and 1.9%, respectively (p = 0.004). Coexisting fatty liver was an independent risk factor of HCC (aHR = 3.005, p = 0.03) after adjusting for age and cirrhosis, but it was no longer (aHR = 1.709, p = 0.47) after balancing with inverse probability weighting for metabolic factors, suggesting that coexisting NAFLD potentiates the risk for HCC in CHB patients as a hepatic manifestation of metabolic syndrome.25
Otherwise, some studies have shown that concurrent NAFLD does not increase the risk of HCC in CHB patients, and even protects them from HCC. A retrospective cohort study from Singapore showed that biopsy-proven hepatic steatosis was found in 185 (64.0%) patients of 289 CHB patients without significant alcohol intake at baseline and 21 (77.8%) of 27 incident HCC patients at a median follow-up of 111.1 months.67 Concurrent hepatic steatosis in patients with CHB infection was not associated with HCC development (HR = 2.445, p = 0.056).67 The aforementioned prospective study from Taiwan including 2903 male CHB patients showed ultrasonographically diagnosed concurrent fatty liver was associated with a lower risk of HCC development (aHR = 0.24, 95% CI: 0.14–0.42).61 A prospective cohort study from Hong Kong showed that CAP diagnosed steatosis was found in 1156 (48.1%) patients of 2403 CHB patients without significant alcohol intake at baseline and 12 (25.0%) of 48 incident HCC patients at a median follow-up of 21.7 months.68 The cumulative probability of HCC increased with the decrease of hepatic steatosis (no steatosis vs. mild to moderate steatosis vs. severe steatosis = 2.88% vs. 1.56% vs. 0.71%, p = 0.01). CAP value was negatively associated with HCC development in multivariate Cox regression analysis (HR = 0.993, 95% CI: 0.987–0.999) and hepatic steatosis was negatively associated with HCC development in propensity score matching analysis (HR = 0.41, 95% CI: 0.21–0.83). The authors speculated that the negative association between hepatic steatosis and HCC development was as a result of: (1) suppression of HBsAg and HBV DNA or any upstream steps of viral lifecycle by steatosis, (2) “burnt-out NASH” usually with low CAP value but more severe fibrosis even cirrhosis.68 Similarly, a retrospective cohort study from South Korea also demonstrated that among 1823 CHB patients with HBV replication suppressed by antiviral therapy, a low CAP value predicted a higher risk for HCC among patients with LS above 10 kPa (aHR 0.47, p = 0.003).69
The impact of NAFLD on the recurrence of CHB-related HCC has also been explored. A retrospective cohort study from South Korea including 338 CHB-related HCC who underwent surgical resection found the patients with NAFLD had significantly better overall survival (OS) (log-rank, p = 0.004) and tended to have better recurrence-free survival (RFS) (log-rank, p = 0.16) than those without.70 However, the OS benefit of the concurrent NAFLD no longer existed after multivariable Cox analysis (aHR 0.94, p = 0.84) or after propensity score matching (log-rank, p = 0.57).70
These different conclusions may be related to the different study design, sample size, patient population and diagnose method of NAFLD. But more importantly, it reflects the battle of the inhibition of the HBV virus, the promotion of fibrosis, and other complex changes caused by concurrent NAFLD. Additional high-quality large-scale prospective studies are needed to explore the exact impact of NAFLD on HCC development in CHB patients.
3.5 Impact of concurrent NAFLD on all-cause mortality in CHB patients remains controversial
It has been demonstrated that NAFLD can increase mortality from cardiovascular diseases and extrahepatic tumours in the general population.71 The aforementioned retrospective cohort study of 524 treatment-naïve CHB patients from Israel with a mean follow-up of 73.62 months found 62 composite outcomes, including 20 mortality events and 42 newly diagnosed cancer.13 Liver steatosis was significantly associated with composite endpoint (HR = 4.35, p < 0.001) whilst baseline HBV viral load wasn't (HR = 1.65, p = 0.298).13
However, some studies have reached different conclusions. The aforementioned prospective study of 2903 Taiwanese male CHB patients showed that the aHRs for liver-related mortality were 0.18 (95% CI: 0.08–0.41) in patients with ultrasonographically diagnosed concurrent fatty liver, 1.74 (95% CI: 1.15–2.65) in overweight patients, and 1.50 (95% CI: 0.36–6.19) in obese patients.61 It indicated that excess body weight promoted liver-related mortality, whilst the fatty liver was the opposite.61 Similarly, the aforementioned study of 4376 asymptomatic HBeAg negative HBsAg carriers from Taiwan demonstrated that hepatic steatosis (aHR = 0.317, p = 0.001) and HBsAg clearance were negatively associated with mortality, while liver cirrhosis and age at entry were positively associated with mortality.52 Recently, a study from North America and Europe included 1089 CHB patients, 57% of whom were Asians. 52 (5.7%) of no-NASH (n = 904) and 27 (14.6%) of NASH (n = 185) experienced at least one outcome events during a median follow-up of 10 years, including 20 decompensation, 35 HCC, 24 deaths.72 The presence of advanced fibrosis and concomitant NASH (aHR = 4.8, p < 0.01) or advanced fibrosis alone (aHR = 2.3, p = 0.004) significantly increased the risk of outcome events when compared to the absence of NASH and advanced fibrosis, but NASH alone did not (aHR = 1.23, p = 0.796). It suggests that advanced fibrosis, but not NASH, is the major driver of poor outcomes.72 Therefore, the impact of concurrent NAFLD on all-cause mortality in CHB patients has not been fully clarified.
3.6 Impact of concurrent NAFLD on antiviral therapy response in CHB patients remains controversial
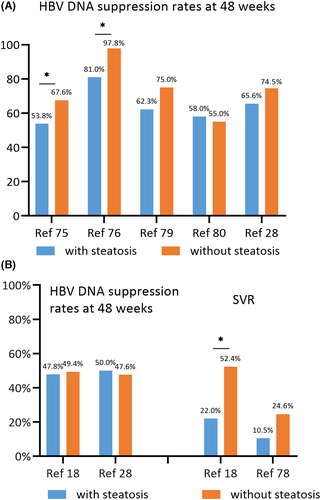
CHC patients with concurrent fatty liver have a poorer response to PEG-IFN and ribavirin combination therapy73 but the similar response to direct-acting antivirals (DAAs).74 The impact of concurrent NAFLD on antiviral therapy of CHB is controversial. Some studies have demonstrated that concurrent NAFLD weakens the antiviral efficacy of CHB. A nested case-control study from Mainland China including 267 entecavir-treated CHB patients found that 54.9%, 63.8%, and 74.2% of patients had HBV DNA clearance at 24, 48 and 96 weeks.75 Hepatic steatosis was significantly associated with treatment failure at these timepoints (all p<0.05).75 Another study from Mainland China including 153 entecavir-treated CHB patients reported that the rates of HBV DNA clearance and ALT normalization were significantly lower in patients with high CAP compared with those with normal CAP at 12, 24 and 48 weeks (all p<0.05).76 A study from Mainland China including 89 CHB patients treated with PEG-IFN reported that patients with hepatic steatosis had a similar end-of-treatment response (ETR) at 48 weeks (p > 0.05) but lower sustained viral response (SVR) at 96 weeks than those without (p < 0.05).18 Multivariate analysis showed that steatosis was negatively associated with SVR (OR = 0.012, p = 0.020).18 A meta-analysis of eight prospective cohort studies and 1087 CHB patients also demonstrated that patients with hepatic steatosis had a lower biochemical and virological response at weeks 48 and 96 when compared to those without.77
But more studies have not found an association between concurrent NAFLD and the efficacy of antiviral therapy. Cinodruk et al.30 (n = 140) and Ates et al.78 (n = 84) from Turkey did not discover a correlation between hepatic steatosis and SVR of PEG-IFN based antiviral treatment. A retrospective study of 125 entecavir-treated CHB patients from Mainland China observed patients with concurrent NAFLD had a similar virologic response rate (p > 0.05) but lower ALT normalization rate (p < 0.05) at 48 and 96 weeks when compared to those without.79 A retrospective study from the United States included 555 NAs-treated CHB patients, 87.6% of whom were Asians.80 Patients with concurrent NAFLD achieved similar rates of complete viral suppression (86% vs. 88%, p > 0.05) and biochemical response (38% vs. 41%, p > 0.05) during the follow-up of up to 60 months, and NAFLD was not a predictor for treatment outcomes in multivariate analysis.80 The aforementioned study from Thailand (33 treated with PEG-IFN and 79 treated with NAs) showed the virologic response combined with aminotransferase normalization rates at 48 weeks were not different between the steatohepatitis and non-steatohepatitis groups (43% vs. 53%, p < = 0.475).28 Figure 2 shows the response rates to NAs or PEG-IFN in CHB patients with and without hepatic steatosis.
Of note, hepatic steatosis and obesity may hinder fibrosis regression in CHB patients during antiviral treatment. The aforementioned study from Hong Kong including 235 NAs-treated HBeAg negative patients found that hepatic steatosis was independently associated with fibrosis progression (OR = 7.799, p = 0.001).60 Another study from the same team including 123 severe liver fibrosis demonstrated that 29.3% of the total had fibrosis regression after median NAs treatment of 87.5 months, with lower rates of 17.9%, 14.9% and 11.5% in patients with BMI ≥ 25 kg/m,2 metabolic syndrome and DM, respectively.81 Lower BMI was the only factor independently associated with fibrosis regression (OR = 0.68, p = 0.034).81 It suggests that antiviral therapy alone cannot solve all the problems and control of metabolic factors will be beneficial to the reversal of liver fibrosis in CHB patients.
4 CONCLUSIONS
Although CHB patients tend to have a lower prevalence and incidence of NAFLD than the general population, concurrent NAFLD among CHB patients is still common and has an upward trend over time in Asia. Clinical studies have demonstrated that concurrent NAFLD can promote HBsAg seroclearance, and might inhibit HBV replication but exacerbate liver fibrosis. Additional high-quality large-scale prospective studies are needed to explore the exact impacts of concurrent NAFLD on HCC risk, all-cause mortality, and antiviral treatment response in CHB patients.
ACKNOWLEDGEMENTS
We thank Prof. Yangfeng Wu and Dr. Yanjun Ma for their contribution to figure making.
CONFLICT OF INTEREST
MY does not have any disclosures to report. LW has received research grants from Abbvie, Bristol-Myers Squibb, and Gilead, and consulting for Gilead, Huahui, MSD, Pfizer, speaker for Ascletis Pharma, Bristol-Myers Squibb, Gilead, and Kaiyin.