Novel and emerging therapies for cholestatic liver diseases
Abstract
While bile acids are important for both digestion and signalling, hydrophobic bile acids can be harmful, especially when in high concentrations. Mechanisms for the protection of cholangiocytes against bile acid cytotoxicity include negative feedback loops via farnesoid X nuclear receptor (FXR) activation, the bicarbonate umbrella, cholehepatic shunting and anti-inflammatory signalling, among others. By altering or overwhelming these defence mechanisms, cholestatic diseases such as primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) can further progress to biliary cirrhosis, end-stage liver disease and death or liver transplantation. While PBC is currently treated with ursodeoxycholic acid (UDCA) and obeticholic acid (OCA), many fail treatment, and we have yet to find an effective therapy for PSC. Novel therapies under evaluation target nuclear and surface receptors including FXR, transmembrane G-protein–coupled receptor 5 (TGR5), peroxisome proliferator-activated receptor (PPAR) and pregnane X receptor (PXR). Modulation of these receptors leads to altered bile composition, decreased cytotoxicity, decreased inflammation and improved metabolism. This review summarizes our current understanding of the role of bile acids in the pathophysiology of cholestatic liver diseases, presents the rationale for already approved medical therapies and discusses novel pharmacologic therapies under investigation.
Abbreviations
-
- AASLD
-
- American Association for the Study of Liver Diseases
-
- ACG
-
- American College of Gastroenterology
-
- AE2
-
- Apical chloride/bicarbonate exchanger
-
- ALP
-
- Serum alkaline phosphatase
-
- ALT
-
- Alanine transaminase IL 12
-
- AMA
-
- Antimitochondrial antibody
-
- ASBT
-
- Apical sodium-dependent bile acid transporter
-
- ATRA
-
- All-trans retinoic acid
-
- BSEP
-
- Bile salt export pump
-
- C4
-
- 7a-hydroxy-4-cholesten-3-one
-
- CD
-
- Crohn's disease
-
- CFTR
-
- Cystic fibrosis transmembrane conductance regulator
-
- CTLA-4
-
- Cytotoxic T-lymphocyte antigen 4
-
- CVC
-
- Cenicriviroc
-
- CYP3A4
-
- Cytochrome P450 3A4
-
- CYP7A1
-
- Cytochrome P450 7A1-hydroxylase
-
- FGF-19
-
- Fibroblast growth factor 19
-
- FXR
-
- Farnesoid X nuclear receptor
-
- GLP-1
-
- glucagon-like peptide 1
-
- HDL
-
- High-density lipoprotein
-
- IBD
-
- Inflammatory bowel disease
-
- LOXL2
-
- Lysyl oxidase-like 2
-
- MDR1
-
- Multidrug resistance protein 1
-
- MDR3
-
- Multidrug resistance protein 3
-
- MRP2
-
- Multidrug resistance-associated protein 2
-
- MRP3
-
- Multidrug resistance-associated protein
-
- MRS
-
- Mayo Risk Score
-
- NF-κB
-
- Nuclear factor kappa B
-
- norUDCA
-
- 24 nor-ursodeoxycholic acid
-
- NTCP
-
- Sodium-taurocholate cotransporting polypeptide
-
- OATP
-
- Organic anion-transporting polypeptide
-
- OCA
-
- Obeticholic acid
-
- OST
-
- Organic solute transporter
-
- PBC
-
- Primary biliary cholangitis
-
- PPAR
-
- Peroxisome proliferator-activated receptor
-
- PSC
-
- Primary sclerosing cholangitis
-
- PXR
-
- Pregnane X receptor
-
- QALY
-
- Quality-adjusted life year
-
- RXR
-
- Retinoid X receptor
-
- STAT
-
- Signal transducer and activator of transcription
-
- SULT2A1
-
- Sulphotransferase family 2A member 1
-
- TGR5
-
- Transmembrane G-protein–coupled receptor 5
-
- TUDCA
-
- Tauroursodeoxycholate
-
- UC
-
- Ulcerative colitis
-
- UDCA
-
- Ursodeoxycholic acid
-
- UGT1A1
-
- UDP glucuronosyltransferase family 1 member A1
-
- ULN
-
- Upper limit of normal
Key points
- Patients with primary biliary cholangitis (PBC) who do not respond to ursodeoxycholic acid (UDCA) have an increased risk of developing complications of cirrhosis, including clinical decompensation, hepatocellular carcinoma and death.
- Obeticholic acid is approved as the second-line therapy for PBC and is indicated for non-responders to UDCA or patients who are intolerant to UDCA.
- There is no approved medical therapy for primary sclerosing cholangitis.
- Many novel therapies targeting nuclear and surface receptors involved in bile acid signalling are currently under evaluation for cholestatic liver diseases, including agonists of FXR, PPAR, PXR and TGR5, among others, with promising results.
1 INTRODUCTION
Cholestasis is defined as a condition where bile flow is decreased, either due to impaired secretion by hepatocytes or by obstruction to the bile flow. Primary cholestatic liver diseases affecting adults are mainly primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC). PBC is a rare disease in which a chronic, immune-mediated injury to the small intrahepatic bile ducts leads to an imbalance between cholangiocyte proliferation and apoptosis, with resulting ductopaenia, fibrosis and eventually biliary cirrhosis.1 The diagnosis is made by a combination of unexplained biochemical cholestasis and a positive antimitochondrial antibody (AMA) titre, which is found in greater than 90% of patients.2 When AMAs are not detected, a PBC diagnosis can be confirmed by the presence of PBC-specific antinuclear antibodies or typical findings on histology. Only 2 drugs have received FDA approval for the treatment of PBC: ursodeoxycholic acid (UDCA), as the first-line therapy, and obeticholic acid (OCA), for UDCA non-responders or intolerance to UDCA. Approximately 40% of patients with PBC fail to respond to UDCA, and only half of those respond to OCA.3 Non-responders to treatment with UDCA and/or OCA are at risk for progression to biliary cirrhosis, end-stage liver disease and death or liver transplantation.4
Primary sclerosing cholangitis affects predominantly medium- to large-sized bile ducts, with progressive inflammation and fibrosis leading to multifocal biliary strictures. Effective medical therapy is not available for PSC; therefore, the disease invariably progresses towards biliary cirrhosis at variable rates. Moreover, patients with PSC may develop dominant strictures and bacterial cholangitis in addition to having an increased risk for malignancies. Liver transplantation is the only definitive treatment and its rates have remained stable since the 1990s.5 Identifying an effective medical therapy for PSC is one of the greatest unmet needs in hepatology. A comparison of the clinical features of PBC and PSC is highlighted in Table 1.
| Primary biliary cholangitis1, 109, 110 | Primary sclerosing cholangitis111, 112 | |
|---|---|---|
| Pathogenesis | Multifactorial; genetic susceptibility, immune mediated with loss of tolerance to self-antigen (pyruvate dehydrogenase complex), defective bicarbonate umbrella.1, 113 | Multifactorial; genetic susceptibility, increased exposure to bacterial products, abnormal lymphocyte homing, toxic bile.111, 113 |
| Disease hallmark | (+) Antimitochondrial antibodies | (+) Multifocal biliary strictures/beading of bile ducts on cholangiogram |
| Size of bile ducts involved | Small to medium | Medium to large |
| Classic histopathology | Florid duct lesion114 | Onion skinning115 |
| Gender predominance | Women >>> men | Men > women |
| Average age of onset (y) | 40-50 | 30-40 |
| Incidence (per 10 000 people) | 0.33-5.8116 | 0-1.3116 |
| Prevalence (per 10 000 people) | 1.91-40116 | 0-16.2116 |
| Association with IBD | No | Yes, in 50%-75% |
| Complications | Biliary cirrhosis, portal hypertension | Biliary cirrhosis, portal hypertension, dominant bile duct strictures (in 50%), bacterial cholangitis |
| Risk for malignancy | Hepatocellular carcinoma in cirrhotics, especially males and non-responders to UDCA. | Cholangiocarcinoma (lifetime risk up to 20%), gallbladder adenocarcinoma, colorectal neoplasia (in patients with PSC-IBD). Rarely, hepatocellular carcinoma. |
| Disease-modifying treatment |
First line: UDCA Second line: OCA |
No approved therapy |
| Transplant-free survival | UDCA-treated patients: 90% at 5 y, 78% at 10 y and 67% at 15 y.117, 118 | 10-21 y from diagnosis to transplant or PSC-related death.5, 119, 120 |
| Ranking as indication for liver transplantation in the US (2016-2017)a | 6th | 5th |
| 10-y patient survival after liver transplantation | 76%-94%121, 122 | 83%123, 124 |
| Disease recurrence after liver transplantation | Yes, 9.6%-34% at 5 y121 | Yes, 20%-37% at 5 y121, 123 |
- IBD, inflammatory bowel disease; OCA, obeticholic acid; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; UDCA, ursodeoxycholic acid.
- a Based on OPTN data as of 1 January 2018 (https://optn.transplant.hrsa.gov/data/view-data-reports/build-advanced/).
2 BILE ACID HOMOEOSTASIS, SIGNALLING PATHWAYS AND TARGETS FOR THERAPY
Given the liver involvement in their synthesis and transport, the liver is exposed to very high concentrations of bile acids. While their detergent-like properties help with digestion, leakage and accumulation of bile acids in hepatocytes at higher concentrations may lead to activation of cholangiocytes, causing chronic inflammation, proliferation, apoptosis and subsequently fibrosis.6-8 Bile acids are thought to play a key role in the progression of both PBC and PSC.
The primary bile acids, cholic acid and chenodeoxycholic acid, are synthesized in the liver from cholesterol by the rate limiting enzyme cytochrome P450 7A1-hydroxylase (CYP7A1). Prior to secretion into the bile canaliculi, they undergo conjugation with taurine or glycine to form bile salts which decrease passive reabsorption. Once in the bowel, these primary bile salts undergo dehydroxylation and deconjugation by the gut microbiota, forming the secondary acids deoxycholic acid, lithocholic acid and UDCA. Although a small amount of bile acids are reabsorbed passively in the upper intestine, 90%-95% of them are reabsorbed in the terminal ileum through the apical sodium-dependent bile acid transporter (ASBT).9, 10 Bile acids then enter the portal circulation via transport by organic solute transporter (OST) α/β and are subsequently taken back into the hepatocyte through the sodium-taurocholate cotransporting polypeptide (NTCP) or the organic anion-transporting polypeptide (OATP). This process is known as the enterohepatic circulation and it is tightly regulated by numerous feedback mechanisms to protect the hepatocytes from bile acid-induced cytotoxicity (Figure 1).
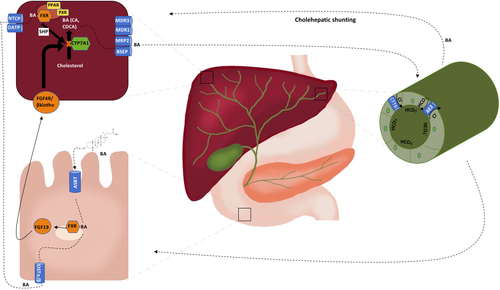
The main negative feedback mechanism regulating bile acid homoeostasis is via the farnesoid X nuclear receptor (FXR). In the enterocytes, bile acids bind FXR, triggering a signalling cascade which ultimately downregulates CYP7A1, thus decreasing the synthesis of bile acids. Importantly, one of the pathways to suppress CYP7A1 activity is through upregulation of the enteral hormone fibroblast growth factor 19 (FGF-19). Once secreted in the portal circulation, FGF-19 reaches the hepatocyte, where it binds to its receptor, the fibroblast growth factor 4/β klotho complex, to inhibit CYP7A1 gene transcription. Furthermore, FXR activation leads to downregulation of the intestinal bile acid transporter ASBT, the hepatic uptake transporters NTCP and OATP and upregulation of the hepatic efflux transporters bile salt export pump (BSEP) and multidrug resistance-associated protein 2 (MRP2).11, 12
Other important defence mechanisms include (1) the bicarbonate umbrella, (2) cholehepatic shunting, and (3) anti-inflammatory signalling. The bicarbonate umbrella, thought to be defective in PBC and PSC, refers to the alkalinization of bile via increased bicarbonate secretion. The elevated pH shifts bile acids towards an ionized form, decreasing their ability to diffuse and lessening their cytotoxic effects.13, 14 Similarly, cholehepatic shunting occurs when bile acids are recirculated between hepatocytes and cholangiocytes through the periductular capillary plexus, thus increasing bile flow and augmenting bicarbonate-rich choleresis.11, 15 Finally, bile acids can modulate inflammatory pathways via binding to a variety of nuclear and surface receptors (ie FXR, TGR5 and PXR) throughout the intestines and bile ducts.16 By altering or overwhelming these defence mechanisms, PBC and PSC can further progress.
3 IMMUNOPATHOLOGY
Cholangiocytes recognize the presence of pathogens through pattern recognition receptors (PRR), such as the toll-like receptors, which are activated in the presence of pathogen-associated molecular patterns (PAMPs). Activation of these PRRs triggers a signalling cascade which results in the expression of various cytokines and chemokines, immunoglobulins and adhesion molecules.17 Histological samples from PBC and PSC patients show a strong lymphocytic predominance, with the majority being CD4+ T cells, along with the presence of CD8+ T cells, B cells, macrophages and natural killer cells. These immune cells are able to release a number of mediators with diverse activity including pro- and anti-inflammatory, angiogenic, fibrogenic and proliferative, which are directly affected by bile acids.18 For instance, accumulation of hydrophobic bile acids can blunt the inflammatory cytokine response, inhibit lymphocyte proliferation and impair clearance of apoptotic cholangiocytes. The ineffective removal of apoptotic bodies is important in the pathogenesis of immune-mediated liver diseases, since the exposure of intracellular components generates activation of the immune system by unrecognized self-antigens. This understanding remains the basis for consideration of immunomodulatory therapies for cholestatic liver diseases, including drugs that regulate T-cell activation, such as abatacept, or deplete the pool of B cells, such as rituximab.
4 EXISTING THERAPIES
4.1 Ursodeoxycholic acid
Ursodeoxycholic acid was approved in 1997 for use in PBC at a dose of 13-15 mg/kg/d. The beneficial effects of UDCA are due to an increase in the ratio of hydrophilic to hydrophobic bile acids, stimulation of bile flow through BSEP, stabilization of the bicarbonate umbrella and antiapoptotic as well as anti-inflammatory effects.16, 19, 20 In addition to improvement in liver biochemistries, studies have shown that UDCA delays histological progression, delays the development of oesophageal varices and improves survival free of liver transplantation.21-23 UDCA should be used as the first-line therapy for all patients with PBC.
The American Association for the Study of Liver Diseases (AASLD) recommends against the use of UDCA as medical therapy in PSC, as beneficial data regarding its long-term use are lacking and doses >28 mg/kg/d have been associated with worse outcomes compared to placebo.24, 25 However, some experts still use UDCA for PSC in dosages of 17-23 mg/kg/d in hopes of decreasing serum alkaline phosphatase (ALP),26 as studies have shown a survival benefit in patients who normalized ALP either spontaneously or as a result of treatment with UDCA.27-29 Notably, recent guidelines from the American College of Gastroenterology (ACG) emphasize that doses greater than 28 mg/kg/d should not be used, but defer to the treating physician whether or not to use median doses (17-23 mg/kg/d).30
4.2 Obeticholic acid
The natural ligand for FXR is chenodeoxycholic acid. Obeticholic acid (OCA), a 6-ethyl derivative of chenodeoxycholic acid with 100 times its potency, functions as a strong FXR agonist.31 In addition to modulation of bile acid homeostasis, as previously discussed, FXR also regulates lipid metabolism and gluconeogenesis as well as inflammation and fibrosis pathways.32
Obeticholic acid was granted conditional approval in May 2016 for use in PBC. It should be prescribed in conjunction with UDCA for patients with inadequate response to UDCA, or as monotherapy for those who are unable to tolerate UDCA. This approval was based on results of the POISE trial, where 216 patients with PBC and an inadequate response to UDCA received either placebo, OCA 10 mg/d or OCA 5 mg/d with the possibility to titrate to 10 mg/d, for 12 months. In this study, OCA or placebo was given in combination with UDCA in 93% of patients and as monotherapy in 7%. The primary endpoint was a composite of 3 criteria: ALP < 1.67× upper limit of normal (ULN), total bilirubin ≤ULN and an ALP reduction of at least 15%. Nearly half of OCA-treated patients met the primary endpoint compared to only 10% of patients on placebo.3 In a separate phase II study, ALP reductions of 53.9% and 37.2% were observed in patients receiving 10 mg/d and 50 mg/d of OCA monotherapy, respectively.33
The most common side effect of OCA is a dose-dependent development of itching. This is minimized by starting with the lowest recommended dose, 5 mg/d, and increasing to 10 mg/d after 3 months if the medication is well tolerated and the ALP remains elevated. Use of OCA has also been associated with a dose-dependent reduction in high-density lipoprotein (HDL) cholesterol and this should be monitored during treatment.34 Importantly, the recommended starting dose is reduced in cirrhotics with moderate or severe hepatic impairment. Hepatic decompensation, liver failure and death have been reported when Child B or C cirrhotics are dosed more frequently than recommended. As a result, the FDA issued a black box warning to the OCA label highlighting prescribing recommendations for patients with decompensated liver disease (https://www.fda.gov/Drugs/DrugSafety/ucm594941.htm). These recommendations are listed in Table 2.
| OCA dose | Disease stage | |
|---|---|---|
| Non-cirrhotic or compensated cirrhosis (Child-Pugh A) | Decompensated cirrhosis, child B or C (including prior decompensation) | |
| Starting dose: 1st 3 mo | 5 mg/d | 5 mg/wk |
| Dose titration at 3 mo | 10 mg/d |
5 mg twice a week, at least 3 d apart May increase to 10 mg twice a week, at least 3 d apart |
| Maximum dose | 10 mg/d | 10 mg twice a week |
- mg, milligramme; OCA, obeticholic acid.
Further studies are needed to examine the long-term effect of OCA therapy on mortality and liver transplantation as well as its adequacy for patients with more advanced liver disease. Using data from the POISE trial in a microsimulation model, Samur and colleagues determined that the combination of UDCA and OCA could decrease the 15-year cumulative incidence of decompensated cirrhosis, hepatocellular carcinoma, liver transplants and liver-related deaths, thus increasing transplant-free survival. However, adding OCA to UDCA led to an incremental cost-effectiveness ratio of $473 400/quality-adjusted life year (QALY) gained, which is deemed not cost effective when using a willingness-to-pay threshold of $100 000/QALY.35 While OCA is promising in terms of its ability to significantly decrease ALP levels in PBC patients and possibly improving survival free of liver transplantation, it is not economically feasible in the long term and a significant reduction in cost would be required to achieve cost-effectiveness.
In regard to the use of OCA in PSC, the phase II AESOP trial looked at the effect of OCA on 77 patients with PSC over 24 weeks (NCT02177136). Investigators noted a statistically significant decrease in baseline ALP of 22% in both the low-dose (1.5-3 mg) and high-dose (5-10 mg) groups,36 and a long-term extension phase is ongoing. While encouraging, further trials are needed to assess if these results translate into a clinically significant endpoint such as increased time to transplantation or death.
5 NOVEL THERAPIES
5.1 Bile acids: TUDCA and norUDCA
Tauroursodeoxycholate (TUDCA) is the taurine conjugated form of UDCA, which has increased hydrophilicity. TUDCA was evaluated in a non-inferiority phase III trial including 199 Chinese patients with PBC treated over a 24-week period with TUDCA or UDCA. A similar proportion of patients in both groups achieved a 25% or 40% reduction in ALP compared to baseline values. Likewise, rates of study-related adverse events were similar except for itching, which appeared to occur more frequently in UDCA-treated subjects.37 The study suffered from several limitations including a heterogeneous population, short treatment duration, lack of bioequivalence between doses of TUDCA and UDCA and lack of appropriate instruments to evaluate pruritus and other quality of life measures. Overall, the drug offers no advantage over the well-established use of UDCA.
Twenty four nor-ursodeoxycholic acid (norUDCA) is a side-chain shortened UDCA, which prevents it from undergoing amidation with glycine or taurine.16 This modification is thought to reinforce the bicarbonate umbrella via cholehepatic shunting. Since this molecule escapes conjugation with glycine or taurine, it is passively reabsorbed within the biliary canaliculi, augmenting the secretion of bile acids and bicarbonate.10 norUDCA is also more hydrophilic and therefore less toxic to the epithelial cells. A phase II study of 159 patients with PSC treated with placebo vs 500, 1000 or 1500 mg of norUDCA showed that, at 12 weeks, norUDCA reduced ALP levels in a dose-dependent manner.38 The dose reductions were 12.3%, 17.3%, and 26.0% in the 500, 1000, and 1500 mg groups, respectively, compared to a 1.2% ALP increase in the placebo group. Notably, this effect was independent of disease stage, duration of disease or prior use of UDCA. Adverse events and pruritus occurred at similar rates in all groups. NUC-5, a larger phase III randomized controlled trial with norUDCA, is in progress in Europe (EudraCT Number: 2016-003367-19).
5.2 FXR agonists: LJN-452, GS-9674, EDP-305
In addition to OCA, other FXR agonists are emerging as potential therapies for cholestatic liver diseases. In mouse models, 2 non-steroidal FXR agonists have shown promising results with reduction in liver biochemistries, improvement of inflammatory infiltrates and improvement in fibrosis (Figure 2).39 These FXR agonists, GS-9674 by Gilead Sciences and Tropifexor (otherwise known as LJN-452) by Novartis, are currently undergoing phase II trials for PBC. Both are non-bile acid formulations, thus expected to cause less pruritus and hyperlipidaemia compared to OCA.40 In addition, a newer non-bile acid FXR agonist, EDP-305, recently demonstrated a promising safety and tolerability profile in a phase I study including healthy individuals and patients with presumed non-alcoholic fatty liver disease.41 The drug is now under evaluation for patients with PBC (NCT03394924).
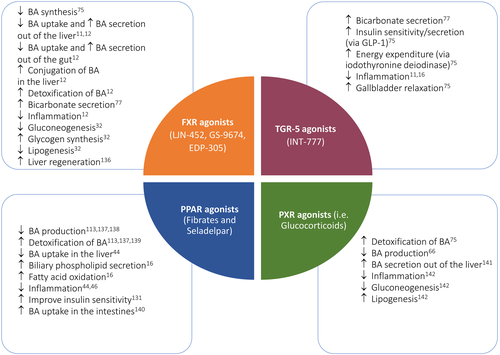
A proof-of-concept phase II trial assessing GS-9674 in patients with PSC in an open-label fashion is also ongoing (NCT02943460). All ongoing trials for PBC and PSC are summarized in Tables 3 and 4.
| Mechanism | Drug | Primary outcomes | Phase | Clinical trial/EudraCT number |
|---|---|---|---|---|
| ASBT inhibitor | GSK2330672 | Pruritus | II | NCT02966834 |
| Bile acid | Median dose UDCA (18-22 mg/kg/d) | Biochemical | IV | NCT03345589 |
| CX3CL1 monoclonal antibody | E6011 | Biochemical | II | NCT03092765 |
| FXR agonist | EDP-305 | Biochemical | II | NCT03394924 |
| FXR agonist | LJN452 | Biochemical | II | NCT02516605 |
| FXR agonist | OCA | Clinical outcomes | IV | NCT02308111 |
| FXR agonist | GS 9674 | Biochemical | II | NCT02943447 |
| Kappa opioid receptor agonist | Nalfurafine Hydrochloride | Pruritus | NCT02659696 | |
| NADPH oxidase NOX1/NOX4 inhibitor | GKT137831 | Biochemical | II | NCT03226067 |
| PPAR-α and gamma agonist | Saroglitazar magnesium | Biochemical | II | NCT03112681 |
| PPAR-α agonist | Fenofibrate | Biochemical | I/II | NCT02965911 |
| PPAR-α agonist | Fenofibrate | Biochemical | III | NCT02823353 |
| PPAR-α agonist | Fenofibrate | Biochemical | III | NCT02823366 |
| PPAR-α, γ, & δ | Bezafibrate | Biochemical | III | NCT02937012 |
| PPAR- α & δ agonist | Elafibranor | Biochemical | II | NCT03124108 |
| PPAR-δ agonist | Seladelpar | Biochemical | II | NCT02955602 |
| PPAR-δ agonist | Seladelpar | Biochemical | II/III | NCT03301506 |
| Selective S1P receptor modulator | Etrasimod (APD334) | Biochemical | II | NCT03155932 |
| PPAR- α, γ, & δ | Bezafibrate | Pruritus | III | NCT02701166 |
| Corticosteroid | Budesonide | Clinical outcomes | III | 2007-004040-70 |
- ASBT, Apical sodium-dependent bile acid transporter; CX3CL1, chemokine (C-X3-C motif) ligand 1; CTLA-4, cytotoxic T-lymphocyte antigen 4; EudraCT, European clinical trials database; FXR, farnesoid X receptor; IgG1, immunoglobulin G1; NADPH, nicotinamide adenine dinucleotide phosphate; NOX, NADPH oxidase; OCA, obeticholic acid; PPAR, peroxisome proliferator-activated receptor; S1P, sphingosine 1-phosphate; UDCA, ursodeoxycholic acid.
- a From https://clinicaltrials.gov.
| Mechanism | Drug | Primary outcomes | Phase | Clinical trial/EudraCT number |
|---|---|---|---|---|
| Retinoic acid receptor agonist | All-trans retinoic Acid | Biochemical | II | NCT03359174 |
| FXR agonist | OCA | Biochemical | II | NCT02177136 |
| FXR agonist | GS-9674 | Biochemical | II | NCT02943460 |
| Inhibitor of hyaluronan synthesis | Oral hymecromone | Safety, biochemical, MRCP changes | I/II | NCT02780752 |
| VAP-1 monoclonal antibody | BTT1023 | Biochemical | II | NCT02239211 |
| PPAR- α, γ, & δ | Bezafibrate | Pruritus | III | NCT02701166 |
| Altering the microbiome | Faecal microbiota transplantation | Biochemical | I/II | NCT02424175 |
| Undefined | HTD1801 | Biochemical | II | NCT03333928 |
| Undefined | DUR-928 | Biochemical | II | NCT03394781 |
| Antibiotic | Vancomycin | Biochemical | IV | NCT02605213 |
| Antibiotic | Vancomycin | Biochemical | III | NCT01802073 |
| Bile acid derivative | NorUDCA | Biochemical | III | 2016-003367-19 |
- FXR, farnesoid X receptor; NorUDCA, 24 nor-ursodeoxycholic acid; OCA, obeticholic acid; PPAR, peroxisome proliferator-activated receptor.
- a From https://clinicaltrials.gov.
5.3 FGF-19 analogues: NGM282
As previously mentioned, FGF-19 expression results directly from FXR activation and leads to downregulation of CYP7A1.42 In a phase II multicenter randomized controlled trial of NGM282, a synthetic analogue of FGF-19 with a modified N terminus, 45 patients with PBC and an incomplete response to UDCA received UDCA plus NGM282 in doses of 0.3 mg or 3.0 mg vs placebo for 28 days. A dose-dependent reduction in ALP and other liver biochemistries was observed in treated patients at day 28: 15.2% in the 0.3 mg group, 19.2% in the 3.0 mg group and 1.2% in the placebo group. The ALP reduction was more pronounced in the subgroups where the baseline ALP was greater than 3× the ULN. Main side effects included diarrhoea, nausea and headaches.43 NGM282 was also evaluated in a randomized controlled trial including 62 patients with PSC (NCT02704364). A press release note from NGM states that the study did not meet the primary endpoint of a statistically significant change in ALP (http://www.ngmbio.com/media/press-releases/020518/).
5.4 PPAR agonists: Fibrates, Seladelpar
The nuclear receptor peroxisome proliferator-activated receptor (PPAR) occurs in 3 different isoforms: α, γ and δ. PPAR agonists have different affinities for the various isoforms. For instance, fenofibrate binds PPAR-α with the greatest specificity, bezafibrate binds all 3 subtypes comparably, and seladelpar binds the δ isoform selectively. Furthermore, each isoform has variable expression in different organs, with PPAR-α being predominantly expressed in the liver and other organs participating in fatty acid catabolism, PPAR-γ is found in adipose tissues and immune cells and the δ isoform being ubiquitously expressed.44, 45 PPAR agonists will have different effects depending on which isoforms are activated.
Peroxisome proliferator-activated receptor agonists have a multitude of effects thought to be beneficial in cholestatic diseases. First, they upregulate MDR3, which promotes excretion of phosphatidylcholine in bile causing reduced bile salt cytotoxicity. Furthermore, they modulate expression of MRP2, MDR1, NTCP and ASBT and upregulate sulphotransferase family 2A member 1 (SULT2A1) and UDP glucuronosyltransferase family 1 member A1 (UGT1A1), among other genes which help detoxify bile acids. Fibrates also suppress NF-κB transcription (Figure 2), thus interfering with inflammatory pathways, and cross-react with other receptors, including pregnane X receptor (PXR) and FXR, further enhancing its anticholestatic and anti-inflammatory properties.16, 46
5.4.1 Fibrates
Several studies evaluated the use of bezafibrate alone or in combination with UDCA for PBC and consistently showed improvement in serum liver biochemistries.47-56 Typically, these studies included a small sample size (10-30 patients) and were of relatively short duration. The recently completed BEZURSO trial, included a much larger sample size of 100 PBC patients (50 in each group), treated for 2 years with either UDCA/bezafibrate 400 mg/d or UDCA/placebo. Normalization of ALP and normalization of all liver chemistries were seen in 67% and 30% of bezafibrate-treated patients, respectively, compared to none on placebo. Additionally, liver stiffness by transient elastography and the itch scores also improved in treated patients.57
Similarly, Reig and colleagues reported their experience in treating 48 patients with PBC and an incomplete response to UDCA with the addition of bezafibrate 400 mg/d for a median of 38 months. Notably, 54% of patients normalized serum ALP. In this group of so-called responders, investigators observed significant improvement in pruritus, jaundice and liver stiffness measured by elastography. Predictors of non-response to bezafibrate included liver stiffness >7.3 kPa and younger age (<53 years). The drug was safe; serious adverse events were not reported; and the kidney function was stable. Side effects were infrequent and included mild gastrointestinal discomfort and myalgias.58
The long-term efficacy and safety of bezafibrate should be evaluated further. In that regard, a trial by Hosonuma and colleagues randomized 27 patients with PBC and incomplete response to UDCA to receive UDCA + bezafibrate (400 mg/d) vs UDCA alone for up to 8 years.59 Despite improvement in serum ALP and Mayo risk score, survival was not better in bezafibrate-treated patients. In addition, patients receiving bezafibrate were more likely to discontinue the study due to renal dysfunction or myalgias. However, one should recognize that this study enrolled only patients with PBC who also had dyslipidaemia; thus, the patient population may not be representative of all PBC patients.
While bezafibrate is not available in the United States, fenofibrate is available. Treatment of PBC patients refractory to UDCA monotherapy with the addition of fenofibrate 160 mg/d led to significant reductions in serum ALP and other liver biochemistries as well as improvement in serum IgM and inflammatory markers. Side effects included mostly heartburn, myalgias and transient transaminase elevation.60
In contrast to the growing experience with PPAR agonists in PBC, summarized in Table 5, our understanding of their effect on patients with PSC is just beginning. A Japanese open-label study prospectively evaluated use of bezafibrate 200 mg twice daily for 12 weeks in 11 patients with PSC.61 Serum ALP and ALT improved in 7 patients (64%). Use of fenofibrate was also evaluated for PSC in 2 small open-label studies from France and the US published only in abstract format, again showing improvement of serum liver biochemistries.62, 63 Additional studies including larger sample size and longer follow-up are warranted.
| Drug | PPAR isoforms affected | Mechanism | Summary of findings |
|---|---|---|---|
| Fenofibrate | α; expressed predominantly hepatocytes only80 | ||
| Bezafibrate | Pan-PPAR; expressed in adipose tissue and immune system45 | ||
| Seladelpar | δ; expressed in hepatocytes, cholangiocytes, macrophages and stellate cells45, 80 | ||
| Elafibranor (NCT03124108) | α and δ; expressed in hepatocytes, cholangiocytes, macrophages and stellate cells80 |
|
- CYP7A1, cholesterol 7 alpha-hydroxylase; HNF4, hepatocyte nuclear factor 4; IgM, immunoglobulin M; LDL-C, low-density lipoprotein cholesterol; MDR3, multidrug resistance protein 3; PPAR, peroxisome proliferator-activated receptor; PXR, pregnane X receptor; TG, triglycerides; TNF-α, tumor necrosis factor α.
5.4.2 Other PPAR agonists
Seladelpar is a selective PPAR-δ agonist. It was recently evaluated in a multicenter trial for PBC patients with an incomplete response to UDCA, where addition of seladelpar 50 mg/d or 200 mg/d was compared to placebo. The study showed a significant reduction in ALP of 53% and 63% in the 50 and 200 mg groups, respectively, compared to 2% in the placebo group. However, the trial was stopped early after 1 patient in the 50 mg group and 2 in the 200 mg group developed grade 3 increases in aminotransferases.64 An ongoing phase II/III trial is testing seladelpar at lower doses of 2, 5 and 10 mg (NCT03301506 & NCT02955602). Interim analyses suggest maintained efficacy at these lower doses, without the previously observed elevation in transaminases.65
Elafibranor (GFT505), a dual PPAR-α and PPAR-γ agonist, is thought to have antifibrotic activity and is currently being evaluated in non-alcoholic fatty liver disease and in PBC (NCT03124108).
5.5 PXR agonists: Budesonide
The main role of PXR is in detoxification and metabolism of bile acids via CYP3A4 and SULT2A1, decreasing bile acid synthesis through CYP7A1, and upregulation of MRP3, MRP2 and MDR1 (Figure 2).16, 66 Glucocorticoids are thought to possibly improve liver chemistries and histology in PBC but are not recommended given their extensive side effect profile.67 However, budesonide, a steroid with greater than 90% first-pass hepatic metabolism, may be a more tolerable alternative. In vitro, use of budesonide in combination with UDCA caused stimulation of the apical chloride/bicarbonate exchanger (AE2), producing a bicarbonate-rich choleresis and strengthening the bicarbonate umbrella.68 Three small studies have evaluated the use of budesonide in PBC and suggest that budesonide + UDCA may be slightly superior to UDCA alone in non-cirrhotic patients with respect to improvement in inflammation and fibrosis.67, 69, 70 Despite the high first-pass metabolism, side effects are still reported after long-term use of budesonide, including bone mass loss, hyperglycaemia and cosmetic changes.69 Furthermore, increased rates of portal vein thrombosis have been reported in cirrhotic patients receiving budesonide.71 Results of an European phase III multicentre randomized controlled trial comparing UDCA + budesonide to UDCA + placebo are awaited (Eudra CT number 2007-004040-70).
Budesonide has been previously evaluated in patients with PSC and found not to be beneficial at doses of 3 mg/d or 9 mg/d.72, 73
5.6 TGR5 agonists: INT-767 & INT-777
Transmembrane G-protein–coupled receptor 5 (TGR5) is a cell surface receptor which is widely distributed in the gastrointestinal tract, especially in the submucosal nervous plexus in the small and large intestine.74 In the liver, we find TGR5 in the sinusoidal endothelial cells, cholangiocytes and Kupffer cells, but not in the hepatocytes. Bile acid binding to TGR5 results in activation of intracellular cAMP signalling and other cell-specific signalling cascades. Among other effects, there are inhibition of NF-kB pathway, secretion of glucagon-like peptide 1 (GLP-1) and activation of iodothyronine deiodinase, which result in anti-inflammatory properties, improvement of diabetes control and enhancement of basal metabolism.75 In the biliary epithelial cell, TGR5 also mediates increased bicarbonate secretion through the cystic fibrosis transmembrane conductance regulator (CFTR), thus protecting against bile cytotoxicity. Furthermore, activation of TGR5 has been linked to the known immunosuppressive effect that bile acids exert on immune cells.74
In mice, TGR5 was found in dorsal root neurons that mediate itch and pain, and mice overexpressing TGR5 have spontaneous itching. Bile acids can activate TGR5 and induce secretion of neuropeptides that transmit itch and analgesia.76 Therefore, modulating TGR5 could represent a novel approach to treat itching in cholestatic diseases. Mouse models with overexpression of TGR5 have shown protective effects in 3,5-diethoxycarbonyl-1,4-dihydrocollidine induced sclerosing cholangitis, and likewise, mice with lower levels of TGR5 expression showed worsening fibrosis.16
The wide ranging and favourable activity of TGR5 makes it a potential treatment target, with 2 drugs currently being investigated. INT-767, a 23-sulphate derivative of OCA, is an agonist of both FXR and TGR5, and is 3 times as potent an FXR agonist as OCA. INT-767 has been shown to decrease bile acid synthesis, increase bicarbonate excretion and inhibit NF-kB.77 INT-777, α-ethyl-23(S)-methylcholic acid, is specific for TGR5. Both molecules have shown promising results in animal studies.78 However, while TGR5 agonists are potentially beneficial to their anti-apoptotic and proliferative effects in cholangiocytes, certain cancerous cells including cholangiocarcinoma tissue have increased expression of TGR5 receptors. Consequently, activation by TGR5 agonists may promote cholangiocarcinoma progression, thus making these drugs less attractive for patients with PSC.79
5.7 All trans retinoic acid (ATRA)
As FXR, PPAR and PXR all work via forming heterodimers with retinoid X receptor (RXR), with subsequent binding to promoter regions on DNA,80 retinoic acid has been evaluated as potential therapy for PSC. A pilot study treated 15 patients with PSC who failed UDCA monotherapy with combination UDCA + ATRA (45 mg/m2/d divided into 2 doses) for 12 weeks. Investigators observed a significant decrease in the bile acid precursor C4 as well as in ALT levels, but no significant change was noted in ALP levels. Additionally, 63% and 26% of patients experienced headaches and tinnitus respectively.81 A phase II study is ongoing to evaluate lower dose ATRA (10 mg twice daily) over 24 weeks in 20 PSC patients (NCT03359174).
5.8 Immunomodulatory Therapies
5.8.1 Ustekinumab
Genome-wide association studies found significant associations with the interleukin 12 (IL 12)—signal transducer and activator of transcription (STAT) 4 signalling pathway in PBC.82 Furthermore, mouse models with deletion of IL-12p40 have marked reduction of pro-inflammatory Th1 cytokines in the liver and less pronounced destructive cholangitis.83 With that in mind, a phase II open-label trial evaluated the effect of ustekinumab, an IL12/23 monoclonal antibody, in 20 patients with PBC and failed to demonstrate a sizable improvement in ALP after 28 weeks of treatment.84 Ustekinumab is not being evaluated further for PBC.
5.8.2 Abatacept
Abatacept is a fusion protein of the Fc region of IgG1 and cytotoxic T-lymphocyte antigen 4 (CTLA-4), where CTLA-4 is required for costimulatory activation of T cells via CD80/CD86. In a mouse model of PBC, pretreatment with a CTLA-4 antibody abrogated development of cholangitis, and treatment after cholangitis had already developed led to reduction of the intrahepatic T-cell infiltrates and bile duct damage.85 A phase II study of abatacept 125 mg weekly for 24 weeks in 20 patients with PBC failed to meet the primary endpoint of either normalization or a decrease >40% in ALP from baseline.86
5.8.3 Rituximab
Rituximab is an anti-CD20 monoclonal antibody which has been evaluated in 2 small studies for PBC, unfortunately with only modest effect on serum ALP.87, 88 Nevertheless, the drug was overall safe and well tolerated.87 While Rituximab may not be beneficial in preventing disease progression, a study was recently completed in the UK exploring the use of rituximab for the treatment of fatigue in PBC (NCT02376335) and results are awaited.
5.8.4 Cenicriviroc
Cenicriviroc (CVC) is an antagonist of chemokine receptor 2 and chemokine receptor 5, which acts by blocking the recruitment of monocytes and macrophages. CVC has shown anti-inflammatory and antifibrotic effects in animal models of fibrosis and NASH.89 A phase II trial (PERSEUS) evaluating the effect of a 24-week treatment with CVC 150 mg/d in patients with PSC (NCT02653625) was completed December 2017, but results are yet to be published.
5.8.5 Vedoluzimab
The aberrant homing theory asserts that extraintestinal manifestations of IBD, including PSC, are secondary to atypically expressed adhesion molecules. The mucosal addressin cell adhesion molecule-1 (MADCAM-1), usually confined to the gut, can be found in the hepatic endothelium in patients with IBD-associated liver disease. MADCAM-1 is bound by α4β7 integrin (found on T cells) causing migration of gut-primed, mucosally activated T cells to the liver.90, 91 These are long-lived memory cells, which can explain why liver disease can occur years apart from the bowel disease and run a separate disease course.
Vedoluzimab is an anti-α4β7 integrin monoclonal antibody. Conceptually, this drug could prevent abnormal lymphocyte trafficking from the gut to the liver and mitigate inflammation, cell death and the eventual development of fibrosis in PSC. A retrospective study of 34 PSC patients receiving vedoluzimab for concomitant IBD [16 with Crohn's disease (CD) and 8 with ulcerative colitis (UC)] did not demonstrate a decline in liver chemistries or Mayo PSC risk score at 30 weeks. However, 55% and 29% of CD and UC patients, respectively, reached clinical remission of their IBD symptoms.92
5.9 Manipulation of gut microbiome
The strong association between PSC and IBD as well as the growing evidence suggesting a role for the microbiome on the pathogenesis of PSC has led many investigators to pursue the use of antibiotics in PSC.93 Several antibiotics have been tested including vancomycin, metronidazole, minocycline and rifaximin. All but the latter have demonstrated at least some beneficial effect on liver biochemistries.94-97 Among the antibiotics in use, vancomycin is the most promising.97 It has been shown to improve liver biochemistries and markers of inflammation, lower the prognostic index mayo risk score (MRS), reduce symptoms and exert immunomodulatory effects on regulatory T cells, especially in the paediatric population without cirrhosis. Vancomycin is also better tolerated, with a significant proportion of patients on metronidazole or minocycline having dropped out of studies due to adverse events. However, long-term use of vancomycin induces drastic changes in the microbiome and prompts concerns about infections with resistant bacteria.98 Furthermore, nephrotoxicity, neutropaenia and ototoxicity are notorious side effects of vancomycin.
Small randomized controlled trials of both low and high doses of vancomycin for PSC over 12 weeks have shown a decrease in baseline ALP of 18%-43%, improvement of the MRS and improvement in symptoms including pruritus, fatigue, diarrhoea and anorexia.99, 100 These results show promise and larger trials over longer periods are needed to assess the efficacy of vancomycin. Currently, 2 clinical trials looking at vancomycin in patients with PSC are underway (NCT02605213 & NCT01802073).
Finally, in an ongoing open-label trial examining the safety and efficacy of faecal microbiota transplant in 6 PSC patients, 5 having concomitant ulcerative colitis, an interim analysis showed that 50% reached the primary endpoint of a decline in ALP ≥50% from baseline, without reported adverse events (NCT02424175).101
5.10 Antifibrotic therapies: Simtuzumab
Lysyl oxidase-like 2 is a copper-dependent amine oxidase which plays an important role in fibrosis by catalyzing the cross links in both collagen and elastin. Simtuzumab is an IgG4 monoclonal antibody directed against lysyl oxidase like-2 (LOXL2). In a phase 2b study which included 234 PSC patients (51% of patients at baseline had bridging fibrosis or cirrhosis), simtuzumab was administered at dosages of 75 mg or 125 mg vs placebo over 96 weeks. After 96 weeks, there was no significant difference in hepatic collagen content, Ishak fibrosis stage, decrease in ALP or adverse event rate compared to placebo. Despite simtuzumab being well tolerated, there was no overall benefit in patients with PSC and there are no other clinical trials evaluating this drug for PBC or PSC.102
6 TREATMENT OF PRURITUS
6.1 ASBT inhibitors: GSK2330672 & Marilixibat
Apical sodium-dependent bile acid transporter (ASBT) is a transporter on the apical border of the terminal ileum which reclaims about 90% of the bile acids.103 ASBT inhibitors decrease absorption of bile acids, hindering intra-ileal activation of FXR and subsequently preventing the FXR induced downstream inhibition of CYP7A1. Also, by blocking ASBT, the overall size of the BA pool decreases causing less of a buildup and decreased toxicity in the bile canaliculi.16, 104 The 2 ASBT inhibitors currently being studied are maralixibat and GSK2330672.
In a phase II trial of maralixibat + UDCA (CLARITY study), 66 PBC patients were treated for 13 weeks to determine the effect on itching. Despite improvement from baseline itching scores, no significant difference was observed in the magnitude of effect between the drug and placebo. Side effects included diarrhoea (61.9%), abdominal pain (23.8%) and nausea (23.8%).105
A phase IIa 14-day crossover study of GSK2330672 in 22 patients with PBC showed a significant reduction in pruritic symptoms. Besides the increased incidence of diarrhoea (7 patients in the GSK2330672 group vs one in the placebo group), there is concern for downstream effects such as vitamin deficiencies, gallstone formation and colonic inflammation.106, 107 A larger phase II study including 118 PBC patients is ongoing to confirm the beneficial effect on itching and further examine drug tolerability (NCT02966834).
Maralixibat was also evaluated in 27 patients with PSC (CAMEO study; NCT02061540). Data published on clinicaltrials.gov shows 25 of 27 patients experiencing adverse events, with the majority of the events being diarrhoea and other gastrointestinal symptoms. While secondary outcomes such as change in bilirubin (from 1.22 to 0.98), ALP (from 471.6 to 434.9) and ItchRO scores (from 15.00 to 7.23) all improved, these do not appear to be clinically meaningful. However, further analysis of the results is warranted.
6.2 Bezafibrate
Currently, underway in the Netherlands is the phase III FITCH trial, comparing placebo vs bezafibrate 400 mg daily over 3 weeks for the treatment of itching in 84 PBC patients (NCT02701166).108
7 CONCLUSION
As animal models and human studies continue to elucidate bile acid physiology, their associated nuclear and surface receptors, transporters and role in cholestatic diseases, the emergence of new therapeutic targets for PBC and PSC is rapidly growing. In addition to bile acid derivatives and FXR agonists, drugs targeting a variety of other signalling pathways are under evaluation, with PPAR agonists possibly showing the most promise so far. The BEZURSO trial and additional studies out of Spain showed pronounced biochemical and symptom improvement in bezafibrate-treated patients with PBC, and, likewise, use of seladelpar led to major improvement in liver biochemistries. Smaller studies suggest biochemical benefit of fibrates and FXR agonists in PSC as well. Unfortunately, experimental therapies which show benefit in PBC have not been as effective for PSC; the lack of adequate animal models and biomarkers in PSC precludes well-designed, hypothesis-driven, trial development. Despite this and other challenges in the design and conduct of clinical trials for cholestatic diseases, much progress has been made. Future trials are likely to evaluate combination therapies of drugs with synergistic mechanisms of action to halt disease progression and improve symptom control.
CONFLICT OF INTEREST
JG none. CL received research grants from Gilead, Intercept, Shire, Tobira, GSK, Cyma Bay, Genfit, Genkyotek, Novartis, High Tide, Durect and Enanta. She participates in advisory boards for Intercept and is a consultant for GSK, Novartis and Intercept.




