Ramucirumab in patients with previously treated advanced hepatocellular carcinoma: Impact of liver disease aetiology
Funding information
This study was funded by Eli Lilly and Company (grant numbers not applicable)
Handling Editor: Pierre Nahon
ABSTRACT
Background & Aims
Hepatocellular carcinoma (HCC) is a common complication of chronic liver disease with diverse underlying aetiologies. REACH/REACH-2 were global phase III studies investigating ramucirumab in advanced HCC (aHCC) following sorafenib treatment. We performed an exploratory analysis of outcomes by liver disease aetiology and baseline serum viral load.
Methods
Meta-analysis was conducted in patients with aHCC and alpha-fetoprotein (AFP) ≥400 ng/mL (N = 542) from REACH/REACH-2 trials. Individual patient-level data were pooled with results reported by aetiology subgroup (hepatitis B [HBV] or C [HCV] and Other). Pre-treatment serum HBV DNA and HCV RNA were quantified using Roche COBAS AmpliPrep/COBAS TaqMan. Overall survival (OS) and progression-free survival (PFS) were evaluated using the Kaplan-Meier method and Cox proportional hazard model (stratified by study).
Results
Baseline characteristics were generally balanced between arms in each subgroup (HBV: N = 225, HCV: N = 127, Other: N = 190). No significant difference in treatment effect by aetiology subgroup was detected (OS interaction P-value = .23). Median OS (ramucirumab vs placebo) in months was 7.7 versus 4.5 (HR 0.74, 95% CI 0.55–0.99) for HBV, 8.2 versus 5.5 (HR 0.82, 95% CI 0.55–1.23) for HCV and 8.5 versus 5.4 (HR 0.56, 95% CI 0.40–0.79) for Other. Ramucirumab showed similar overall safety profiles across subgroups. Worst outcomes were noted in patients with a detectable HBV load. Use of HBV antiviral therapy, irrespective of viral load, was beneficial for survival, liver function and liver-specific adverse events.
Conclusions
Ramucirumab improved survival across aetiology subgroups with a tolerable safety profile, supporting its use in patients with aHCC and elevated AFP.
Abbreviations
-
- AFP
-
- alpha-fetoprotein
-
- aHCC
-
- advanced hepatocellular carcinoma
-
- ALBI
-
- albumin–bilirubin
-
- BCLC
-
- Barcelona Clinic Liver Cancer
-
- CI
-
- confidence interval
-
- ECOG PS
-
- Eastern Cooperative Oncology Group Performance Status
-
- HBV
-
- viral hepatitis B
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- viral hepatitis C
-
- HR
-
- hazard ratio
-
- NASH
-
- non-alcoholic steatohepatitis
-
- OS
-
- overall survival
-
- PCR
-
- polymerase chain reaction
-
- PFS
-
- progression-free survival
-
- TKI
-
- tyrosine kinase inhibitor
Lay summary
Hepatocellular carcinoma (HCC) is a common complication of chronic liver disease with diverse underlying aetiologies such as viral hepatitis, chronic alcohol consumption, and non-alcoholic steatohepatitis. In this analysis of two-phase III studies (REACH and REACH-2), the effects of ramucirumab in relation to liver disease aetiology for patients with advanced HCC are described in detail. In patients with previously treated advanced HCC and elevated alpha-fetoprotein, ramucirumab improved survival irrespective of liver disease aetiology.
1 BACKGROUND
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death globally.1 Rates of HCC are highest in East Asia but are continuing to rise in India and most countries in Europe, North/South America and Oceania.2 HCC commonly occurs as a result of chronic liver disease secondary to viral hepatitis B (HBV) or C (HCV) infection, although it can also be caused by other factors such as heavy alcohol use (more common in developed countries).3 Additionally, the rise of obesity and associated metabolic disorders in developed countries have contributed to an increase of HCC associated with non-alcoholic fatty liver disease and non-alcoholic steatohepatitis (NASH) further adding to the complexity and diversity of HCC disease aetiologies.4
Survival outcomes can differ by HCC disease aetiology with notable differences between systemic treatments. Post-hoc data analyses suggest that sorafenib, a first-generation tyrosine kinase inhibitor (TKI), is less beneficial in patients with HBV compared with other disease aetiologies while second-generation TKI cabozantinib appeared to be less beneficial in patients with HCV.5, 6 Checkpoint inhibitor pembrolizumab works less well in patients with HCV versus other disease aetiologies,7 while patients with HBV loads >100 IU/mL were excluded from the trial, and have been consistently omitted from clinical trials with immune checkpoint inhibitors.7-9 A detectable HBV or HCV load in patients with HCC is associated with poorer outcomes than in patients without detectable viral loads.10, 11 Antiviral treatment has been shown to reduce the risk of developing HCC and clinical guidelines support treatment in patients with HCC and viral hepatitis.12-15
REACH and REACH-2 studied ramucirumab, an immunoglobulin G1 monoclonal antibody to vascular endothelial growth factor 2, in patients with advanced HCC following sorafenib.16, 17 REACH-2 met its primary overall survival endpoint for ramucirumab versus placebo, demonstrating increased overall survival in patients with baseline alpha fetoprotein (AFP) levels ≥400 ng/mL, consistent with outcomes from the pre-specified population of patients in the REACH study with baseline AFP ≥400 ng/mL.16, 17 In both clinical trials, patients with chronic viral hepatitis (HBV or HCV) irrespective of viral load were eligible for enrolment.16, 17 In this exploratory analysis, we investigated the efficacy and safety of ramucirumab in patients with advanced HCC from REACH (patients with AFP ≥ 400 ng/mL) and REACH-2 by liver disease aetiology.
2 METHODS AND MATERIALS
REACH (NCT01140347) and REACH-2 (NCT02435433) were both global, randomised, placebo-controlled, double-blind, phase 3 trials (Figure 1).16, 17 Patients with advanced HCC, Barcelona clinic liver cancer (BCLC) stage C or B disease (refractory or not amenable to locoregional therapy), Child–Pugh class A liver disease (score <7), Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 or 1, and who were intolerant to or progressed on sorafenib were eligible. Patients with chronic viral hepatitis were eligible in both studies, irrespective of viral load. Treatment and management of chronic viral hepatitis was recommended but left up to the investigator’s discretion per local practice. REACH-2 restricted enrolment to patients with baseline serum AFP concentrations of ≥400 ng/mL whereas REACH did not.16, 17 Patients were randomised in REACH (1:1) and REACH-2 (2:1) to receive ramucirumab (8 mg/kg, intravenously) or placebo every 14 days (once every 2 weeks) until disease progression, unacceptable toxicity, or withdrawal of consent. Both studies complied with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and applicable local regulations. Ethics committees at all participating centers approved the protocol, and all patients provided written informed consent.16, 17
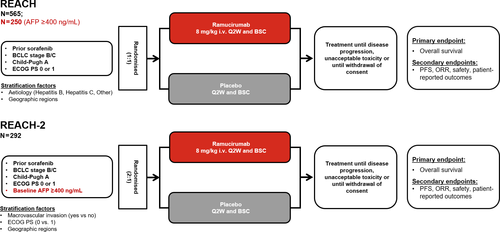
A meta-analysis of individual patient level data from REACH (patients with AFP ≥400 ng/mL) and REACH-2 was performed (pooled population). The pooling of patient-level data provided a substantially larger patient population, enabling a more precise estimation of the treatment effect in aetiology subgroup analyses. All pooled analyses were done at the level of individual patient data, stratified by study.16 The aetiology subgroups included HBV, HCV and Other. For patients where more than one aetiology has been reported, the classification was prioritised according to the order of HBV, HCV and then Other; the Other category refers to all aetiologies other than HBV or HCV (e.g., significant alcohol use, steatohepatitis, and haemochromatosis), including missing aetiology.18 This classification strategy was used for all efficacy and safety analyses. Overall survival (OS) was defined as the time from randomisation to death from any cause, and progression-free survival (PFS) was defined as the time from randomisation to radiographic progression or death.16, 17 Survival was evaluated using the Kaplan-Meier method and Cox proportional hazard model, and log-rank test p-value was used to compare Kaplan-Meier curves. Objective response rate and disease control rate were assessed per Response Evaluation Criteria in Solid Tumors, version 1.1. The aetiology subgroup-by-treatment interaction was tested using Wald test in the Cox model.
Pre-treatment serum HBV DNA was quantified using an HBV-specific polymerase chain reaction (PCR) (Roche COBAS AmpliPrep/COBAS TaqMan HBV Test v2.0) by a central laboratory. HBV DNA >15 IU/mL was considered detectable and <15 IU/mL was considered undetectable. Outcomes were assessed by concomitant antiviral therapy using combined treatment arms—ramucirumab and placebo. Pre-treatment serum HCV RNA was quantified using an HCV-specific PCR (Roche COBAS AmpliPrep/COBAS TaqMan HCV Test v2.0) by a central lab. HCV RNA >20 IU/mL was considered detectable and <20 IU/mL was considered undetectable.
Safety endpoints were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 throughout the study and for 30 days after treatment discontinuation. Laboratory monitoring, including serum albumin and bilirubin levels, were measured within 14 days prior to randomisation and before administration of study drug at each cycle (every 14 days). The ALBI (albumin–bilirubin) linear predictor was used to evaluate overall liver function. Patients were categorised into the ALBI grades by applying cut-offs to the linear predictor: Grade 1: ≤−2.60; Grade 2: > −2.60 and ≤ −1.39 and Grade 3: > −1.39.19
3 RESULTS
Data from a total of 225 patients with HBV, 127 patients with HCV, and 190 patients with “Other” aetiologies (Alcohol, Steatohepatitis and Other) were analysed (Table 1 and Figure S1). Across all aetiologies, most patients were male, had an ECOG PS of 0, BCLC stage C disease, and discontinued sorafenib due to progressive disease with a median duration of prior sorafenib of approximately 3–4 months. In the HBV aetiology subgroup, patients tended to be younger with higher rates of extrahepatic spread, had increased baseline AFP levels, and were primarily from Asia. Significant alcohol use was found to be the primary driver of disease in the other aetiology subgroup with steatohepatitis (NASH, fatty liver) and haemochromatosis following. Baseline characteristics were generally balanced between treatment arms within each aetiology subgroup with some differences observed in baseline AFP levels.
| Hepatitis B | Hepatitis C | Other | ||||
|---|---|---|---|---|---|---|
| n (%) except where indicated | RAM N = 124 | PL N = 101 | RAM N = 76 | PL N = 51 | RAM N = 116 | PL N = 74 |
| Sex, male | 101 (81.5) | 89 (88.1) | 56 (73.7) | 41 (80.4) | 89 (76.7) | 59 (79.7) |
| Age, years, median | 57 | 56 | 65 | 63 | 69 | 69 |
| ECOG PS 0 | 69 (55.6) | 46 (45.5) | 42 (55.3) | 33 (64.7) | 62 (53.4) | 39 (52.7) |
| Geographical region | ||||||
| Region 1 (Americas, Europe, Australia, Israel) | 20 (16.1) | 23 (22.8) | 41 (53.9) | 28 (54.9) | 93 (80.2) | 57 (77.0) |
| Region 2 (Asia excluding Japan) | 85 (68.5) | 64 (63.4) | 9 (11.8) | 6 (11.8) | 7 (6.0) | 8 (10.8) |
| Region 3 (Japan) | 19 (15.3) | 14 (13.9) | 26 (34.2) | 17 (33.3) | 16 (13.8) | 9 (12.2) |
| Child-Pugh Score A-5 | 91 (73.4) | 69 (68.3) | 35 (46.1) | 23 (45.1) | 64 (55.2) | 43 (58.1) |
| Barcelona Clinic Liver Cancer Stage C | 106 (85.5) | 93 (92.1) | 66 (86.8) | 43 (84.3) | 99 (85.3) | 61 (82.4) |
| Macrovascular invasion present | 36 (29.0) | 31 (30.7) | 32 (42.1) | 24 (47.1) | 45 (38.8) | 22 (29.7) |
| Extrahepatic spread present | 99 (79.8) | 84 (83.2) | 47 (61.8) | 36 (70.6) | 80 (69.0) | 51 (68.9) |
| Discontinued sorafenib due to PD | 109 (87.9) | 94 (93.1) | 65 (85.5) | 39 (76.5) | 100 (86.2) | 65 (87.8) |
| Discontinued sorafenib due to intolerance | 15 (12.1) | 7 (6.9) | 11 (14.5) | 12 (23.5) | 16 (13.8) | 9 (12.2) |
| Median duration of prior sorafenib (months) | 3.33 | 3.61 | 3.61 | 4.57 | 4.63 | 4.14 |
| Median alpha-fetoprotein (IQR) (ng/mL) | 5378 (1313–26 910) | 8490 (1325–23 750) | 3666 (1186–17 465) | 4990 (1012–22 711) | 4045 (1167–19 937) | 2790 (1057–12 317) |
| Aetiology of liver disease†, n (%) | ||||||
| Hepatitis B virus | 124 (100.0) | 101 (100.0) | 0 | 1 (2.0)‡ | 0 | 0 |
| Hepatitis C virus | 7 (5.6) | 5 (5.0) | 76 (100.0) | 51 (100.0) | 0 | 0 |
| Significant alcohol use | 6 (4.8) | 4 (4.0) | 12 (15.8) | 11 (21.6) | 53 (45.7) | 28 (37.8) |
| Steatohepatitis (NASH, fatty liver) | 1 (0.8) | 0 | 1 (1.3) | 1 (2.0) | 24 (20.7) | 10 (13.5) |
| Haemochromatosis | 0 | 0 | 0 | 0 | 2 (1.7) | 1 (1.4) |
| Other | 6 (4.8) | 1 (1.0) | 0 | 2 (3.9) | 37 (31.9) | 27 (36.5) |
- ECOG PS, Eastern Cooperative Oncology Group Performance Status; IQR, interquartile range; N, number of patients in intent-to-treat population; n, number of subjects in the specified category; NASH, non-alcoholic steatohepatitis; PD, progressive disease; PL, placebo; RAM, ramucirumab.
- † For patients with multiple aetiologies, the classification was prioritised according to the order of Hepatitis B, Hepatitis C, and Other; the Other category refers to all aetiologies other than Hepatitis B or Hepatitis C, including missing aetiology.
- ‡ Interactive voice response system/IWRS data, entered by the investigator at the time of enrollment in REACH, indicated this patient had aetiology of HCV; however, based on data from the case report form, this patient had HBV with co-infection of HCV.
Aetiology was not found to be a significant prognostic factor for OS in univariate cox regression analysis or in multivariate analyses after adjusting for other baseline prognostic factors. The multivariate Cox regression analysis, adjusted by treatment arm, did identify several baseline factors significantly associated with OS including macrovascular invasion, ECOG PS, and AFP levels (Table S1).
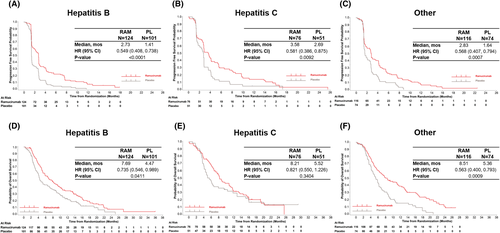
Ramucirumab improved OS across all aetiology subgroups. Ramucirumab also provided consistent PFS benefit irrespective of aetiology (Figure 2A-C). The median OS for ramucirumab versus placebo was 7.7 vs 4.5 months [hazard ratio (HR) 0.7, 95% confidence interval (CI) 0.55-0.99] for HBV, 8.2 versus 5.5 months (HR 0.8, 95% CI 0.55-1.23) for HCV, and 8.5 versus 5.4 months (HR 0.6, 95% CI 0.40-0.79) for Other (Figure 2D-F). No significant difference was detected in treatment effect in OS and PFS by aetiology subgroup (OS/PFS interaction P-value = .23/.39). Objective response rate and disease control rate were greater in the ramucirumab group in all aetiologies (Table S2). A potential imbalance in baseline AFP concentrations between treatment arms could have influenced survival results in each aetiology subgroup (Table 1). When adjusting for baseline AFP concentration, survival outcomes remained improved in ramucirumab treated patients in each aetiology subgroup (Figure S2). The two main subgroups of patients in the “Other” aetiology category, significant alcohol use and steatohepatitis, both had improved survival with ramucirumab consistent with that seen with HBV and HCV (Figure S3).
The most frequently reported treatment-emergent adverse event of Grade ≥3 in the ramucirumab group (Table 2) was hypertension (HBV: 11 [8.9%], HCV: 8 [10.5%], Other: 21 [18.1%]). No new safety signals were detected and Grade ≥3 adverse events were consistent with observations from both REACH and REACH-2.
| Hepatitis B | Hepatitis C | Other | ||||
|---|---|---|---|---|---|---|
| AESI Categories, n (%) | RAM N = 124 | PL N = 99 | RAM N = 76 | PL N = 51 | RAM N = 116 | PL N = 73 |
| Liver injury or failure | 18 (14.5) | 25 (25.3) | 18 (23.7) | 16 (31.4) | 27 (23.3) | 18 (24.7) |
| Ascites† | 3 (2.4) | 4 (4.0) | 5 (6.6) | 1 (2.0) | 7 (6.0) | 4 (5.5) |
| Hepatic encephalopathy† | 2 (1.6) | 0 | 4 (5.3) | 0 | 3 (2.6) | 1 (1.4) |
| Hypertension | 11 (8.9) | 3 (3.0) | 8 (10.5) | 3 (5.9) | 21 (18.1) | 2 (2.7) |
| Bleeding or haemorrhage events | 4 (3.2) | 6 (6.1) | 8 (10.5) | 5 (9.8) | 3 (2.6) | 4 (5.5) |
| Gastrointestinal haemorrhage events | 3 (2.4) | 4 (4.0) | 6 (7.9) | 5 (9.8) | 2 (1.7) | 3 (4.1) |
| Pulmonary haemorrhage events | 1 (0.8) | 1 (1.0) | 0 | 0 | 0 | 0 |
| Proteinuria | 0 | 0 | 3 (3.9) | 0 | 1 (0.9) | 0 |
| Arterial thromboembolic events | 0 | 1 (1.0) | 1 (1.3) | 0 | 2 (1.7) | 1 (1.4) |
| Gastrointestinal perforation events | 1 (0.8) | 0 | 0 | 2 (3.9) | 1 (0.9) | 0 |
| Venous thromboembolic events | 0 | 2 (2.0) | 0 | 2 (3.9) | 1 (0.9) | 1 (1.4) |
| Congestive heart failure | 0 | 1 (1.0) | 0 | 0 | 1 (0.9) | 0 |
| Infusion-related reactions | 1 (0.8) | 0 | 0 | 0 | 0 | 0 |
- AESI, adverse event of special interest; N, number of patients in safety population; PL, placebo; RAM, ramucirumab.
- Data are treatment-emergent adverse events of special interest irrespective of cause, according to either consolidated categories or †preferred terms.
Survival and liver function were also assessed in REACH-2 patients with viral aetiology that were tested by a central laboratory for the presence of serum HBV DNA or HCV RNA at baseline. Central laboratory PCR was not performed in the REACH study, which precluded analysis of HBV DNA or HCV RNA in that trial.
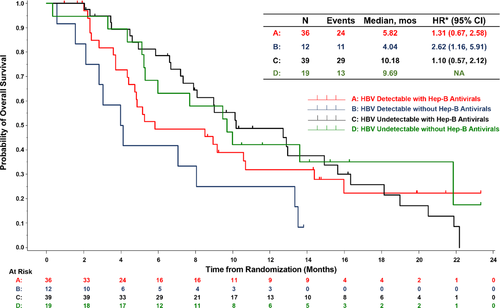
Of the 107 REACH-2 patients with HBV aetiology, 106 had available PCR samples and were included in the combined treatment arm analysis (70 ramucirumab patients and 36 placebo patients). Patients with a detectable HBV load (N = 48) had poorer median OS compared with those with an undetectable viral load (N = 58) (5.3 vs 10.1 months, HR 1.45, 95% CI 0.93-2.28) (Table S3 and Figure S4A). Survival in patients with a detectable HBV load was improved with the use of concomitant antiviral therapy (N = 36) compared with patients who did not receive antiviral therapy (N = 12; 5.8 vs 4.0 months) (Figure 3). No survival difference by antiviral therapy usage was noted for patients with an undetectable HBV load (N = 39 concomitant antiviral vs N = 19 no concomitant antiviral) (10.2 vs 9.7 months, HR 1.10, 95% CI 0.57-2.12). Maximum change in size of targeted lesions from baseline are shown in Figure S5 and appeared to be independent of viral load and antiviral therapy. Liver injury/failure related adverse events were less frequent in patients taking concomitant antiviral therapy (Table S4). During treatment, mean ALBI scores remained stable, with a trend toward improvement in patients receiving antiviral therapy (Figure S6).
Survival outcomes by HCV load were also assessed. Of the 76 REACH-2 patients with HCV aetiology, 67 had available PCR samples. In the HCV group, no difference in OS was detected between patients with and without a detectable HCV viral load (Figure S4B) nor when higher PCR cut-off values (<50 [undetectable], 50–800 000 [low], and >800 000 [high] IU/mL) were examined (data not shown).
4 DISCUSSION
HCC continues to be a global health burden, and has been increasing in areas where populations have traditionally been considered low-risk for developing HCC.20 Chronic viral infection (HBV or HCV), heavy alcohol use, and non-alcoholic fatty liver disease/NASH are known risk factors associated with HCC.21 Recently, survival outcomes for HCC have been shown to differ by disease aetiology with some systemic therapies, such as TKIs and checkpoint inhibitors.5-7 This exploratory analysis of the REACH (AFP ≥400 ng/mL) and REACH-2 studies provides significant evidence that ramucirumab improves survival across aetiology subgroups, with a tolerable safety profile, in patients with advanced HCC and elevated AFP.
The value of aetiology as a prognostic factor has been variable in the literature, with studies showing both an association and lack thereof between disease aetiology and survival outcomes in HCC.22-27 In the analysis presented here, aetiology was not independently prognostic for OS, with a similar treatment benefit across disease aetiologies (OS/PFS interaction P-value = .23/.39). One explanation for this lack of prognostic difference between aetiology subgroups could be the biomarker-selected population in our studies—patients with baseline AFP levels ≥400 ng/mL. It is known that patients with HCC and elevated AFP levels have poorer outcomes than those with lower AFP levels 28 and the contribution of elevated AFP as a prognostic factor might be greater than that of HBV, HCV, and other aetiologies.29
Several other trials have assessed efficacy by baseline aetiology subgroups and the magnitude of benefit was not equivalent across subgroup and agents. In the CELESTIAL phase 3 trial, OS was improved for cabozantinib versus placebo with baseline HBV aetiology (with or without HCV: HR 0.69, 95% CI 0.51–0.94) more so than for patients with HCV aetiology (without HBV; HR 1.11, 95% CI 0.72–1.71).6 In the CheckMate 459 phase 3 trial, OS was improved in patients with a viral aetiology treated with nivolumab versus sorafenib (HBV: HR 0.77, 95% CI 0.56–1.05; HCV: HR 0.71, 95% CI: 0.49–1.01) an equivalent improvement was not observed in patients with a non-viral aetiology (HR 0.95, 95% CI 0.74–1.22).30 In the RESORCE phase 3 trial, HBV aetiology was associated with improved OS (HR 0.58, 95% CI 0.41–0.82) for regorafenib versus placebo more so than HCV (HR 0.79 [95% CI: 0.49–1.26]).31 In the IMbrave150 phase 3 trial, OS was improved in patients with a viral aetiology treated with atezolizumab plus bevacizumab versus sorafenib (HBV: HR 0.51, 95% CI 0.32–0.81; HCV: HR 0.43, 95% CI 0.22–0.87), with less improvement shown for patients with a non-viral aetiology (HR 0.91, 95% CI: 0.52–1.60).32 These studies were not powered for subgroup analyses, including aetiology. However, these results in combination with REACH/REACH-2 data, suggest disease aetiology can affect efficacy outcomes for different systemic therapies, although this needs to be further explored.
Contrary to some contemporary trials, REACH/REACH-2 did not restrict patient enrolment due to viral load levels. HBV load is known to be a poor prognostic factor and the strongest independent predictor of death in HCC, suggesting a connection between HBV viral replication and tumour progression.33, 34 Recently, the use of prophylactic antiviral therapies in patients with HCC have been shown to prevent HBV reactivation and improve outcomes (long-term recurrence-free and OS) after resection of HBV-related HCC.35 In a meta-analysis of 9 HBV-related HCC cohort studies, Wong et al.36 showed that anti-viral treatment after curative treatment of chronic hepatitis B-related hepatocellular carcinoma was associated with a decreased risk of tumour recurrence and liver-related mortality and improved OS. These studies focused on patients with early/recurrent HCC, and the data suggest that anti-viral treatment may prevent reoccurrence, however, REACH/REACH-2 focused on patients with advanced HCC regardless of HBV/HCV loads. Due to low patient numbers in treatment groups, our analysis investigating HBV/HCV status included patients from combined treatment arms. We found that those patients with detectable HBV loads had worse OS than those with undetectable HBV loads. We show that patients with detectable HBV DNA, who received concurrent antivirals, displayed survival benefits compared with those patients who did not receive antiviral therapy. Regarding safety, liver function was preserved with the addition of antiviral therapy and no additional safety signals were detected. Overall, these data provide evidence that patients with detectable HBV loads may benefit from antiviral therapy while receiving systemic therapy. Interestingly, this trend was not seen in patients infected with HCV. No difference in OS was detected when comparing patients with and without a detectable HCV load.
This exploratory analysis has several limitations related to the inclusion and exclusion criteria of both the REACH and REACH-2 studies. Consistent with guidelines for trial exclusion, patients with severe liver cirrhosis (Child Pugh class B or worse) were excluded.37, 38 In addition, our analysis was limited by inclusion of only patients with elevated AFP levels (≥400 ng/mL). Both trials enroled patients irrespective of HBV and/or HCV serum viral load, but only REACH-2 was designed to collect and analyse serum viral load by a central laboratory, which led to limitations in sample size. Furthermore, serum viral loads were only assessed at baseline and we were therefore unable to monitor changes in viral load levels during study treatment or to carefully monitor the effectiveness of antiviral therapy. Finally, although the data for these exploratory analyses were from two phase 3 trials, they were not powered specifically for these post-hoc analyses.
5 CONCLUSIONS
This exploratory analysis demonstrates a consistent treatment benefit with ramucirumab for patients with advanced HCC and AFP ≥400 ng/mL, regardless of aetiology. No significant prognostic difference was observed among different disease aetiologies. Ramucirumab was well tolerated, with a similar safety profile in all aetiology subgroups.
6 Ethics Approval and Patient Consent
Both studies are complied with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and applicable local regulations. Ethics committees approved the protocol followed at all participating centers, and all patients provided written informed consent.
Acknowledgements
The authors gratefully acknowledge Laura Ramsey, David McIlwain, and Louise McGrath, employees of Eli Lilly and Company, and Andrea Humphries of Syneos Health, for providing editorial and process support.
Conflict of Interest
P. Galle reports grants from Bayer and Eli Lilly and Company, personal fees from BMS, AstraZeneca, Sirtex, MSD, Eisai, Ipsen, Bayer, and Roche, and other from Eli Lilly and Company. M. Kudo reports grant and personal fees from Eisai and EA Pharma; grants from Gilead Sciences, Taiho, Sumitomo Dainippon Pharma, Takeda, Otsuka, and Abbvie; and personal fees from Roche, Ono, Eli Lilly and Company, BMS, and Bayer. J. Llovet reports grants and personal fees from Bayer Pharmaceuticals, Eisai, Boehringer Ingelheim, & Ipsen, grants from Bristol Myers Squibb, and personal fees from Eli Lilly and Company, Celsion, Merck, Genentech, Roche, Glycotest, Nucleix, Sirtex, Mina Alpha, Astrazeneca, Axis, and Medscape. R. Finn reports personal fees from AstraZeneca and Cstone; grants and personal fees from Bayer, Eisai, Bristol-Myers Squibb, Roche/ Genentech, Merck, Pfizer and Eli Lilly and Company. M. Karwal reports grants from Eli Lilly and Company, and other from Eisai. D. Pezet has nothing to disclose. T. Y. Kim has nothing to disclose. T. S. Yang has nothing to disclose. S. Lonardi reports grants and other from Amgen, Merck Serono, Eli Lilly and Company, Astra Zeneca, Bristol-Myer Squibb, Roche, grants from Bayer, and other from Incyte, Daiichi Sankyo, Servier, Glaxo-Smith Kline and Pierre-Fabre. J. Tomasek has nothing to disclose. J. M. Phelip reports grants, personal fees and non-financial support from Eli Lilly and Company and personal fees and non-financial support from Servier. Y. Touchefeu has nothing to disclose. S. J. Koh has nothing to disclose. G. Stirnimann reports personal fees from Eli Lilly and Company. K. Liang is a former employee and shareholder of Eli Lilly and Company and has nothing further to disclose. K. Ogburn is a former employee and shareholder of Eli Lilly and Company and is currently employed by AstraZeneca. C. Wang is an employee and shareholder of Eli Lilly and Company. P. Abada is an employee and shareholder of Eli Lilly and Company. R. Widau is an employee and shareholder of Eli Lilly and Company. A. Zhu reports personal fees from Roche, Eli Lilly and Company, Eisai, Bayer, Merck, Exelixis & Sanofi.
Author Contributions
All authors approved the final version of the manuscript for submission to Liver International and have participated sufficiently in the work to agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Conception of the work: R. Finn, R. Widau, A. Zhu and J. Llovet. Design of the work: P. Abada, R. Finn, R. Widau and A. Zhu. Acquisition of data for the work: M. Kudo, P. Galle, S. J. Koh, Y. Touchefeu, D. Pezet, G. Stirnimann, C. Wang, R. Finn, S. Lonardi, A. Zhu, T. S. Yang, T. Y. Kim, J. Tomasek and M. Karwal. Analysis of data for the work: P. Galle, P. Abada, C. Wang, R. Finn, R. Widau, K. Liang and T. Y. Kim. Interpretation of data for the work: M. Kudo, P. Galle, P. Abada, R. Finn, R. Widau, A. Zhu, J. M. Phelip, J. Llovet, T.Y. Kim, K. Ogburn and M. Karwal. Drafting of the work: P. Galle, R. Widau and T. S. Yang. Critical revision of the work for important intellectual content: M. Kudo, P. Galle, S. J. Koh, Y. Touchefeu, D. Pezet, G. Stirnimann, P. Abada, C. Wang, R. Finn, R. Widau, S. Lonardi, A. Zhu, K. Liang, J. M. Phelip, J. Llovet, T. Y. Kim, J. Tomasek, K. Ogburn, and M. Karwal.