Primary biliary cholangitis management: controversies, perspectives and daily practice implications from an expert panel
Handling Editor: Ana Lleo
FUNDING INFORMATION
This working group was supported by Intercept Pharmaceuticals, which had no role in the discussion, writing of the article and in the decision to submit it for publication.
Abstract
Primary biliary cholangitis (PBC) is a rare progressive immune-mediated liver disease that, if not adequately treated, may culminate in end-stage disease and need for transplantation. According to current guidelines, PBC is diagnosed in the presence of antimitochondrial antibodies (AMA) or specific antinuclear antibodies, and of a cholestatic biochemical profile, while biopsy is recommended only in selected cases. All patients receive ursodeoxycholic acid (UDCA) in first line; the only registered second-line therapy is obeticholic acid (OCA) for UDCA-inadequate responders. Despite the recent advances in understanding PBC pathogenesis and developing new treatments, many grey areas remain. Six Italian experts selected the following topics as the most urgent to address in PBC management: diagnosis and natural history of PBC: as a portion of the subjects with isolated AMA, normal alkaline phosphatase (ALP) levels and no symptoms of liver disease could have PBC by histology, defining how to manage and follow this population is crucial; role of liver biopsy: recent evidence suggests that biopsy may provide relevant information for risk stratification and prediction of UDCA response, possibly facilitating personalized approaches; risk stratification: the tools for risk stratification are well established, but some issues (eg bile acid dosage in routine practice) remain controversial; and therapy: those in more advanced stages of development are nuclear receptor modulators and fibrates, but more data are needed to plan personalized strategies. In this manuscript, for each topic, current evidence, controversies and future perspectives are summarized with the possible implications for clinical practice.
Abbreviations
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine aminotransferase
-
- AMA
-
- antimitochondrial antibodies
-
- ANA
-
- antinuclear antibodies
-
- AST
-
- aspartate aminotransferase
-
- BA
-
- bile acid
-
- CK7
-
- cytokeratin 7
-
- CYP7A1
-
- cholesterol 7α-hydroxylase
-
- DR
-
- ductular reaction
-
- EASL
-
- European Association for the Study of the Liver
-
- FGF-15/-19
-
- fibroblast growth factor-15 and −19
-
- FXR
-
- farnesoid X receptor
-
- GGT
-
- gamma-glutamyl transpeptidase
-
- GLs
-
- guidelines
-
- OCA
-
- obeticholic acid
-
- PBC
-
- primary biliary cholangitis
-
- PPARs
-
- peroxisome proliferator-activated receptors
-
- TFS
-
- transplant-free survival
-
- UDCA
-
- ursodeoxycholic acid
-
- VCTE
-
- vibration-controlled transient elastography
Key points
- PBC is diagnosed in the presence of AMA or specific antinuclear antibodies, and of a cholestatic biochemical profile; biopsy is currently recommended in selected cases only
- PBC requires a personalized approach; however, despite recent advances, many grey areas remain
- As a portion of subjects with isolated AMA could have PBC by histology, defining how to manage and follow this population, also reconsidering the role of biopsy, is crucial
- Liver biopsy may be relevant for risk stratification and UDCA response prediction
- More data on therapies are necessary to plan personalized strategies
1 INTRODUCTION
Primary biliary cholangitis (PBC) is a progressive immune-mediated liver disease, characterized by chronic inflammation of interlobular bile ducts that causes bile acid (BA) retention into the liver (cholestasis) and secondary hepatocyte damage. If not adequately treated, it may culminate in end-stage liver disease and need for liver transplantation.1 PBC epidemiology varies widely.2 In Italy, the incidence of PBC has been estimated to range between 2.21 and 5.31 per 100 000 inhabitants, and the prevalence between 3.86 and 27.90 per 100 000.3, 4
Current treatment aims to halt progression, in order to prevent end-stage complications of liver disease and manage associated symptoms.1 It relies on a stepwise approach, starting with administration of ursodeoxycholic acid (UDCA) monotherapy in all patients.1 This is a natural BA that demonstrated long-term efficacy and good safety and tolerability, with a low cost. Indeed, UDCA effectively reduces serum biochemical parameters, slows disease progression, decreases the risk of ascites and the incidence of varices and jaundice, prolongs transplant-free survival (TFS) 5-10 and decreases mortality.11 Unfortunately, 25%-40% of UDCA-treated patients do not experience adequate biochemical response and are therefore considered for second-line therapies: currently, the only registered drug is the farnesoid X receptor (FXR) agonist obeticholic acid (OCA), but novel therapeutic options are under investigation.12, 13
The high heterogeneity of PBC presentation, symptomatology, clinical course and response to therapy requires a personalized, life-long approach to ensure patients’ optimal care, and, despite the recent advances, much work remains to be done.1, 13-15 With this in mind, a board of six experts in the field of PBC gathered in September 2019 to thoroughly review and discuss different aspects of disease management and treatment and share their daily practice experience. Although they acknowledged that many grey areas still exist, the experts selected the following as the most urgent to address: diagnosis and natural history of PBC, role of liver biopsy, risk stratification and therapy. For each topic, current evidence and controversies are summarized, and future perspectives, including novel studies that may help filling some important knowledge gaps, are proposed, together with the possible implications for clinical practice.
2 DIAGNOSIS AND NATURAL HISTORY OF PBC
Serological antimitochondrial antibody (AMA) positivity is the hallmark of PBC, being detected in approximately 95% of patients and being very rare in other diseases.16 The diagnosis is made in the presence of AMA or PBC-specific subtypes of antinuclear antibodies (ANA),17, 18 together with elevation of alkaline phosphatase (ALP).1 ANA may be present in 30%-50% of patients with PBC: two subtypes, anti-Sp100 and anti-Gp210, are considered specific for PBC.17, 18 Anti-Sp100 is the main antigenic target of multiple nuclear dot reactivity. Anti-Gp210 is a glycoprotein integrated in the nuclear pore complex of nuclear membrane. In patients with a clinical suspicion of PBC negative for AMA, it is of fundamental importance to test both Sp100 and Gp210 antibodies.17, 18
Based on autoantibodies’ positivity, liver biochemistry, symptoms and complications, four distinct phases can be recognized (Figure 1): silent, asymptomatic, symptomatic and liver failure. Although progression is generally slow, interindividual variability is high and patients do not necessarily pass through all phases.
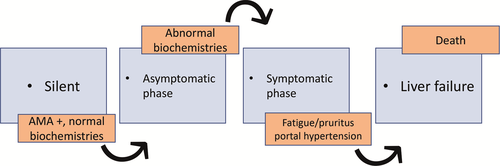
In the last years, patients have been more frequently diagnosed with early or asymptomatic disease,19, 20 likely due to more frequent routine tests and improved AMA detection methods. As a fact, 0.1%-0.8% of healthy individuals are accidentally found with isolated AMA in the absence of clinically apparent liver disease or abnormal biochemistries21 (often in rheumatology settings). Whether and when they will develop overt PBC, however, remains an open question. The 2017 European Association for the Study of the Liver (EASL) guidelines (GLs) state that AMA reactivity alone is not sufficient to diagnose PBC1 and recommend follow-up of these patients with annual biochemical reassessment for the presence of liver disease. This statement is essentially based on two studies: Metcalf JV et al demonstrated that, of 29 AMA + subjects followed for a median of 17.3 years, 76% developed symptoms and 83% cholestatic biochemical profile diagnostic of PBC but no patient developed clinically apparent portal hypertension or cirrhosis.22 The second, more recent study by Dahlqvist G et al demonstrated that, of 66 AMA + subjects with normal ALP followed-up for a median of 7 years after AMA detection, only one in six (16.6%) developed PBC within 5 years.23 In contrast, Mitchinson et al reported that 24/29 (82.8%) AMA + subjects with normal liver function tests and no symptoms of liver disease who had undergone liver biopsy displayed histological findings diagnostic of or compatible with PBC; only 2/29 (6.9%) were fully normal.24 Sixteen subjects were followed-up for a mean of 8.7 years since AMA detection: five (31.3%) developed symptoms and 11 (68.8%) experienced ALP increase, but none had signs or symptoms of major complications.24 In line with these results, a recent Chinese study showed that of 67 subjects with isolated AMA (an incidental finding as part of routine testing for ANA in patients with arthralgia and/or undiagnosed fatigue) who underwent liver biopsy, 55 (82.1%) had histological signs of cholangitis compatible with the diagnosis of PBC, while the remaining 12 (17.9%) displayed normal histology.25 The only significant difference between these groups relied in the titre of AMA and ALP; in particular, although ALP values were in the normal range, they were higher in subjects with vs without PBC.25
On the basis of these results, the authors suggested that a portion of subjects with isolated AMA, normal ALP levels and no symptoms of liver disease could have PBC by histology and that normal values of ALP could be revised for these subjects. Unfortunately, given the normality of liver tests and in the absence of positivity for disease-specific ANA,17, 18 biopsy cannot be routinely indicated and therefore it is a matter of discussion how to manage and follow this AMA+ population and whether or when treatment could be proposed.
2.1 Future perspectives and implications for clinical practice
The panel agreed that normal ALP levels are not sufficient to rule out PBC, and that more reliable markers and methods are needed. They acknowledged the possibility to distinguish ALP values within the normal range,25 although further confirmation is needed, and discussed the possible role of increased gamma-glutamyl transpeptidase (GGT), considering that it is a highly sensitive but poorly specific marker of cholestasis (see below).
- Methods to detect the presence of AMA: it remains an open question whether to standardize such evaluation, especially if rheumatological patients will be routinely screened, since the methods currently in use (immunofluorescence is the gold standard) are not suitable. One approach that may be useful is the Reflex modality,26 including PBC-specific ANAs, which relies on antibody testing at the time of the first evaluation without the need for the specialist's indication.
- Methods to detect liver injury (biliary ducts) and liver stiffness: the panel acknowledged the need for non-invasive and more innovative methods alternative to vibration-controlled transient elastography (VCTE) (eg elastography by nuclear magnetic resonance, which use is, however, challenged by costs). Moreover, to date, VCTE cannot rely on cut-off values to discriminate the stages of PBC. Importantly, the panel agreed on reconsidering the role of biopsy, despite the fact that the 2017 EASL GLs recommend against liver biopsy for the diagnosis of PBC (except in specific situations) (III, 1),1 and that, currently, the procedure is feasible only in the setting of clinical trials approved by ethical committees (difficult to obtain). In the opinion of some experts, biopsy execution would be justified by increased GGT and transaminase levels in subjects with isolated AMA positivity.
The experts deemed as urgent the need to define a management strategy for subjects with isolated AMA, particularly during the follow-up. This is even more important considering that the sooner the UDCA therapy is started, the better the outcome.19, 27
- Evaluate the actual prevalence of isolated AMA: to make it feasible, it would be necessary to involve few pilot centres with a central lab performing immunofluorescence for AMA detection, and limit candidates to high-risk subjects, that is those aged 40-60 years.
- Follow the natural history of subjects with isolated AMA and verify the possible impact of treatment: in the expert opinion, if starting a therapy in subjects with isolated AMA yields a clinical benefit, biopsy will be justified.
- In case of isolated AMA with high GGT in the absence of metabolic syndrome, executing liver biopsy would be justified (a limited sample size would be required).
3 ROLE OF LIVER BIOPSY IN PBC
EASL acknowledges that liver biopsy may be of value for risk stratification in PBC.1
The histologic staging systems currently in use are (1) the Scheuer's classification28 or the Ludwig system, which take into consideration fibrosis, cholangitis or portal hepatitis, periportal fibrosis or hepatitis, septal fibrosis, bridging necrosis and biliary cirrhosis,29 and (2) the Nakanuma system, accounting for fibrosis, bile duct loss and deposition of orcein-positive granules (i.e. cholestasis).30
The Nakanuma system further includes scores for disease grading, based on the assessment of cholangitis and hepatitis activities.30 Remarkably, advanced histological stages are consistently associated with poor prognosis in PBC.31
Recent evidence suggests that integrating these systems with additional histological analyses, such as the c/p ratio and ductular reaction (DR) extent, may provide important information for risk stratification and response to UDCA therapy.27 In particular, DR represents a peculiar histological feature, easily evaluable by immunohistochemical staining for cytokeratin 7 (CK7). It consists in tortuous and irregular conduits close to the portal space, without a well-defined lumen.32 Often, in association with DR, it is possible to identify the so-called intermediate hepatocytes, that is periportal hepatocytes with a specific pattern of CK7 positivity (weakly positive cytoplasm with a stronger signal on the cellular membrane) and positive for the epithelial cell adhesion molecule.32, 33 The origin of such cells is controversial, but they appear exclusively when the parenchymal damage is prominent.
Ductular reaction characterizes almost all chronic liver diseases and represents a response/activation of the progenitor cell niche to a prolonged, chronic damage. Depending on the specific disease, participation of the progenitor cell niche occurs only when mature parenchymal cells (cholangiocytes or hepatocytes) exhaust their capacity of proliferating or are blocked in their cell cycle by specific insults (eg oxidative stress).35 DR has a different molecular profile and phenotype based on the aetiology and cellular target of the specific liver disease.34 Regardless, DR can influence the cells of its own niche, activate myofibroblasts and impact on fibrosis progression.36, 37
Besides the numerous experimental data in rodents, the role of DR in human liver fibrogenesis is demonstrated by the correlation between the extent of DR and of fibrosis in a number of diseases including non-alcoholic fatty liver disease, primary sclerosing cholangitis and PBC itself.34 Moreover, in certain conditions (eg alcoholic hepatitis and acute liver failure), DR correlates with patient outcome and prognosis. More recently, the extent of DR has been shown to correlate with the Global-PBC and UK-PBC prognostic scores,34 and with the response to UDCA.27
3.1 Future perspectives and implications for clinical practice
Liver biopsy is generally not necessary for the diagnosis of PBC, given the high specificity of serological markers. However, it could add relevant information for a personalized approach to the disease. In PBC, the need for second-line therapies is currently based on the assessment of biochemical response after 1 year of therapy with UDCA. Recently, the histologic evaluation of fibrosis has been proved to be an independent predictor of TFS and its association with the outcome persists after 1 year, independent of treatment response38; the prognostic role of fibrosis stage at baseline has recently been confirmed in patients stratified by their biochemical response to 1-year UDCA treatment.39 Moreover, preliminary data from 20 patients showed a correlation between DR and the probability to respond to the first-line therapy (ie UDCA27). Therefore, although no recommendation exists, liver biopsy could provide meaningful information for risk stratification, irrespective of first-line therapy response; moreover, the evaluation of liver biopsy could be implemented with the study of DR, which could result useful for risk stratification and to predict response to UDCA therapy.
The panel highlighted the importance of setting-up a reproducible method to standardize the detection of DR, independently of operators and national and international reference centres, possibly taking advantage of image analysis software commercially available or by the development of artificial intelligence tools. This would allow to evaluate the extent and phenotype of DR through routine immunohistochemistry staining and standardized procedures in every centre, reducing the inter-operator variability.27 In general, to this aim, the development of specific algorithms for analysis, machine learning or artificial intelligence may be of help.
- Collect more data on DR quantification during first-line therapy with UDCA and study the pathogenetic basis underlying the correlation between DR and UDCA therapy, including connection between bile ductular and hepatocyte canalicular system40
- Compare DR after treatment in UDCA responders vs non-responders
- Evaluate the presence of possible DR modifications induced by second-line therapies
4 RISK STRATIFICATION
PBC, even when treated, remains a progressive disease carrying the risk of liver-related complications and death; for this reason, the 2017 EASL GLs recommend evaluating all patients for their risk of developing progressive PBC.1 Currently, patients are stratified in low- and high-risk disease based on UDCA response, and the factors associated with the highest risk of complications are age at onset (the younger the patient, the higher the risk of progression), male gender (although evidence is not solid, males seem to have a higher risk of neoplastic transformation in case of advanced liver disease and when non-responders to UDCA), stage at onset and serum ALP and bilirubin levels.1 Recently, Gatselis et al identified male sex, baseline levels of bilirubin, albumin and ALP, aspartate to alanine aminotransferase ratio (AST/ALT), platelet count and treatment with UDCA as predictive of transition from early to moderate PBC.41 The same variables except male sex were also significantly associated with the transition from moderately advanced to advanced disease.41 It is worth noting that, even if the biochemical response to UDCA is the most important factor for risk stratification, inadequate responders may still experience an improvement in TFS.10
- Age and gender: the likelihood of response to UDCA therapy is <50% for a subject younger than 30 years and >90% for those aged >70 years, and it is significantly higher in females compared to males.42 Recently, Carbone and colleagues developed and validated the UDCA Response Score based on pretreatment variables, and age, but not gender, was significantly associated with UDCA response (ie younger age is predictive of lower response, P < .0001).27
- Liver biochemistry and treatment response in UDCA-treated patients: Bilirubin and ALP levels are the two strongest predictors of PBC prognosis.1 They have been validated in two large cohorts, that is Global-PBC and UK-PBC.42-44 To date, the most important scoring systems for risk assessment are the GLOBE score and the UK-PBC score.42-44 Validation in large cohorts has confirmed that they are good predictors of cirrhosis-related complications, with similar and excellent prognostic performance.45 However, they seem to better predict the risk of progression in large cohorts than in single patients.45 More recently, the UDCA Response Score was developed and validated in a discovery cohort of PBC patients in the UK; in this scoring system, the probability of response to UDCA has been directly correlated with ALP (P < .0001) and bilirubin levels (P = .0003), and inversely correlated with transaminase concentration (P = .0012), which is included in the UK-PBC but not in the GLOBE score; however, the physiopathological meaning of the latter is unclear.27
- Treatment time lag: The longer the interval from PBC diagnosis to the start of UDCA treatment (time lag), the lower the probability of UDCA response (P < .0001).27 These data confirm previous data showing that timely treatment may positively affect the patient outcome.19
- Liver histology: Despite the possible predictive role of biopsy (see section 3)38, 39, the invasiveness of the procedure and the operator-dependent nature of the evaluation to date limit its use.
- Non-invasive methods: liver stiffness measurement by elastography has been shown to predict poor outcome 46; in particular, Corperchot et al identified a cut-off of liver stiffness measurement equal to 9.6 KPa as highly predictive of adverse events and reduced survival,47 and EASL GLs consider this cut-off to discriminate between early and advanced disease.1 Therefore, although elastography is not precise, it may provide useful information.
4.1 Future perspectives and implications for clinical practice
With regard to liver biochemistry and UDCA response in risk stratification, the experts discussed on the role of increased GGT and BA dosage, which remains controversial in routine practice; they agreed that GGT elevation can be useful only if included as part of a panel. Moreover, they acknowledged that BA dosage, frequently prescribed by general practitioners, should be discouraged, as it is misleading especially during UDCA treatment.
Next, the panel underlined that, by histology, the main staging systems are based on the identification of the fibrosis pattern and localization (e.g. portal, periportal and bridging fibrosis) rather than the actual amount of collagen fibres deposited within the liver parenchyma; as elastography reflects the latter, it seems rationale that it would not be reliable in discriminating fibrosis stages, particularly the early ones. The experts advised to longitudinally monitor patients by VCTE every 2 years in case of early disease and annually in case of more advanced disease.
- To correlate the amount of collagen fibre deposition with data from elastography measurement
- To tailor risk management strategy to patient-specific features (eg by age and gender) 48
- Repeat risk stratification according to treatment response over time, as disease control in some patients is transient.
- Search for novel biomarkers to identify, at the time of PBC diagnosis, patients at high-risk
5 THERAPIES IN PBC: FOCUS ON NUCLEAR RECEPTOR AGONISTS
Recent advances have clarified some mechanisms underpinning PBC development and progression, but the primum movens remains to be defined. Regardless, it is now well established that cholangiocyte injury can be triggered by both immune-mediated and BA-mediated mechanisms.49 As a result, ductopenia and cholestasis occur, that may cause fibrosis, cirrhosis and, ultimately, liver failure (Figure 2).49
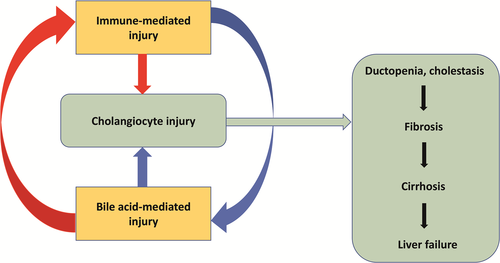
UDCA, the mainstay of PBC treatment in first line, contributes to limit cholangiocyte stress by shifting the composition of the BA pool towards a less toxic phenotype; in addition, it may stimulate biliary secretion, BA detoxification and inhibition of hepatocyte apoptosis;50 which mechanism determines the therapeutic effect of UDCA likely depends on the stage of PBC.50
In second line, the most studied therapeutic approaches rely on immunomodulatory agents and agonists of nuclear receptors. Immunomodulatory agents include biologics (such as the anti-CXCL-10 monoclonal antibody NI-0801 and the anti-IL-12/23 monoclonal antibody ustekinumab), and budesonide, a synthetic corticosteroid with high first-pass metabolism within the liver. Preliminary results on biologics have been disappointing, probably because these agents are effective only in early disease.49, 51, 52 As for budesonide, in small studies, it was shown to yield some benefit in combination with UDCA as second-line treatment.53, 54 However, it was recently demonstrated that, despite a clinically meaningful improvement in biochemical markers of disease activity, add-on budesonide did not improve liver histology.55 Moreover, its use is contraindicated in case of cirrhosis due to increased risk of portal vein thrombosis.56
With regard to the nuclear receptors involved in PBC, we focused on FXR (target of OCA) and on peroxisome proliferator-activated receptors (PPARs) (targets of fibrates) (Figure 3). As a BA sensor, FXR plays a crucial role in BA homeostasis, which is tightly regulated via a negative feedback mechanism between liver and intestine. BAs are synthesized from cholesterol in the liver by the rate-limiting enzyme cholesterol 7α-hydroxylase (CYP7A1). In the liver, FXR downregulates CYP7A1 expression and promotes BA secretion via upregulation of specific BA transporters. In the intestine, following the binding to BA, FXR induces the expression of fibroblast growth factor-15/19 (FGF-15/19), which is released into the portal circulation, reaches the liver and triggers a cascade that culminate in CYP7A1 downregulation.57, 58 In this way, the total pool of BA is reduced. FXR acts also as a negative regulator of liver inflammation and proliferation 59; therefore, the pharmacological modulation of FXR and of the FGF-19 axis in the intestine may represent an optimal option also for patients with liver cancer.59, 60
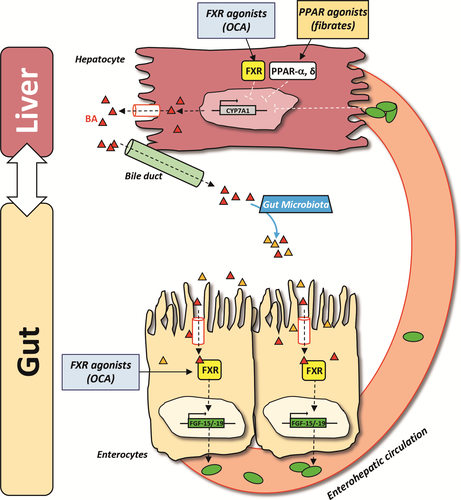
PPARs are involved in BA synthesis, anti-inflammatory responses and in T-cell function and autoimmune diseases.61, 62 These nuclear receptors exist in three isoforms (α, β/δ and γ), which differ in their expression pattern and function: PPAR-α is mostly expressed in hepatocytes and primarily downregulates BA synthesis through inhibition of CYP7A1; PPAR-δ and PPAR-γ are present at a lower level in the liver; PPAR-δ is expressed in hepatocytes, cholangiocytes, Kupffer cells and stellate cells and controls BA homeostasis and inflammation; PPAR-γ is mainly expressed in Kupffer cells and exerts anti-inflammatory actions.49, 63-66
Hereinafter, we summarize the available data of the nuclear receptor agonists in more advanced stages of development.
5.1 FXR agonists: obeticholic acid
Obeticholic acid is the first in class steroidal FXR agonist approved as second-line treatment in PBC. Its use is currently recommended in adult patients in combination with UDCA in inadequate responders or as monotherapy for those intolerant to UDCA.1 It has anticholestatic and hepatoprotective properties, and preclinical studies demonstrated that it can regulate lipid and glucose metabolism, reduce liver fibrosis and prevent development of cirrhosis and intestinal inflammation.67
Approval was based on the results from the 12-month, double-blind, placebo-controlled phase 3 trial POISE, in which 217 patients who had an inadequate response or intolerance to UDCA were randomized to receive OCA at a dose of 10 mg (10-mg group), 5 mg with titration to 10 mg if applicable (5- to 10-mg group) or placebo. The primary end point (ie ALP reduction and normal bilirubin level) occurred significantly more frequently upon OCA than placebo (46% in the 5- to 10-mg group and 47% in the 10-mg group vs 10% in the placebo group, P < .001 for both comparisons), together with greater decreases in ALP level and total bilirubin level (P < .001 for all comparisons).68 The most common adverse event was pruritus, that occurred more frequently in OCA-treated groups (56% of patients in the 5- to 10-mg group and 68% of those in the 10-mg group vs 38% in the placebo group); however, it is worth mentioning that 59% of patients (53% of those in the 5- to 10-mg group, 60% in the 10-mg group and 64% in the placebo group) reported pruritus at baseline.68 During the open-label extension phase, patients received up to 10 mg/die OCA, with dosage variability based on efficacy and tolerability: the 3-year interim data demonstrated the long-term efficacy of OCA; the drug was generally well-tolerated; and 77% of patients reported pruritus with an intensity, as measured by mean visual analogue scale score, that increased up to month 3 and then returned to baseline level over the open-label extension.69 The sustained improvement in liver biochemistry was recently confirmed by the 5-year open-label extension data.70 Interestingly, data from 17 patients included in POISE show that, following 3 years of OCA treatment, 12 (71%) patients displayed improvement or no progression in fibrosis stage compared to five (29%) patients who worsened.71 Finally, a recent study using data from POISE demonstrated that OCA has a positive impact on the long-term risk of death and liver transplantation as predicted by the GLOBE and UK-PBC risk scores (P < .0001). In case of OCA, the only evaluation of TFS was performed through simulation models with data from the POISE study, and demonstrated that TFS would improve from 61% to 73% with the combination of UDCA and OCA.72
As for the real-world efficacy and safety of OCA in PBC patients, preliminary data seem to confirm the rapid improvement in liver biochemistry and that pruritus is the most common adverse event.73-76 It is worth noting that OCA induced a biochemical response also in high-risk PBC patients with PBC-autoimmune hepatitis overlap syndrome,77 with efficacy and safety results similar to those observed in PBC patients.78
In addition to OCA, three newer FXR agonists, cilofexor (NCT02943447), tropifexor (NCT02516605) and EDP-305 (NCT03394924), are currently being investigated for use in PBC.
5.2 PPAR agonists: fibrates and rationale for their use in PBC
Fibrates (fenofibrate, bezafibrate, ciprofibrate and gemfibrozil) are an old class of hypolipidaemic agents. They act as PPAR agonists and bind preferentially the α-isoform, except for bezafibrate that is a pan-PPAR agonist.66 Their use in the setting of PBC can be traced back to the 1990s, when a number of small-sized studies were undertaken in Japan.79-85 Although fibrates demonstrated the potential to reduce symptoms and ALP levels in PBC, either in association with UDCA or alone, no clear-cut evidence exists and more powered randomized studies are needed.9, 84-88
The 2017 EASL GLs do not recommend fibrates as second-line therapy in PBC and their use remains off label.1, 66 In 2018, the results from BEZURSO were made available:89 the trial included 100 patients randomized to receive 400-mg bezafibrate or placebo over a 24-month, double-blind phase.89 The primary endpoint was a complete biochemical response, defined as normal levels of total bilirubin, ALP, aminotransferases and albumin, as well as a normal prothrombin index, and occurred in 31% of bezafibrate-treated patients and in 0% of placebo-treated patients (P < .001). No significant difference between bezafibrate- and placebo-treated patients was found with regard to histologic stage, fibrosis stage and activity grade,89 but more data are needed. As for safety, the following adverse events were reported: 5% increase in mean creatinine levels upon bezafibrate and 3% decrease upon placebo starting from month 3 and throughout the trial duration; increase in aminotransferase levels beyond 5X the upper limit of the normal range in 6% of bezafibrate-treated patients (resolved within 3 months in all cases) and 2% of placebo-treated patients; and increase in creatine kinase levels beyond 5X the upper limit of the normal range in 2% of bezafibrate-treated patients and 0% of placebo-treated patient.89 Interestingly, there was a marked reduction in pruritus, and this beneficial effect on this symptom was also observed in a Spanish cohort of 48 patients treated with bezafibrate over a median period of 38 months.90 Honda et al recently published their retrospective data from the Japan PBC Study Group comparing long-term outcomes using the GLOBE and UK-PBC scores in 118 patients after treatment with UDCA monotherapy for 1 year vs after combining UDCA and bezafibrate therapy for another year. GLOBE score decreased from 0.508 to 0.115 (P < .0001), and 34.2% of patients were able to drop their GLOBE score to ≤0.30, suggesting comparable life expectancy to the matched general population.91
In clinical practice, fibrates are frequently misused. FDA underlines that there are no pharmacokinetic data of patients with liver failure treated with fenofibrate, and thus recommends avoiding its use in case of cirrhosis.92 According to EMA, fenofibrate use is discouraged in case of reduced liver function and contraindicated in case of liver failure.93 As for bezafibrate, data are almost exclusively with simvastatin; according to its label, the drug must not be administered in patients with liver diseases, exception made for hepatic steatosis as it is frequently associated with hypertriglyceridaemia.94
Other compounds targeting PPARs are the new-generation dual PPAR (PPAR-α and -δ) agonist elafibranor and the PPAR-δ agonist seladelpar. Elafibranor is currently in clinical development for different therapeutic indications. It has recently been granted breakthrough therapy designation by the FDA for the treatment of PBC in adults with inadequate responders to UDCA. Its safety and efficacy in PBC have been explored in a double-blind, randomized, placebo-controlled phase 2a trial.95 Results showed a significantly more frequent ALP reduction at 12 weeks (primary endpoint) in patients receiving elafibranor vs placebo (41% of patients receiving 80 mg and 48% of those receiving 120 mg, vs 3%, P < .001). Also bilirubin and GGT decreased significantly in the treatment group, and lipid markers, inflammation markers and pruritus improved. Treatment with both doses of elafibranor was generally well-tolerated.95 Recently, the clinical development in PBC of seladelpar has been recently halted because of the finding of interface hepatitis in concurrent trials evaluating the use of seladelpar for non-alcoholic steatohepatitis and PBC.96
5.3 Future perspectives and implications for clinical practice
- Define how to treat naive patients: in the experts’ opinion, it is important to change the actual treatment paradigm and apply different therapeutic strategies to different subgroups: patients with AMA positivity and normal cholestatic enzymes; symptomatic patients with compensated liver disease: waiting to evaluate the response after 1 year?; symptomatic patients with portal hypertension: different therapeutic approach?
- Collect prospective data of OCA-treated patients
- Collect solid real-world and long-term data on large cohorts treated with OCA. Results are awaited from TARGET-PBC (ClinicalTrials.gov Identifier: NCT02932449), a 5-year, longitudinal, observational study that will describe the real-world practice of diagnosis, management (including therapies) and natural history of PBC 97, 98;
- Confirm the therapeutic efficacy of OCA on clinical outcomes. COBALT (ClinicalTrials.gov Identifier: NCT02308111) is an ongoing phase 4, double-blind, randomized, placebo-controlled multicentre study that is being undertaken at up to 170 sites internationally to evaluate the effect of OCA on clinical outcomes in 428 subjects with PBC over a minimum follow-up of 6 years.99
- In case of fibrate therapy, develop criteria to define hepatotoxicity in subjects who already have a liver disease.
- Collect data on the effects of fibrates on histology and liver biochemistry in subjects with liver diseases, as well as safety data
- Evaluate the efficacy and safety of combination therapies, such as FXR with PPAR agonists. Recently, encouraging data from a multicentre retrospective cohort study showed that, in 50 poor responders to UDCA, addition of OCA and fibrates to UDCA significantly decreased the levels of GGT (P < .0001), ALT (P <.0001), AST (P < .01) and total bilirubin (P = .02), and increased the rate of ALP normalization compared to dual therapy UDCA/OCA or UDCA/fibrates.100
- Assess the impact of therapies on fibrosis
- Collect safety data of the drugs currently in use in patients with advanced disease
- Collect data on patient-reported outcomes to gain insights into the impact of therapy on patient's disease perception
6 CONCLUSIONS
PBC requires a personalized, life-long approach. Despite the recent advances in understanding its pathogenesis and developing new treatments, many grey areas remain that hamper the provision of optimal care to patients. In the management of PBC, it is important to define strategies to manage and follow subjects with isolated AMA, starting from diagnosis that may imply to reconsider biopsy and evaluate surrogate markers other than ALP. Moreover, from the most recent evidence, a possible role of biopsy in risk stratification and prediction of response to UDCA emerged, that may, therefore, be implemented in routine practice. Finally, more data on therapies (especially for hard end points) are needed, including assessment of the impact on fibrosis, that may further contribute to plan personalized strategies.
ETHICS APPROVAL STATEMENT
Not applicable.
PATIENT CONSENT STATEMENT
Not applicable.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
ACKNOWLEDGEMENTS
This working group has been unconditionally supported by Intercept Pharmaceuticals.
CONFLICT OF INTEREST DISCLOSURE
DA declares consulting activities for Intercept Pharmaceuticals; GC declares speaker's activities for Intercept Pharmaceuticals; AC declares consulting activities for Intercept Pharmaceuticals; AF declares consulting activities for Intercept Pharmaceuticals; AM received a project grant from Intercept Pharmaceuticals on the role of Fxr in hepatocarcinoma; PI received grant supports from Intercept Pharmaceuticals, Gilead, Abbvie and Bruschettini.