Treatment of hepatocellular carcinoma: beyond international guidelines
Abstract
Treatment of hepatocellular carcinoma (HCC) is guided by the tumour stage. The Barcelona clinical liver cancer (BCLC) score endorsed by the European Society of the Liver EASL divides patients into five prognostic categories, each with a distinct treatment indication. Hepatic resection, orthotopic liver transplantation and percutaneous local ablation are strongly indicated in accurately selected patients with very early (BCLC 0) and early stage (BCLC A) tumours providing a survival rate of between 50 and 75% at year five. In patients with a large tumour burden such as those with intermediate stage BCLC B, repeated treatments with transarterial chemoembolization (TACE) are advocated with clinical benefits (from 16 to 22 months). Survival may also improve in patients who are in poor condition or who do not respond to TACE and those with an advanced HCC (BCLC C), following oral therapy with the multikinase inhibitor, sorafenib. However, most recommendations are based on uncontrolled studies and expert opinions rather than well-designed controlled trials, and up to one-third of patients do not fit recommendations because of advanced age, the presence of significant comorbidities or a strategic location of the nodule. For these patients, treatment of HCC beyond guidelines is often advocated.
Abbreviations
-
- BCLC score
-
- Barcelona Clinic Liver Cancer score
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- RFA
-
- radiofrequency abalation
-
- SOC
-
- standard of care
-
- TACE
-
- transarterial chemoembolization
Key points
- In daily practice, up to one-third of the patients with HCC do not fit recommendations drafted by Scientific International Societies for the treatment of the cancer.
- Treatment of HCC is multimodal in patients who do not reach radiological complete response with one type of treatment alone. For these patients, treatment of HCC beyond guidelines is often advocated.
- The use of therapeutic algorithms outside recommendations results in lower survival compared to a guideline-driven approach to the treatment of HCC.
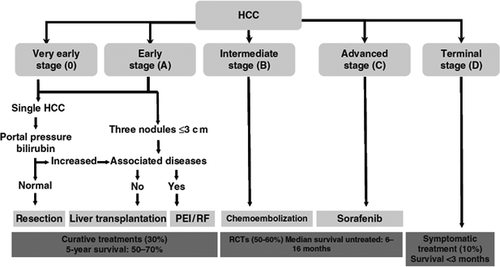
Hepatocellular carcinoma (HCC) is a lethal tumour whose prognosis largely depends on tumour stage at diagnosis and patient access to radical treatment 1. The treatment of HCC has progressed from an empirical choice of surgical resection in selected patients with small tumours to numerous options from orthotopic liver transplantation to locoregional ablation in patients who do not meet criteria for resection 1. More recently, the multikinase inhibitor, sorafenib, has been validated for the treatment of patients with advanced HCC, for whom no therapy was previously available 2. Management of HCC has been standardized according to clinical staging systems such as the Barcelona clinical liver cancer (BCLC) classification, where patients are stratified by tumour stage and underlying liver disease. The life expectancy of HCC patients can be reliably predicted and appropriate treatment can be identified according to stage 1, 3 (Table 1, Fig. 1), thus treatment of HCC based on the BCLC score has resulted in increased survival, mainly by preventing treatment failures from faulty staging of patients and empirical decisions. However, in daily practice a significant number of patients do not match the therapeutic criteria corresponding to their BCLC stage for reasons from advanced age to the anatomical location of the hepatic tumour. These patients may still be effectively treated outside current guidelines.
| BCLC stage | Performance status | Tumour volume, number and invasiveness | Child–Pugh |
|---|---|---|---|
| 0 – Very early | 0 | <2 cm | A |
| A – Early | 0 | Single or three nodules <3 cm each | A and B |
| B – Intermediate | 0 | Large/multinodular | A and B |
| C – Advanced | 1–2 | Vascular invasion and/or extrahepatic spread | A and B |
| D – End-stage | 3–4 | Any of the above | C |
The standard of care
BCLC 0 and A
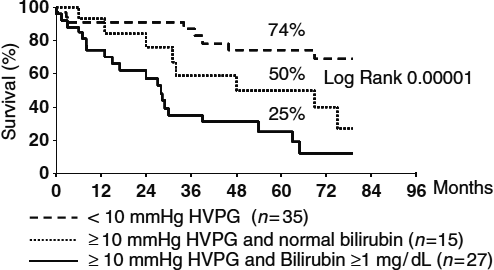
This stage includes both very early and early HCC, that is, a tumour <2 cm confined to the liver in a patient with well-compensated cirrhosis 4. Very early HCC is a small tumour that is usually not diagnosed on dynamic imaging techniques such as contrast-enhanced CT or gadolinium MRI, while early HCC is a distinctly nodular tumour that can be detected on contrast imaging because of arterial neovascularization. The BCLC A stage also includes patients with a single nodule, <5 cm or up to three nodules 3 cm or less (Milan criteria) 3. Thus, BCLC A is stratified into four classes according to the number and size of the tumours and other predictors of survival after surgical resection such as the portocaval gradient HVPG and serum bilirubin levels (Fig. 2). Patients with nodules ≤2 cm (80% of all tumours detected during surveillance in patients with cirrhosis) have a low risk of extranodal spread 5, and are theoretically ideal candidates for radical therapies as local ablation, resection and liver transplantation (LT). The final therapeutic decision, however, is based on patient characteristics such as age, liver status, the presence of comorbidities, the potential of improving underlying liver disease and the length of the transplantation waiting list. In carefully selected patients with a tumour ≤2 cm, survival is >70% at 5 years with all techniques, but the costs and risks of intervention-related mortality differ. The latter is very low for percutaneous ablation, while it is 1–3% for resection and 10% for LT 6. Antiviral therapy reduces the risk of postoperative decompensation and late onset recurrence from second primary tumours in patients with hepatitis B or C related tumours undergoing resection or local ablation of HCC 7, 8. In patients transplanted for end-stage hepatitis C virus (HCV), all oral agents against HCV prevent and successfully treat recurrent HCV, which is a major cause of graft failure and early death in these patients 9.
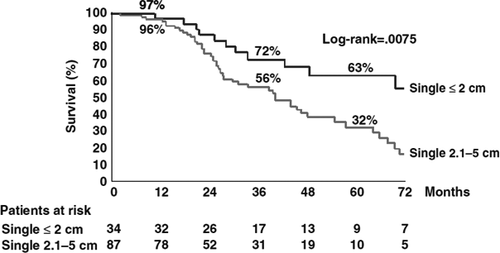
Percutaneous local radiofrequency ablation (RFA) is preferable to ethanol injections (PEI) in 2 and 3 cm tumours because of improved prevention of tumour recurrence on the periphery of the nodule 10, 11. While the efficacy of both ablative techniques is suboptimal for the treatment of tumours >3 cm because of an increased risk of tumour recurrence 12, resection and percutaneous ablation can be proposed to patients with up to three nodules ≤3 cm, but with less encouraging survival rates than in patients with a single ≤2 cm nodule, once again because of the increased risk of postoperative recurrence 13 (Fig. 3).
The Milan criteria are universally recognized as the guideline to list patients for orthotopic LT with an overall predicted survival of 75% at 5 years 14. In patients with >2 cm tumours who are on the waiting list, tumour ablation by RFA or transarterial chemoembolization (TACE) is currently recommended as a bridge to LT to prevent tumour progression and removal from the list or an increased risk of recurrence. Living donor liver transplantation has gained popularity to compensate for donor shortage, but it is associated with high complication rates (>30%) in recipients and a small, but non-negligible, risk of donor death (0.5%) 15. The limited number of donor livers does not favour strategies to extend the oncological criteria beyond the Milan criteria for transplantation, since extending criteria is associated with an increased risk of tumour recurrence and shortened survival 16.
In daily practice the treatment of HCC is multimodal in patients with more than one nodule who do not reach the end-point of a radical cure with one type of treatment alone. TACE has been used in combination with RFA or PEI with promising results, although the reported benefits to survival compared to either treatment alone has not been validated 17.
BCLC B
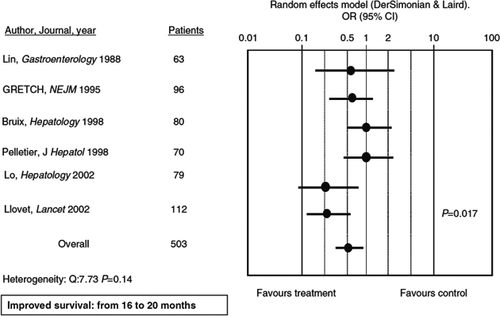
Transarterial chemoembolization is the standard of care (SOC) in these patients. This is based on a meta-analysis of seven studies 18 showing that repeated treatment with TACE was associated with improved survival of an average of 20 months in patients with intermediate HCC (Fig. 4). However, TACE is limited to patients with compensated cirrhosis (Child–Pugh A or B), without portal invasion and with small oesophageal varices to minimize the risk of hepatic decompensation following procedure-induced liver ischaemia. Tumour response after TACE should be evaluated by EASL or modified RECIST criteria combining assessment of tumour shrinkage and loss of viable cells 19, 20.
Transarterial chemoembolization with doxorubicin-loaded (DEB-TACE) beads is a new paradigm, since it can cause complete necrosis of <5 cm HCC nodules, suggesting that TACE may be a radical treatment modality in selected patients 21. While DEB-TACE is generally as well tolerated as conventional TACE, incremental survival of DEB-TACE vs. conventional TACE was not demonstrated.
The efficacy of adjuvant pharmacological therapies has not been confirmed in patients treated by RFA or in studies with targeted therapies (STORM) 22.
Radioembolization (TARE) is gaining popularity as an alternative treatment in patients with intermediate and advanced tumours. Based on the injection of microspheres loaded with Yttrium-90, a pure beta emitter with short tissue penetration, TARE can also be offered to patients with portal thrombosis. However, the real limitation is the presence of arteriovenous shunts in the lungs which increases the risk of pulmonary embolization and advanced liver disease. TARE has been used for bridge therapy in patients on the waiting list for liver transplantation and as down staging therapy to bridge local ablation, resection or liver transplantation.
BCLC C
The multikinase inhibitor, sorafenib, is the SOC for these patients 2, 23 (Table 2) and is restricted to patients with compensated liver disease (Child–Pugh A) with either a clinical or radiological response during the first 2 months of therapy. As a result, the recently updated RECIST radiological criteria have been replaced by the EASL criteria, which include early sorafenib-induced modifications in arterial vascularization of the tumour and better identification of patients who respond to sorafenib 2, 24. All of the trials with new agents inhibiting either the same pathways as sorafenib or different pathways such as mTOR and growth factors involved in liver cell carcinogenesis and angiogenesis, have failed.
| Study characteristics | SHARP study | Asia study |
|---|---|---|
| Median age | 65 years | 51 years |
| BCLC-B stage | 18% | 4% |
| Previous treatments | 67% | NA |
| HBV aetiology of cirrhosis | 19% | 71% |
| TTP | 5.5 months (2.8 months) | 2.8 months (1.4 months) |
| Median survival (control) | 10.7 months (7.9 months) | 6.5 months (4.2 months) |
| Grade 3/4 toxicity | 30% | 24% |
BCLC D
These patients have tumours of any size with symptoms of neoplasia and disturbed liver function (Child–Pugh C), with an average predicted survival of 3 months. There is no specific treatment available in these cases, except the best palliative care.
HCC treatment beyond the guidelines
The SOC Milan criteria for listing are felt to be too restrictive by many, since they prevent many patients from receiving LT, a life-saving treatment. However, approaches to expand criteria for LT listing are limited by the shortage of donated livers and lack of robust pretransplant imaging findings to identify predictors of tumour recurrence such as microscopic vascular invasion and tumour satellites that prevail among patients exceeding Milan criteria.
Based on explant histology, surgeons at the University of California (UCSF) proposed expanded criteria for listing that were prospectively validated by radiological imaging in clinical practice 25, 26. Listing for transplantation based on UCSF criteria, which include 1 nodule ≤6.5 cm, or 2–3 nodules ≤4.5 cm to reach a total tumour diameter ≤8 cm, resulted in a comparable 5-year recurrence-free probability (90% and 94%) in patients within and outside Milan criteria, while the risk of pretreatment tumour understaging was similar for patients within the Milan (20%) and UCSF (29%) criteria 26.
In a retrospective multicenter study of explanted livers that included 1156 BCLC- A patients who underwent OLT for HCC in 36 centres 27, patients outside the Milan criteria were analysed in two subgroups ‘Up-to-seven’ (7 cm being the sum of the size of the largest tumour and the number of tumours) and ‘Exceeding up-to seven’. The 5-year overall survival was 73.3% for patients who fulfilled the Milan criteria and 53.6% in those who were transplanted outside the criteria. Interestingly, the survival of 283 patients in the Up-to-seven group lacking microvascular invasion was as high as 71.2%, suggesting that more patients with HCC could be candidates for LT if the current dual approach (Milan in/Milan out) for candidacy was replaced with a more precise estimation of survival in relation to tumour characteristics and the up-to-seven criteria. However, the assessment of tumour burden for this expanded criterion was based on pathology at explant, not on pretreatment radiology, limiting its clinical application for listing. Other studies have addressed the survival benefits of extended criteria for listing, but without any external validation 28-30.
Survival benefits have also been reported in patients with intermediate and advanced cancer 31-33. In a multinational, retrospective study including nine tertiary referral centres for hepatic surgery, the 1-, 3- and 5-year overall survival rates were 87%, 66% and 55% for 360 BCLC B patients with cirrhosis and 71, 42 and 31% for 169 BCLC C patients who underwent hepatic resection, with corresponding 1-, 3- and 5-year disease-free survival rates of 66%, 42% and 26% for BCLC B and 42%, 28% and 17% for BCLC C 34. Another study in Taiwan supports these results showing significantly better survival in 268 patients with intermediate HCC who underwent surgery compared to 455 patients who were conventionally treated by TACE, with 5-year survival rates of 43% vs. 15% P < 0.001 35. However, it should be noted that enrolment of patients with advanced cirrhosis was significantly skewed towards the TACE group. In a single centre study in Italy, hepatic resection of 247 patients with cirrhosis and intermediate HCC resulted in satisfactory 3- and 5- year survival rates of 49% and 34%, respectively, which was considered to be comparable to TACE 36. The major caveat in hepatic resection in patients with cirrhosis, that is, how to avoid liver failure caused by the loss of viable liver cells, has mainly been evaluated in relation to the predictive power of portal hypertension, since patients with > 10 mm Hg HVPG have an increased risk of postoperative decompensation. In a recent randomized-controlled trial comparing hepatectomy vs TACE for resectable multiple HCCs exceeding the Milan criteria, 1-, 2- and 3- year overall survival was 76%, 63% and 51%, respectively, for the resected patients, compared to 52%, 35% and 18% for the TACE group (P < 0.001) 37.
The BCLC-A patients who are not suitable for hepatic resection can be considered for RFA. In the gray zone of tumours >3 cm with an increased risk of satellite nodules, techniques to enlarge the ablation field or combination therapy with other modalities such as TACE, should be considered. Survival benefits provided by combination therapy with RFA plus TACE compared to RFA alone in HCCs between 3 and 5 cm have been demonstrated in an RCT in Japan 38 and a consensus-based clinical practice manual of the Japanese Society of Hepatology recommends RFA combined with TACE for HCC > 3 cm 39. The recent development of the microwave technique provides larger areas of tumour necrosis which modifies these recommendations, however, this approach needs to be validated.
Chemoembolization of HCC has been used to treat patients with early HCC in whom surgical or ablative therapies are not applicable for technical reasons and/or with comorbidities. A phase III RCT (PRECISION V) failed to show that DEB-TACE was better than conventional TACE 40 while a recent international study 41 showed a 5-year overall survival of 22.5%, with better responses in Child–Pugh A than in Child–Pugh B patients (29.4% vs. 12.8%).
In two studies in Italy, up to 40% of patients with HCC were treated outside recommended guidelines 42, 43, because of tumour characteristics/location or comorbidities that made it difficult to classify patients in accordance with international recommendations. In our centre, where the 2005 AALSD recommendations have been the SOC for the treatment of HCC, 29% of 227 patients consecutively treated in the last 5 years did not receive a SOC treatment, including 21% of the BCLC A patients with the best prognosis, who ultimately did not receive radical treatment (Sangiovanni et al., unpublished data).
Conclusions
While the decision-making process for the treatment of HCC is guided by the individual evaluation of the patient, only half the patients referred to a tertiary centre will have an early tumour and fulfil the criteria for radical therapy with resection, local ablation or transplantation. One-third of the patients will have intermediate HCC, which can benefit from treatment with TACE in the presence of compensated cirrhosis (Child–Pugh status A or B) and an absence of portal vein complications such as thrombosis or large oesophageal varices. In the remaining patients with advanced HCC, sorafenib is a SOC option as long as cirrhosis is well compensated (Child–Pugh A) and a radiological or a clinical response can be achieved within the first 2 months of treatment. A significant number of patients do not fit treatment eligibility criteria drafted by the international societies, thus creating the need for therapy outside these guidelines. The use of therapeutic algorithms outside recommendations results in lower survival compared to a guideline-driven approach to the treatment of HCC.
Acknowledgment
Conflict of interest: Massimo Colombo: Advisory committees: Merck, Roche, Novartis, Bayer, BMS, Gilead Science, Tibotec, Vertex, Janssen Cilag, Achillion, Lundbeck, GSK, GenSpera, AbbVie, AlfaWasserman, Jennerex. Speaking and teaching: Tibotec, Roche, Novartis, Bayer, BMS, Gilead Science, Vertex, Merck, Janssen, Sanofi, AbbVie. Angelo Sangiovanni: nothing to disclose.