Hepatic manifestations of inflammatory bowel diseases
Abstract
Inflammatory bowel diseases are associated with various hepatobiliary disorders, reported both in Crohn's disease and ulcerative colitis. They may occur at any moment in the natural course of the disease. The prevalence of liver dysfunction rises from 3% to 50% accordingly to definitions used in different studies. Fatty liver is considered as the most common hepatobiliary complication in inflammatory bowel diseases while primary sclerosing cholangitis is the most specific one. Less frequently, inflammatory bowel diseases-associated hepatobiliary disorders include: autoimmune hepatitis/ primary sclerosing cholangitis overlap syndrome, IgG4-associated cholangiopathy, primary biliary cholangitis, hepatic amyloidosis, granulomatous hepatitis, cholelithiasis, portal vein thrombosis and liver abscess. The spectrum of these manifestations varies according to the type of inflammatory bowel diseases. Treatments of inflammatory bowel diseases may cause liver toxicity, although incidence of serious complications remains low. However, early diagnosis of drug-induced liver injury is of major importance as it affects future clinical management. When facing abnormal liver tests, clinicians should undertake a full diagnostic work-up in order to determine whether the hepatic abnormalities are related to the inflammatory bowel diseases or not. Management of hepatic manifestations in inflammatory bowel diseases usually involves both hepatologists and gastroenterologists because of the complexity of some situations.
Abbreviations
-
- AIH
-
- autoimmune hepatitis
-
- AIHC
-
- autoimmune sclerosing cholangitis
-
- AIHG
-
- International Autoimmune Hepatitis Group
-
- ANA
-
- antinuclear antibodies
-
- 5-ASA
-
- 5-aminosalicylic acid
-
- AZA
-
- Azathioprine
-
- CD
-
- Crohn's disease
-
- DILI
-
- drug-induced liver injuries
-
- EASL
-
- European Society of Hepatology
-
- ERCP
-
- endoscopic retrograde cholangiopancreatography
-
- GD
-
- gallstones disease
-
- GETAID
-
- Groupe d'Etude Thérapeutique des Affections Inflammatoires du Tube Digestif
-
- HBVr
-
- HBV reactivation
-
- HD
-
- hepatobiliary disorders
-
- HSTCL
-
- hepatosplenic T-cell lymphoma
-
- IBD
-
- inflammatory bowel diseases
-
- 6-MMP
-
- 6-methylmercaptopurine
-
- 6-MP
-
- 6-mercaptopurine
-
- MRCP
-
- magnetic resonance cholangiography
-
- MS
-
- metabolic syndrome
-
- NAFLD
-
- non-alcoholic fatty liver disease
-
- NASH
-
- non-alcoholic steatohepatitis
-
- NRH
-
- nodular regenerative hyperplasia
-
- PA
-
- alkaline phosphatase
-
- pANCA
-
- antineutrophil cytoplasmic perinuclear antibodies
-
- PBC
-
- primary biliary cholangitis
-
- PML
-
- progressive multifocal leukoencephalopathy
-
- PSC
-
- primary sclerosing cholangitis
-
- SMA
-
- smooth muscle antibodies
-
- SOS
-
- sinusoidal obstruction syndrome
-
- S-PSC
-
- small-duct primary sclerosing cholangitis
-
- 6-TGN
-
- 6-thioguanine
-
- TPMT
-
- thiopurine (S)-methyltransferase
-
- UC
-
- ulcerative colitis
-
- UDCA
-
- Ursodeoxycholic acid
Key points
- Approximately 5% of IBD patients will develop liver disease.
- Abnormal liver tests results in IBD patient require a full diagnostic work-up in order to define, whether it is related to IBD or not , and to begin appropriate management.
- Fatty liver is considered as the most common hepatobiliary disorder related to IBD, and PSC the most specific one.
- Most of the drugs used in IBD are potentially hepatotoxic but have a low incidence of serious complications. However, early diagnosis of drug liver injury is of major importance as it affects clinical management.
1 Introduction
Inflammatory bowel diseases (IBD) are associated with a variety of extraintestinal manifestations, including hepatobiliary disorders (HD).1 HD are reported in both ulcerative colitis (UC) and Crohn's disease (CD), but are more commonly related to UC.2 Their clinical course is often independent from that of the associated IBD. Screening for HD in IBD patients is important, as approximately 5% of adults will develop liver disease.3 Moreover, chronic liver disease is also found in IBD patients with normal liver biochemical tests.3 Because of the large spectrum of hepatobiliary disorders in IBD, the prevalence of liver dysfunction reported in the literature varies greatly. Differences in the definition across studies may also account for the prevalence variability. Indeed, when transient elevation of liver tests is also taken into consideration, the prevalence rises accordingly from 3% to 50%.4
Non-alcoholic fatty liver disease (NAFLD) is considered as the most common liver disease in IBD, even if most epidemiological studies are inconclusive.2 Primary sclerosing cholangitis (PSC) is the most specific hepatobiliary complication associated with IBD, particularly in UC. PSC patients are prone to develop cholangiocarcinoma and colon cancer.3 Less frequently, IBD-associated hepatobiliary disorders include: autoimmune hepatitis/PSC overlap syndrome, IgG4-associated cholangiopathy, primary biliary cholangitis (PBC), hepatic amyloidosis, granulomatous hepatitis, cholelithiasis, portal vein thrombosis and liver abscess. The spectrum of these manifestations varies according to the type of IBD. Granulomas, hepatic abscess, amyloidosis and gallstones are conventionally observed in CD while PSC and autoimmune hepatitis are usually described in UC.5
The pathogenesis of liver injury in IBD is not completely understood. Genetic, immunological and environmental factors that contribute to IBD pathogenesis may also contribute to associated hepatobiliary disorders.6 Some complications are related to IBD itself and can be subsequently related to inflammation or malabsorption. Finally, some hepatobiliary disorders are caused by IBD treatment, as most of them are potentially hepatotoxic. Hepatitis B reactivation during immunosuppressive therapy is also a major concern.
The main goal of this review is to summarize the most recent knowledge regarding hepatobiliary manifestations related to IBD with a particular focus on the hepatotoxicity of concomitant drugs used in the treatment of IBD.
2 Abnormal liver tests in IBD
Transient or persistent elevation of liver tests are frequently encountered in IBD as described in a recent review (Table 1).2
| Disease | Prevalence in general Population | Prevalence in UC | Prevalence in CD |
|---|---|---|---|
| Cholelithiasis | 1.8%-22.4% | 4.6%-36.4% | 11%-34% |
| Primary sclerosing Cholangitis | NA | 0.76%-5.4% | 1.2%-3.4% |
| Fatty liver | 6%-35% | 1.5%-55% | 1.5%-39.5% |
| Hepatic amyloidosisa | NA | 0.07% | 0.9%-3% |
| Granulamatous hepatitis | 0.45%-0.7% (incidence) | NAb | NAb |
| Liver abscess | 2.3/100'000 (incidence) | NA | NA |
| Portal vein thrombosis | 1% | NAc | NAc |
- a overall IBD: 0.5%.
- b overall IBD: less than 1%.
- c 39%-45% of IBD patients undergoing proctocolectomy.
In Sweden, a cohort study that included 1274 patients found that 11% of patients (n=134) experienced liver laboratory abnormalities, defined by an increase in the alkaline phosphatase (PA) activity or transaminases greater than two times the normal value and confirmed at least 2 months apart.7 The abnormalities were transient in 60 patients (5% of the studied population) and observed mainly during a flare of colitis. Abnormal liver tests were considered most commonly transient and generally thought to be related to the activity of IBD, without impact on long-term prognosis. Nevertheless, these abnormalities persisted in 74 patients (6% of the total Swedish cohort) and were related to PSC in 29 cases (40% of the subgroup with persistent abnormal test) and to various other causes (steatosis, excessive alcohol consumption, etc.).
Conversely, a more recent cohort of 544 IBD patients reported no correlation between IBD activity and prevalence of abnormal hepatic tests: 27% patient with active disease and 36% in those in remission (P=.06).3 In this study, abnormal hepatic tests were encountered in one-third of patients with IBD, with similar frequency in CD and UC. The presence of mild transient abnormalities appeared to have a negative impact on the long-term prognosis of these patients as the risk of death was over four times higher for those with abnormal hepatic biochemistries than for those with normal hepatic biochemistries (HR 4.8, 95%CI 1.54-16.40). This has a significant implication for everyday clinical management since current clinical practice does not routinely recommend monitoring of mild liver test abnormalities in IBD patients, as these are often attributed to a flare of the disease.
3 Primary sclerosing cholangitis
Primary sclerosing cholangitis is the most specific hepatobiliary manifestation of IBD, and is viewed as the archetypal hepatobiliary manifestation of UC.8This disease is characterized by chronic inflammation leading first to damage of intra- and/or extrahepatic bile ducts and finally to fibrotic strictures and saccular dilatations. Subsequent secondary biliary cirrhosis and cholangiocarcinoma are of particular concern.9
3.1 Epidemiology
The classical form of PSC affects mainly middle-aged men.8 The annual incidence of PSC is estimated at 0.77 cases per 105 patient-years, whereas the prevalence ranges between 8 and 14/100 000, among Caucasians in North American studies.10, 11 Patients with UC pancolitis represent 85-90% of cases. The methods of IBD diagnosis may partly explain these differences, as colonoscopy was not systematic in all studies. Interestingly, a systematic screening by magnetic resonance cholangiography (MRCP) in a large cohort of IBD patients found a high prevalence (25/322, 7.8%) of PSC-like lesions, two-thirds of them lacking liver test abnormalities.12
3.2 Aetiology
Primary sclerosing cholangitis is classically considered an autoimmune disease. Various hypotheses have been raised such as: chronic portal bacteraemia, abnormal metabolism of bile acids by the intestinal microbiota, direct production of toxins by the microbiota, unrecognized chronic viral infections, ischaemia of the biliary tract and finally, genetic abnormalities of immunoregulation or bile transport.
There is more than 80-fold increased risk of PSC among first-degree relatives.13 Multiple genetic factors of susceptibility have been reported, such as HLA-B8, HLADRB1* 0301 (DR3), HLADRB3*0101 (DRw52a) and HLA-DRB1*0401 (DR4).14 In addition, three UC susceptibility loci have been associated with PSC. These loci harbour the presumed candidate genes REL, IL2 and CARD9.15 In 2013, the largest genetic study identified 12 genome-wide significant associations outside the HLA complex increasing the number of known PSC risk loci to 16. Six of them had much strong association with PSC than with IBD, suggesting distinct disease processes in PSC than IBD.16
Antinuclear antibodies (ANA) are found only in 24%-53%, smooth muscle antibodies (SMA) in 13%-20% and antineutrophil cytoplasmic perinuclear antibodies (pANCA) in 65%-88% of patients, precluding firm conclusion from the presence of autoantibodies.17
Accumulating evidence suggests a potentially important role for intestinal microbiota as a central pathobiological driver of PSC independently of comorbidity with IBD.18 Three genera—Enterococcus, Lactobacillus and Fusobacterium seem over-represented in patients with PSC. However, it remains unclear if these alterations in the gut microbiota are a cause or an effect of liver disease. Further studies are warranted to confirm this challenging task to link dysbiosis with disease pathogenesis.
3.3 Diagnosis
The vast majority of IBD patients with PSC are initially asymptomatic and present abnormal liver tests with a cholestatic pattern.19 Diagnosis is based on the combination of four types of parameters (biochemical, radiological, histological and association with IBD). According to the European Society of Hepatology (EASL) recommendations, PSC diagnosis in its classic form can be retained if the following association is present: chronic cholestasis without other identifiable aetiology, typical MRCP abnormalities and lack of argument for secondary sclerosing cholangitis.20 MRCP is the preferred non-invasive radiological diagnostic tool,21 as its diagnostic yield is high (sensitivity 88-90% and specificity 91-97%), without safety concerns of endoscopic retrograde cholangiopancreatography (ERCP). Radiological picture suggests PSC in the presence of diffuse, multifocal strictures and dilations in the intra- and extrahepatic bile ducts22 (Figure 1).
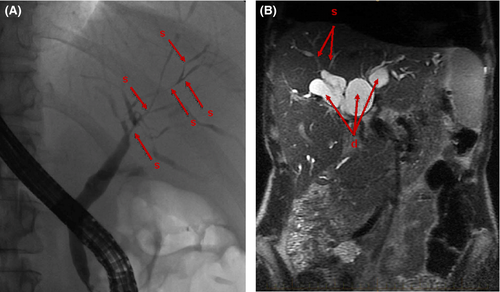
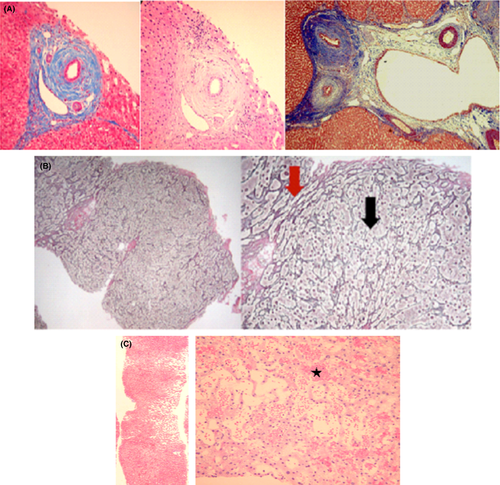
Liver biopsy is not mandatory, but may provide diagnostic and prognostic information especially in atypical forms such as small-duct PSC (S-PSC).23 The most specific histological finding in PSC is fibrous obliteration of small bile ducts, with periductal concentric fibrosis in an “onion skin” pattern, but those findings are absent in more than two-third of the cases because of the heterogeneous distribution of the lesions within the liver (Figure 2). Of note, 5%-10% of biopsies are normal24 and a normal liver biopsy cannot exclude the diagnosis of PSC. A classification into four stages has been proposed by Ludwig et al. based on morphological features.25
3.4 Variant forms of PSC
3.4.1 Small-duct PSC
S-PSC is defined by laboratory abnormalities (cholestasis pattern) and histological findings of PSC, but with normal cholangiography result.26 This form of cholangitis paves way for a differential diagnosis with other causes of cholestasis being macroscopically normal bile ducts, especially PBC, sarcoidosis and drug-induced cholangitis. In this situation, ABCB4 genotyping is now recommended.20 S-PSC could represent the initial stage of PSC and progression to PSC is observed in 12%-23% of cases.27 Long-term prognosis is better than the “classical PSC” and, to the best of our knowledge, no case of cholangiocarcinoma has been described outside the setting of large-duct PSC. The presence of IBD does not appear to change the prognosis. Interestingly, the association with Crohn's disease is more frequent than in the classic form of PSC.
3.4.2 Overlap forms: PSC-autoimmune hepatitis (AIH)
PSC can be very difficult to distinguish from AIH in children and young adults. Autoimmune sclerosing cholangitis (AIHC) represent a distinctive subset of patients having clinical overlap features with PSC and AIH.28 AIHC mainly affects the paediatric population. Cholangiography in children with abnormal liver test results associated with presence of autoantibodies has shown abnormalities of the biliary tract in half of the cases.29 AIHC has also been reported in young adults 30 and the development of PSC was described as a factor associated with resistance to treatment of AIH. Patients with AIHC seem to benefit from treatment with immunosuppressive medications and may have a better prognosis as compared to patients with PSC alone.28
3.4.3 IgG4 cholangitis
IgG4 cholangitis is a particular form of cholangitis that can mimic PSC. Because of the low number of reported cases, it is unclear whether this is an atypical form of PSC or a different entity.31 It is defined by increased serum IgG4, infiltration of biliary tract by IgG4 plasma cells, preferential involvement of the extrahepatic biliary tract, frequent association with another fibrotic pathology and especially type I autoimmune pancreatitis (> 50%) and dramatic regression of biliary strictures with corticosteroids.32 The diagnosis is often challenging when autoimmune pancreatitis is lacking and measuring serum IgG4 levels to exclude IgG4-associated sclerosing cholangitis is recommended at the time of diagnosis of “PSC”.23 The forms without pancreatic involvement can be observed in association with UC.
3.5 Prognosis
Primary sclerosing cholangitis is a progressive disease leading to portal hypertension, cirrhosis and hepatic failure. The median survival time is 18 years. This estimation varies between patients, as survival is significantly worse in symptomatic patients at the time of diagnosis.33 Coexisting IBD has been reported as a factor of poorer prognosis.34
The median survival after diagnosis of cholangiocarcinoma is 9 months.35 Cholangiocarcinoma is not necessarily a complication observed in advanced PSC as 30%-50% of patients are diagnosed during the following year after the discovery of the PSC.36 The diagnosis is very difficult because of pre-existing abnormalities of the biliary tract. Effective screening and monitoring strategies have not been validated nor clearly evaluated in practice.20 Various prognostic models for PSC have been proposed. The Mayo Risk Score based on age, serum bilirubin, albumin, aspartate aminotransferase and history of variceal bleeding are the most popular.37 Recently, transient elastography has shown some promise.38
3.6 Treatment
The treatment of PSC associated with IBD is not different from PSC without IBD. Different immunosuppressive or antifibrotic therapies including D-penicillamine, steroids, cyclosporine, tacrolimus, mycophenolate mofetil, methotrexate, infliximab, and colchicine have been tested in randomized studies but have not demonstrated any beneficial effect on morbidity and mortality.39 Ursodeoxycholic acid (UDCA) has been proposed because of PSC similarity with PBC. Unfortunately, large randomized studies (UDCA 13-23 mg/kg/day) demonstrated an improvement of liver biochemistry but failed to show a benefit in terms of survival without transplantation.40 The largest randomized controlled study evaluating the efficacy of 28-30 mg/kg/d of UDCA vs placebo, has been interrupted because of excess mortality in the UDCA group, including increase in portal hypertension.41 Consequently, administration of UDCA>20 mg/kg/day is not recommended, but there is still controversy regarding the use of UDCA at lower doses.20, 23
Clinical trials testing new drugs targeting fibrosis (simtuzumab), immunity (vedolizumab) and cholestasis (nor-ursodeoxycholic acid, obeticholic acid (FXR agonist) or, analogues of FGF19 decreasing the synthesis of bile acids, are ongoing. Orthotopic liver transplantation remains the only established option of treatment for PSC with a 5-year survival estimated at 85%–90%.42 However, 20% of patients will relapse after transplantation.43
Invasive treatment should be avoided in PSC patients because of significant risk of complication. However, dominant bile duct strictures with significant cholestasis should be treated with biliary dilatation, while biliary stent insertion can be considered if stricture dilatation and biliary drainage are unsatisfactory.20
3.7 IBD associated with PSC: a distinct behaviour?
Primary sclerosing cholangitis diagnosis can be done before or after the appearance of digestive disease, and even after colectomy.44 UC is diagnosed before the PSC in more than two-third of the cases; but the reverse sequence is also possible, and UC may even begin after liver transplantation.44 The prevalence of CD in the PSC varies from 1% to 17%. CD with PSC has, almost constantly, a colonic involvement. Among colonic CD, the prevalence of PSC can reach 9%. Clinical course of PSC is not related to the activity of IBD and vice versa. IBD associated with PSC has a distinct behaviour.
3.7.1 Location
Ulcerative colitis associated with PSC presents mostly as pancolitis, as it extends beyond the splenic flexure in 90% of the cases. The prevalence of PSC is about 5% when the colitis extends beyond the splenic flexure and only 0.5% when colitis is restricted to the descending colon.45 IBD associated with PSC is also remarkable for the frequency of the ileal involvement (backwash ileitis) and a relative rectal sparing compared to the classic UC. Crossing the ileocecal valve is then mandatory during colonoscopy.
3.7.2 Activity
There is no correlation between the severity of the UC and the severity of the PSC.46 Digestive disease can be very active or totally quiescent. Colonoscopy with biopsies should therefore be performed routinely if PSC is suspected because of the possible asymptomatic nature of colitis. Interestingly, colectomy does not appear to alter the natural history of PSC.47
3.7.3 Malignant risks
Colonic dysplasia and cancer
Combination of IBD and PSC increases the risk of colon cancer in patients with UC, especially in the right localization.48 The cumulative risk of developing dysplasia or colon cancer is 9%, 31% and 50%, respectively, after 10, 20 and 25 years of evolution of colitis, requiring strict annual endoscopic surveillance, starting from PSC diagnosis.49 Of note, the severity and the duration of PSC have not been significantly associated with the risk of colon cancer. This risk persists even after liver transplantation.50
Immunosuppression
Patients with IBD have an additional risk of developing cancer compared with the general population. However, the use of immunosuppressive treatments seem not to be related to the development of PSC, cholangiocarcinoma and colorectal cancer.51
3.7.4 Surgery
Pouchitis and pouch dysplasia
In patients with ileoanal anastomosis, the risk of pouchitis is increased in the presence of PSC.52 Pouchitis risk persists even after liver transplantation.53 Furthermore, the risk for dysplasia persists after colectomy.54
Peristomal varices
The occurrence of portal hypertension related to advanced PSC can have serious consequences in IBD patients with ileostomy. The development of peristomal varices causing untreatable bleeding may require the realization of a portosystemic shunt or liver transplantation.55 Subsequent ileostomy should be avoided in patients with PSC.
Liver transplantation
Inflammatory bowel diseases do not affect the survival of patients transplanted for PSC. However, a British study suggested that the risk of PSC recurrence on the graft could be significantly decreased in patients who had colectomy before transplantation.56 Despite immunosuppression, UC may start after transplantation. The activity of IBD may increase following transplantation especially after stopping corticosteroids (up to 50% of cases).57 The risk of colon cancer is particularly important after transplantation (cumulative cancer incidence: 1.25%/person/year, and incidence of dysplasia: 15% at 5 years). Consequently, experts recommend an annual surveillance colonoscopy with chromoendoscopy and, in case of dysplasia, to perform a total colectomy.58
4 Other hepatic manifestations than PSC affecting IBD patients
4.1 Non-alcoholic fatty liver disease
NAFLD is a clinicopathological syndrome with a broad continuum histological spectrum extending from benign steatosis to non-alcoholic steatohepatitis (NASH). NAFLD is the leading cause of elevated liver enzymes in adults and remains largely asymptomatic until end-stage complications occur. The co-existence of NAFLD with IBD emerged recently with an increasing prevalence ranging between 6.2% and 40%,59 (Table 2).
| Authors | N of patients | Type of study | Location | Diagnostic method | IBD type | NAFLD prevalence |
|---|---|---|---|---|---|---|
| Sourianarayanane et al. 2013 | 928 | Case-control | USA | Ultrasound/CT/MRI | 53% (CD)47% (UC) | 8.2% (overall) |
| Gisbert et al. 2007 | 786 | Retrospective cohort | Spain | Ultrasound | 51% (UC)49% (CD) | 40.8% (overall) |
| Bargiggia et al. 2003 | 511 | Prospective cohort | Italy | Ultrasound | 61% (CD)39% (UC) | 39.5% (CD)35.5% (UC) |
| Riegler et al. 1998 | 484 | Prospective cohort | Italy | Ultrasound | 65% (UC)35% (CD) | 13.6% (UC)8.9% (CD) |
| Bessisow et al. 2016 | 321 | Retrospective cohort | Canada | Hepatic steatosis index/ Fibrosis-4 score | 68% (CD)32% (UC) | 33.6% (overall) (Incidence) |
| Yamamoto-Furusho et al. 2010 | 200 | Prospective cohort | Mexico | Ultrasound | 100% (UC) | 11.2% (UC) |
| De Fazio et al. 1992 | 74 | Prospective cohort | Italy | Ultrasound | 68% (UC)32% (CD) | 16.6% (UC)12% (CD) |
Elevated liver enzymes are frequently used to detect NAFLD in IBD, with a poor negative predictive value. Estimation of NAFLD with ultrasonography has a sensitivity and specificity of 85% (95% CI: 79.5-88.9%) and 94%(95% CI: 87.2-97%) respectively.60 There are limited data on liver fibrosis, which is reported in 6.4-10% of IBD patients.59
A recent study reported an even higher incidence rate of NAFLD in IBD patients without underlying liver disease, using the validated Hepatic Steatosis Index.61 NAFLD was reported in one-third of the patients, accounting for an incidence rate of 9.1/100 patient-years (95% CI, 7.4-10.9), which is higher than the general population (.029-3.1/100 patient-years). Among patients with NAFLD, 7.4% developed advanced liver fibrosis.
Transient elastography (TE) represents an alternative imaging method for the detection of liver fibrosis and has been validated in patients with chronic hepatitis C. Few studies have explored diagnostic yield of TE on IBD population. Available studies have focused on CD patients treated with methotrexate and revealed that the prevalence of liver fibrosis associated to methotrexate is low and the abnormal TE was rather because of alcohol use or NAFLD.62, 63
Pathogenesis influencing the co-existence of NAFLD related to IBD is poorly understood but includes factors such as metabolic syndrome (MS), microbial dysbiosis, immune activation, medications, disease activity and duration, prior IBD-related surgical intervention and parenteral nutrition.61 The presence of steatosis appears to be correlated with patient's general condition and severity of IBD.64
Impact of MS in IBD patients is debated as this population can develop NAFLD, even without classical metabolic risk factors. The prevalence of MS in IBD seems to be comparable to the general population (18.6%), with an imbalance in favour of CD (7.1%) compared to UC (23%).65 The overall prevalence of MS was even lower in IBD patients compared to a general US population, highlighting a potentially more complex pathogenesis explaining the relationship between the two diseases.64 One hypothesis is that steatosis may develop from insulin resistance and associated metabolic disturbances, leading to fatty infiltration in the liver.
Intestinal microbiota has emerged as a key player in the pathogenesis of both NAFLD and IBD, where alteration of gut microbiota has been associated with disease activity.66 Alteration of gut microbiota may act as a pathogenic link between IBD and NAFLD and an active underlying inflammatory process could drive fatty infiltration of the liver.
Duration of IBD is probably another independent predictor of NAFLD development.61 Disease exposes patients to multiple risk factors for NAFLD, including chronic relapsing inflammation, alteration of gut microbiota and hepatotoxic drugs. In particular, oxidative stress from reactive oxygen species may also be the common pathogenic factor contributing the co-existence of NAFLD and IBD. Prior surgery is also estimated as an independent predictor of NAFLD, but this is most likely a surrogate marker of the disease with a more active inflammatory condition and a repeated exposition to hepatotoxic drugs.61
Exposition to some IBD-related treatments may also predispose the development of NASH. Conversely, it has been postulated that anti-TNFα may protect against NASH, as TNFα participates to the pro-inflammatory pathways in the development of hepatic inflammation and development of NASH in NAFLD patients. Infliximab has been shown to reduce steatosis and increase insulin signal transduction in rodents on high-fat diet.67 Infliximab also reduced hepatic inflammation, necrosis and fibrosis in NASH rodents induced by methionine- and choline-deficient diet.59 Similar effects were also shown with the use of adalimumab.68 In humans, pentoxifylline, a non-selective phosphodiesterase inhibitor that reduced TNF production, has also been reported to induce biochemical liver enzymes improvement in NASH patients. Conversely, some case series reported the development of biopsy-proven NAFLD in patients receiving anti-TNFα, without changes in metabolic profiles and nutrition. There is some controversy in the literature concerning the role of anti-TNFα as a risk factor, with conflicting results.59
4.2 Cholelithiasis
The relationship between IBD and gallstones disease (GD) has been well recognized, principally in CD. However, data are based on small hospital-based cohort studies. Gallstones are seen in 13%-34% of patients with ileitis or ileal resection,69, 70 with a considerable regional variation.71 Site of disease at diagnosis, lifetime surgery, extent of ileal resections, number of clinical occurrences, total parenteral nutrition, frequency and duration of hospitalization were independently associated with gallstone development.72 Risk is clearly increased in CD but the association with UC is debated. However, a recent meta-analysis did not find significant difference in the prevalence of GD between patients with UC and control group.73 The fact that CD predominantly affects the terminal ileum, resulting in bile reabsorption disruption and increased enterohepatic circulation may explain the formation of cholesterol-supersaturated bile.72 After ileal resection, anaerobic bacteria colonization can also deconjugate bile acids decreasing the absorptive capacity of mucosa. Furthermore, CD patients have reduced gallbladder motility owing to prolonged fasting period, which may also lead to the development of cholelithiasis.74 Systematic cholecystectomy after an ileal resection is, however, not recommended.75
4.3 Autoimmune hepatitis
Autoimmune hepatitis is an immune-mediated chronic disease characterized by continuous hepatocellular inflammation and necrosis. The diagnosis of AIH must be suspected according to International Autoimmune Hepatitis Group (AIHG)-simplified criteria, including autoantibodies, immunoglobulin G, histology and exclusion of viral hepatitis 76. Most of the data concerning AIH in IBD come from studies focusing on PSC, with cases of AIH/PSC overlap syndromes as described above.2, 28, 77, 78
Only few series described patients with IBD and AIH alone, and cases were mainly described in UC adults and paediatric patients.79 In one adult study, AIH was associated with UC in 16% of the cases.80 Some experts suggest that IBD patients with co-existence of AIH are more likely to relapse and progress to cirrhosis.81 The response to treatment of AIH is not affected by the presence of IBD.80
4.4 Primary biliary cholangitis
Primary biliary cholangitis associated with IBD is rare and concerns mostly patients with UC.82, 83 Co-existence of PBC and UC was reported in approximately 20 sporadic cases in the literature and association with CD patients has been described in only few case reports.84 Compared with classical PBC, patients seem to be more often young males with usually mild and non-extensive mucosal lesions, such as proctitis.
4.5 Granulomatous hepatitis
Granulomatous hepatitis is another rare complication of IBD that affects mainly CD, characterized by granulomas on the liver biopsy. It should be suspected when there is an increase in cholestatic enzymes, such as PA.85 The estimated prevalence is less than 1% of cases of IBD. The disease is most often asymptomatic but can manifest by fever or hepatosplenomegaly. It can also be induced by mesalazine and sulfasalazine therapy.86, 87 Development of severe liver disease is unusual. After intestinal resection, regression of liver damage has been reported.88 Prognosis is usually benign.
4.6 Hepatic amyloidosis
Hepatic amyloidosis can be observed, especially in cases of severe CD with infectious complications and/or bowel resection.89 Secondary (reactive or AA) amyloidosis is seen in 0.9% of patients with CD and 0.07% with UC.90 There is a male predominance and prominent colonic involvement. Chronic activity in the bowel contributes to amyloid deposition in the vessels and sinusoids of almost any organ, including the liver, and thus leading to asymptomatic hepatomegaly. Treatment aims to control gut inflammation in order to decrease the release of the acute-phase reactant serum amyloid AA.91
4.7 Portomesenteric vein thrombosis
Patients of IBD are at risk for the development of venous thrombosis, as a consequence of inflammatory hypercoaguable state.92 Independent risk factors are: acquired prothrombotic factors, such as inflammation, immobilization, extent of colon disease, surgery, central catheters, corticosteroids and smoking. IBD patients also have elevated platelet counts, fibrinogen, factor V and VIII levels and concomitant decrease in antithrombin III levels.93, 94 Some experts argue that IBD may be an independent and disease-specific risk factor for thromboembolism.95
The portal vein is a common site of thrombosis in IBD. It occurs most frequently in the post-operative setting and has been reported in patients with UC after restorative proctocolectomy.96 A special feature is thrombosis caused by infection (pyelophlebitis) that is usually seen in patients with infectious complications of IBD, including abscesses.97 Interestingly, a retrospective GETAID study of inactive IBD patients found 40% of fortuitous portomesenteric thrombosis. Authors challenged the classical inflammatory aetiology and questioned the utility of screening for all patients. Anticoagulation remains the mainstay of therapy, even in the setting of gastrointestinal bleeding.98
4.8 Budd–Chiari syndrome
Several case reports of Budd–Chiari syndrome occurring in patients with IBD have been published.99 This is a thromboembolic phenomenon that can occur at baseline in patients with UC but in the setting of an acute flare, the risk is eight times higher. Perioperative period is also a significant risk period for thromboembolism.
4.9 Pyogenic liver abscess
Liver abscesses can reveal IBD, particularly in CD. Their overall incidence reaches 7 per 10 000 person-years.100, 101 Independent risk factors of developing pyogenic abscess in IBD patients include diabetes and endoscopic insertion of biliary drainage.101 Therapeutic benefit of corticosteroids, immunosuppressant or anti-TNF is widely assumed but has not been evaluated specifically. Abscesses are often multiple and located more frequently in the right hepatic lobe.100 Clinical symptoms are fever, abdominal pain, jaundice, diarrhoea or hepatomegaly. Leukocytosis and elevated PA concentrations are frequently seen. Unlike liver abscesses observed in other diseases, infection is often mono-microbial, caused by milleri Streptococcus, Fusobacterium nucleatum, Bacteroides fragilis or Staphylococcus aureus. The mechanism can be a direct extension of intra-abdominal abscesses via portal pyaemia or secondary to an increase in intestinal mucosa permeability.102 Treatment consists of antibiotic therapy guided by culture results, ideally with a guided puncture and drainage in case of large abscesses.
5 Hepatic complications of IBD related to treatment
Most of the drugs used in IBD are potentially hepatotoxic but have a low incidence of serious complications (Table 3). Acute or chronic hepatic injury can be attributable to the drugs used to treat IBD, or to treat complications of immunosuppression, as treatments for reactivation of tuberculosis.
| DILI and IBD medications | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug category | drugs | Acute hepatitis | Chronic hepatitis | Other | Delay | |||
| HC | Chol | Mix | Fulm | |||||
| S-ASA | SULF | ++ | ++ | ++ | + | Granuloma | W-M | |
| MES | + | + | + | D-Y | ||||
| Immuno | AZA | +++ | +++ | +++ | ++ | + | SD-PL-SOS-NRH | D-Y |
| 6-MP | ++ | + | ++ | + | SD-PL-SO S-NRH | D-Y | ||
| METHO | ++ | + | + | ++ | NASH | D-Y | ||
| Anti-TNF | OVERALL | ++ | ++ | ++ | ++ | HSTCL if combined with immuno | W | |
| Anti-Integrins | VDZ | Cases of severe hepatitis reported | ||||||
- 5-ASA, 5-aminosalicylic acid; Immuno, immunomodulators; SULF, sulfasalazine; MES, mesalazine; AZA, azathioprine; 6-MP, 6-mercaptopurine; METHO, methotrexate; VDZ, vedolizumab; HC, hepatocellular; Chol, cholestatic; Mix, mixte; Fulm, fulminant; SD, sinusoidal dilatation; PL, peliosis; NRH, nodular regenerative hyperplasia; SOS, sinusoidal obstruction syndrome; NASH, non-alcoholic steatohepatitis; HSTCL, hepato-splenic T-cell lymphoma.
Causality may be particularly difficult to establish, since IBD patients often are taking many other hepatotoxic drugs or have comorbidities that affect the liver or bile ducts.
5.1 Aminosalicylic acid derivatives
This group includes three major drugs: sulfasalazine, mesalazine and olsalazine. Sulfasalazine is the first aminosalicylate commercialized for treatment of IBD patients for induction as well as for maintenance of remission. This drug is an association of sulphapyridine linked to 5-aminosalicylic acid (5-ASA) via an azo bond 103 and can induce liver damage by hypersensitivity reactions. Sulfasalazine can cause cholestatic liver disease in about 10% of cases. Clinical manifestations include rash, elevation of liver aminotransferases, hyperbilirubinaemia, fever, hepatomegaly or lymphadenopathy. It usually occurs within the first 2 months after treatment onset. Granulomatous hepatitis has also been reported as a complication of sulfasalazine.104 Rare cases of fulminant hepatitis have been described.105-107
The use of sulfasalazine has decreased with the development of aminosalicylates (mesalazine, olsalazine) lacking the sulphonamide group. Immunoallergic hepatitis is rarely associated with mesalazine and olsalazine.108, 109
An audit from the UK reported an incidence of hepatitis in 3.2 and 6 cases per million of prescriptions for mesalazine and sulfasalazine respectively.110 This low risk of hepatotoxicity explains why close monitoring of liver biochemical is not mandatory with those treatments.
5.2 Corticosteroids
Corticosteroids represent the gold standard of IBD treatment. During a short administration for induction therapy, the effect on the liver is negligible. Extended high dose administration may cause macrovesicular steatosis and hepatomegaly. It may also predispose the development of NASH in high-risk patients by exacerbating insulin resistance, hypertriglyceridemia, obesity and diabetes.111
Even though no clear guidelines have been established, corticosteroid should be cautiously used in patients with existing metabolic risk factors.
5.3 Thiopurines
Azathioprine (AZA) and its principal metabolite 6-mercaptopurine (6-MP) are immunomodulators that are associated with a range of drug-induced liver injuries (DILI) including asymptomatic liver enzyme elevations, hepatocellular necrosis, cholestasis and even mixed injuries.112 Liver histology can reveal sinusoidal dilatation, peliosis, nodular regenerative hyperplasia (NRH) and sinusoidal obstruction syndrome (SOS) 113 (Figure 2).
The annual incidence of hepatotoxicity can reach 13% in prospective studies.112, 114 Liver damage often occurs beyond 6 months of treatment. During follow up, abnormal liver tests requiring drug discontinuation is rare (<4%) and can resolve spontaneously.115
6-thioguanine (6-TGN) is the active metabolite responsible for the clinical efficacy of the treatment but at a high concentration can cause liver toxicity and myelotoxicity. However, most liver toxicity is caused by another metabolite: 6-methylmercaptopurine (6-MMP).116 DILI related to thiopurine can be partly explained by the concentration of thiopurine metabolites mediated by activity of the enzyme, thiopurine (S)-methyltransferase (TPMT).117 TPMT is subject to genetic polymorphism with a dozen allele variants described so far. High TPMT activity can lead to lower 6-TG levels, resulting in a suboptimal clinical response, as well as higher levels of 6-MMP that are associated with hepatotoxicity.118 These genetic variations are routinely taken into account to adjust drug doses in patients treated for IBD.119, 120 Allopurinol, a xanthine oxidase inhibitor, is used to alter metabolite levels and reduce hepatotoxicity induced by thiopurines.121 Cases of Stevens Johnson are described with Allopurinol and this drug is contraindicated during pregnancy.
Thiopurine-induced vascular lesions can develop within 3 months after the beginning of treatment or even later, as some cases appeared more than 4 years later, and can cause non-cirrhotic portal hypertension.122,123
The development of NRH appears to be dose-related but independent of the duration of medication usage. Discontinuation of these medications is usually beneficial within 1 year,124 but severe cholestasis or portal hypertension may persist.125
Peliosis is histologically defined by the rupture of reticulin fibres that can lead to hepatic haematomas, and exceptionally to hepatic rupture with hemoperitoneum.
Cases of peliosis in young patients treated by AZA after “binge drinking” alcohol have been described.126 Acute SOS has been reported in IBD patients after 14 months of thiopurines treatment.127 SOS can also present initially as Budd–Chiari-like syndrome with ascites, jaundice and liver failure. The mechanism of SOS induced by AZA involves the depletion of glutathione in sinusoidal endothelial cells as well.128
5.4 Methotrexate
Methotrexate can be involved in acute and chronic liver diseases. The prevalence of hepatotoxicity varies according to therapeutic indication and seems less frequent in CD in comparison to other diseases, such as psoriasis or rheumatoid arthritis. Liver damage induced by methotrexate can range from macrovesicular steatosis, to hepatic fibrosis and even cirrhosis in a cumulative manner.129-131 A moderate increase in transaminases ranges from 19%-30% of CD. Cases of jaundice and acute hepatitis with transaminases increased to >40 times the normal value have been described when using high doses of the drug.
The mechanism of methotrexate hepatotoxicity is not fully understood, but data suggest that stellate cells may play a role. Liver toxicity can also be modulated by genetic factors and folate deficiency as supplementation has been shown to reduce hepatic adverse events.132 Recommendations of follow up of patients on methotrexate are highly debated. Liver biopsy is not recommended in IBD patients, as studies found no association between cumulative dose of methotrexate and development of fibrosis. The role of non-invasive technologies such as transient or MRI elastography warrants further investigations.133, 134
5.5 Anti-TNF agents
Several anti-TNF agents are used in IBD: infliximab, adalimumab, certolizumab, golimumab. TNF plays a key role in liver regeneration and anti-TNF can contribute to liver toxicity by at least three mechanisms: precipitation of “de novo autoimmune hepatitis”, cholestasis and direct hepatocellular necrosis.116, 135 Several reports of probable infliximab hepatitis have been published during infliximab therapy, but of weak significance 135-137 since other confounding factors were observed.138
The literature concerning the hepatotoxicity of other anti-TNF agents is also sparse.139, 140 Experts recommend that anti-TNF agents can be used even in patients with liver disease, but they should be avoided in patients with aminotransferases levels over three times the normal value.135
Finally, HSTCL (hepatosplenic T-cell lymphoma) is a very rare but fatal medical condition that has been described with combination of anti-TNF and other immunosuppressive therapy mostly in young males.141
5.6 Anti-integrins
5.6.1 Natalizumab
Natalizumab, an α4-integrin monoclonal antibody, was withdrawn from the European market for IBD patient's treatment because of its association with severe adverse events such as progressive multifocal leukoencephalopathy (PML).142 Approximately, 30 cases of liver failure because of natalizumab are listed in post-marketing FDA adverse event reporting system and 12 patients with severe liver injury have been reported.143
5.6.2 Vedolizumab
Vedolizumab is a gut-specific anti-integrin, only targeting α4β7 binding with MAdCAM1.144 Pre-licensure controlled trials report ALT elevations above 5 × ULN in <2% of patients with vedolizumab, and a similar proportion of placebo recipients. No case reports of significant liver toxicity were attributed to vedolizumab in the literature so far.
5.6.3 Treatment of IBD and risk of viral hepatitis reactivation
Prevalence of hepatitis B and hepatitis C in IBD is similar to that in the general population.145 HBV reactivation (HBVr) is a major concern in IBD before starting immunosuppressor treatment. Reactivation is defined as an increase in HBV viraemia, usually more than 1 log10 IU/mL, in a patient known for chronic HBV infection or even with a previously cured infection (positive anti-HBs and anti-HBc).146 The natural history of HBV depends on a complex interaction between the virus and the host immune system. Any situation decreasing the immunity of the host is likely to lead to HBV reactivation. The clinical repercussions of HBVr may lead to severe acute viral hepatitis, liver failure and even death. This risk of HBVr in IBD patients varies depending on the type of immunosuppressive drug used, and HBV phase prior to treatment. Patients who are HBsAg-positive are 5-8 times more likely to develop HBVr than those with resolved infection who are HBsAg-negative but anti-HBc positive.147 Managing the risk of HBVr involves screening patients at risk, stratifying patients for risk based on HBV serological status and type of immunosuppression (Table 4).148 Among immunosuppressors agents, high-dose steroids, anti-TNF and anti-integrins are of major concern. Risk appears lower for methotrexate and thiopurine, whereas aminosalycilates seems not even an issue 147 (Table 4). Patients at high risk of reactivation (anticipated incidence of HBVr in >10% of cases) require prophylactic treatment, while low-risk patients (risk of HBVr <1%) do not.148 It is less clear for patients at intermediate risk (risk of HBVr 1-10%). The American Gastroenterological Association suggests antiviral prophylaxis over monitoring for patients at moderate risk undergoing immunosuppressive drug therapy, but the recommendation is weak. As the evidence for low antiviral prophylaxis for HBVr is weak, the management should be individualized and discussed in a shared decision process with each patient.148, 149 Conversely, immunosuppressive therapy does not seem to promote reactivation of hepatitis C, and hepatitis C antiviral treatment does not influence IBD natural history.150
| Type of immunosuppression | ||||||||
|---|---|---|---|---|---|---|---|---|

|
||||||||
| HBV serological status | Corticosteroids high dose>10-20mg prednisone daily or equivalent for >4 weeks | TNF α inhibitorinfliximab, adalimumab, certolizumab, golimumab | Anti-integrinsnatalizumab, vedolizumab | Corticosteroids low dose<10mg prednisone daily or equivalent for >4 weeks | Thiopurinesazathioprine, 6-mercaptopurine | Methotrexate | Aminosalicylic acid derivativessulfasalazine, mesalazine, olsalazine | |
| HBsAg+/anti-HBc+ | HIGH | MODERATE | MODERATE | MODERATE | LOW | LOW | VERY LOW | |
| HBsAg-/anti-HBc+ | MODERATE | MODERATE | MODERATE | LOW | LOW | LOW | VERY LOW | |
- High risk= anticipated incidence of HBVr in >10% of cases, Moderate risk= anticipated incidence of HBVr in >1%<10%, Low risk= anticipated incidence of HBVr in <1%, Very low= negligible risk.
6 Conclusion
Elevation of liver enzymes is frequent in IBD patients and may occur at any moment in the natural course of the disease. When facing abnormal liver tests, a full diagnostic work-up should be immediately undertaken to determine the cause, whether it is related or not to the IBD, and to begin appropriate management (Figure 3). So far, most IBD treatments have been associated with liver toxicity, although incidence of serious complications is low. However, early diagnosis of DILI is of major importance as it affects clinical management (i.e. such as stopping or modifying the supportive treatment).
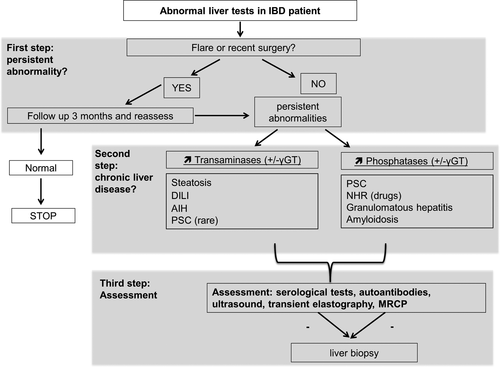
Acknowledgements
We thank Dr Laura Rubbia-Brandt and Dr Alan Barkun who collaborated to illustrate this review.
Conflict of Interest
The authors do not have any disclosures to report.




