Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification
Online ISSN: 1439-0469
[Correction added on 27 October 2021, after first online publication: The Abstract and numbering of items in Section 3.3 have been corrected in this version.]
Abstract
We advance on the knowledge of Eigenmanniinae by proposing a hypothesis of phylogenetic relationships based on the parsimony analysis of a diverse set of 144 anatomical characters, 12% of them treated as quantitative and 88% treated as qualitative (8% external morphology, 51% osteology, 21% myology, and 8% neuroanatomy). Thirty-seven of 45 valid species of Eigenmanniinae are examined in the study, including the incertae sedis species “Eigenmannia” goajira. The final tree yields new insights on species relationships, thus, producing a new classification to Eigenmanninae. Our analysis recovered the monophyly of Eigenmanniinae, Archolaemus, Eigenmannia, and Rhabdolichops. Eigenmannia is proposed as monophyletic based on four morphological synapomorphies, one of which exclusive to the genus. Japigny is proposed to be the sister group of all remaining Eigenmanniinae and “E.” goajira to be the sister group of Archolaemus. The hypothesis of monophyly of Distocyclus including D. conirostris and D. guchereauae is rejected. Consequently, D. guchereauae is included in Eigenmannia, and a new genus is established to include “E.” goajira. A taxonomic key to all genera is provided. In addition, this study highlights the critical role played by a diverse set of anatomical and quantitative characters without discretization on phylogenetic reconstructions.
1 INTRODUCTION
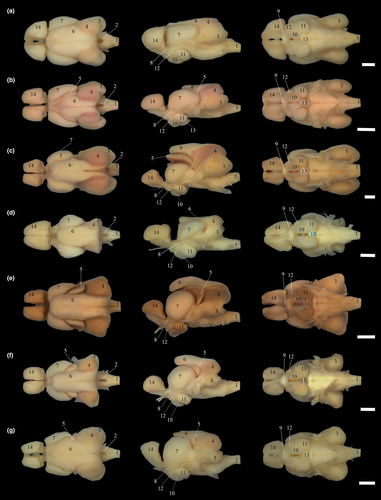
Sternopygidae comprises seven genera and 55 valid species of glass knifefishes included in Sternopyginae and Eigenmanniinae (Ferraris et al., 2017; Fricke et al., 2020). The family is characterized by fishes exhibiting multiple rows of teeth in both jaws, enlarged lateral line canals, and that produce monophasic weak electric organ discharges, ranging from 100 to 780 Hz (Crampton & Albert, 2006; Mago-Leccia, 1978). As also characteristic for other Gymnotiformes, glass knifefishes use their electrogenic-electrosensorial system to communicate and navigate in a variety of aquatic environments in the Neotropical region (Moller, 1995), including floodplains, rapids, river channels, and subterranean drainages (Crampton, 2007; Peixoto et al., 2015; Triques, 1996). Within Sternopygidae, the subfamily Sternopyginae (sensu Albert, 2001) was created to include Sternopygus Müller & Troschel, and †Humboldtichthys (Gayet & Meunier) [formerly proposed as Ellisella Gayet & Meunier, preoccupied by Ellisella Gray, 1858 (Cnidaria)]. In turn, Eigenmanniinae (sensu Meunier et al., 2011) was raised by Mago-Leccia (1978) to comprises five valid living genera (Archolaemus Korringa, Distocyclus Mago-Leccia, Eigenmannia Jordan & Evermann, Japigny Meunier, Jegú & Keith, and Rhabdolichops Eigenmann & Allen—Figure 1), with 45 species and one incertae sedis species—“Eigenmannia” goajira Schultz (Table 1; Ferraris et al., 2017).

| Current name | Valid name |
|---|---|
| Archolaemus blax | Archolaemus blax Korringa, 1970 |
| Archolaemus ferreirai | Archolaemus ferreirai Vari et al., 2012 |
| Archolaemus janeae | Archolaemus janeae Vari et al., 2012 |
| Archolaemus luciae | Archolaemus luciae Vari et al., 2012 |
| Archolaemus orientalis | Archolaemus orientalis Stewart et al., 2012 |
| Archolaemus santosi | Archolaemus santosi Vari et al., 2012 |
| Distocyclus conirostris | Distocyclus conirostris (Eigenmann & Allen, 1942) |
| Distocyclus guchereauae | Eigenmannia guchereauae (Meunier et al., 2014) |
| Eigenmannia antonioi | Eigenmannia antonioi Peixoto et al., 2015 |
| Eigenmannia besouro | Eigenmannia besouro Peixoto & Wosiacki, 2016 |
| Eigenmannia camposi* | Eigenmannia camposi Herrera-Collazos et al., 2020 |
| Eigenmannia desantanai | Eigenmannia desantanai Peixoto et al., 2015 |
| Eigenmannia dutrai* | Eigenmannia dutrai Peixoto et al., 2021 |
| Eigenmannia correntes | Eigenmannia correntes Campos-da-Paz & Queiroz, 2017 |
| Eigenmannia guairaca | Eigenmannia guairaca Peixoto et al., 2015 |
| Eigenmannia humboldtii | Eigenmannia humboldtii (Steindachner, 1878) |
| Eigenmannia limbata | Eigenmannia limbata (Schreiner, Miranda Ribeiro, 1903) |
| Eigenmannia loretana* | Eigenmannia loretana Waltz & Albert, 2018 |
| Eigenmannia macrops | Eigenmannia macrops (Boulenger, 1897) |
| Eigenmannia magoi* | Eigenmannia magoi Herrera-Collazos et al., 2020 |
| Eigenmannia matintapereira | Eigenmannia matintapereira Peixoto et al., 2015 |
| Eigenmannia meeki | Eigenmannia meeki Dutra et al., 2017 |
| Eigenmannia microstoma | Eigenmannia microstoma (Reinhardt, 1852) |
| Eigenmannia muirapinima | Eigenmannia muirapinima Peixoto et al., 2015 |
| Eigenmannia nigra | Eigenmannia nigra Mago-Leccia, 1994 |
| Eigenmannia pavulagem | Eigenmannia pavulagem Peixoto et al., 2015 |
| Eigenmannia sayona | Eigenmannia sayona Peixoto & Waltz, 2017 |
| Eigenmannia sirius* | Eigenmannia sirius Peixoto & Ohara, 2019 |
| Eigenmannia trilineata | Eigenmannia trilineata López & Castello, 1966 |
| Eigenmannia oradens | Eigenmannia oradens Dutra et al., 2018 |
| Eigenmannia virescens | Eigenmannia virescens (Valenciennes, 1836) |
| Eigenmannia vicentespelaea | Eigenmannia vicentespelaea Triques, 1996 |
| Eigenmannia waiwai | Eigenmannia waiwai Peixoto et al., 2015 |
| Eigenmannia zenuensis* | Eigenmannia zenuensis Herrera-Collazos et al., 2020 |
| “Eigenmannia” goajira | Rhinosternarchus goajira (Schultz, 1949) |
| Japigny kirschbaum | Japigny kirschbaum Meunier et al., 2011 |
| Rhabdolichops caviceps | Rhabdolichops caviceps (Fernández-Yépez, 1968) |
| Rhabdolichops eastwardi | Rhabdolichops eastwardi Lundberg & Mago-Leccia, 1986 |
| Rhabdolichops electrogrammus | Rhabdolichops electrogrammus Lundberg & Mago-Leccia, 1986 |
| Rhabdolichops jegui* | Rhabdolichops jegui Keith & Meunier, 2000 |
| Rhabdolichops lundbergi | Rhabdolichops lundbergi Correa et al., 2006 |
| Rhabdolichops navalha* | Rhabdolichops navalha Correa et al., 2006 |
| Rhabdolichops nigrimans | Rhabdolichops nigrimans Correa et al., 2006 |
| Rhabdolichops stewarti* | Rhabdolichops stewarti Lundberg & Mago-Leccia, 1986 |
| Rhabdolichops troscheli | Rhabdolichops troscheli (Kaup, 1856) |
| Rhabdolichops zareti | Rhabdolichops zareti Lundberg & Mago-Leccia, 1986 |
- Names in bold indicate generic changes. An asterisk indicates species absent in the present study.
Eigenmanniinae has been historically characterized by the presence of a scapular foramen entirely included within the scapula, the fusion of the post-temporal and the supracleithrum into a single ossification, and the presence of 11 to 15 precaudal vertebrae (e.g., Lundberg & Mago-Leccia, 1986; Mago-Leccia, 1978; Vari et al., 2012—Table S1). Recently, Tagliacollo et al. (2016b) proposed additional synapomorphies for Eigenmanniinae (Table S1).
Mago-Leccia (1978) proposed the first phylogenetic hypothesis on interrelationships within Eigenmanniinae (Figure 2). Therein, Rhabdolichops is the sister group of all remaining genera based on the retention of putative plesiomorphic conditions, such as the presence of ossified first basibranchial, larger gill rakers on first gill arch, and of posttemporal fossa. Mago-Leccia’s hypothesis supported a clade compounded by Archolaemus, Distocyclus, and Eigenmannia characterized by sharing cartilaginous gill rakers, mouth and gill opening reduced, and a variable position of the anus. Fink and Fink (1981) discussing the interrelationships within Sternopygidae based on Korringa (1970) and Mago-Leccia (1978), offered an alternative hypothesis, considering Archolaemus as the sister group of the remaining Eigenmanniinae, and Rhabdolichops as the sister group of Eigenmannia + Distocyclus. The monophyly of Eigenmannia, Distocyclus, and Rhabdolichops would be supported by the presence of a reduced number of pleural ribs, length of the anterior two to three pleural ribs subequal to the depth of the abdominal cavity, the fusion of the two posterior pectoral proximal radials, and a subcutaneous eye (Fink & Fink, 1981; Lundberg & Mago-Leccia, 1986). Conversely, Triques (1993) rejected the monophyly of Eigenmanniinae based on Rhabdolichops’s position as the sister group of all Sternopygids (including Sternopygus), sustained by the forward displacement of maxilla, and the elongation of palatine and maxillary cartilages. Nevertheless, Triques (1993) emphasized that his hypothesis was incongruent with that of Lundberg and Mago-Leccia (1986), and it would require further analysis.
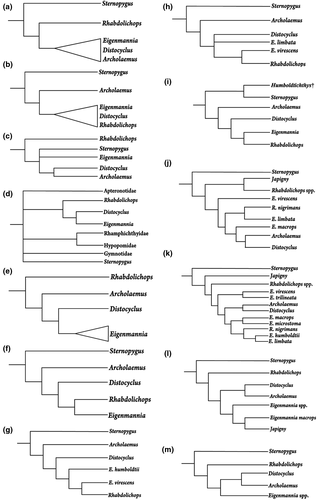
Alves-Gomes et al. (1995) provided the first Gymnotiformes’ hypothesis based on molecular evidence (mitochondrial DNA—mtDNA). The monophyly of Sternopygidae was not recovered with Sternopygus in an uncertain position within Gymnotiformes, and Eigenmanniinae (therein, Eigenmanniidae) as sister group of Apteronotidae. Differently, the relationships of Distocyclus, Eigenmannia, and Rhabdolichops were corroborated as in Fink and Fink (1981), where Rhabdolichops was considered the sister group of Distocyclus plus Eigenmannia. It is noteworthy that Alves-Gomes et al. (1995) did not include Archolaemus in their analyses. A year later, Albert and Fink (1996) also corroborated the hypothesis of Fink and Fink (1981) but Eigenmannia emerged as an unnatural group. Alves-Gomes (1998) used mtDNA to investigate the position of Archolaemus and his results corroborated those of Mago-Leccia (1978), in which Archolaemus emerged as the sister group of Distocyclus plus Eigenmannia, and Rhabdolichops as the sister group of that clade. Like in Alves-Gomes et al. (1995), Alves-Gomes (1998) did not recover the monophyly of Sternopygidae.
Albert and Campos-da-Paz (1998) proposed Rhabdolichops as the sister group of Eigenmannia based on the presence of a short snout, curved frontally in lateral view, the anterior displaced hemal spine as large and straight as the remaining hemal spines, and the lengthy pterygiophores of the anal fin. Eigenmannia virecens, was the sole species of the genus included in the analysis, and none autapomorphy was found to define the genus. A few years later, Albert (2001) added supplementary taxa and characters in the matrix of Albert and Campos-da-Paz (1998) and the monophyly of the clade Eigenmannia plus Rhabdolichops was corroborated by the presence of two additional synapomorphies: fifth epibranchial with a short ascending process, and developmental origin of an adult electric organ from portions of both the hypaxial and anal-fin pterygiophore muscles. The monophyly of Eigenmannia was tested by the inclusion of E. humboldtii in the analysis of Albert (2001) but rejected because of the close relationship between the E. virescens clade and Rhabdolichops, rather than with other members of Eigenmannia. An elongated body, presence of 160 to 199 anal-fin rays, and anal-fin pterygiophores longer than hemal spines supported this relationship. Hulen et al. (2005) during the investigation of the species-level relationships in Sternopygus, corroborated Albert’s (2001) hypothesis of relationships of Eigenmanniinae. Later, Albert and Fink (2007) working on the phylogenetic position of Humboldtichthys also supported Albert (2001) hypothesis.
In the year following the description of Japigny (Meunier et al., 2011), Vari et al. (2012) discussed its phylogenetic position and suggested it to be the sister group of all Eigenmanniinae primarily based on the absence of three synapomorphies that are present in all other genera of the subfamily: the angulo-articular with a distinct socket to receive the condyle of the quadrate; the parapophysis of the second vertebra straight and contacting the parapophysis of the fourth vertebra; and the parapophysis of the fourth vertebra curved ventrally. Tagliacollo et al. (2016a) rejected such hypothesis and proposed Japigny as a member of Eigenmannia despite the lack of genetic information of this taxon. Also, they recovered Archolaemus as the sister group of Distocyclus, as proposed by Triques (1993), and Rhabdolichops as the sister group of all Eigenmanniinae, as proposed by Mago-Leccia (1978) and Alves-Gomes (1995). The monophyly of Eigenmannia was recovered by the first time by Alda et al. (2019) based on the analyses of ultra-conserved elements; however, the authors did not make comments on these results.
Contrasting to its taxa delimitations and relationships (Figure 2), the monophyly of Eigenmanniinae has proven to be well supported by both morphological and molecular datasets (Albert, 2001; Albert & Campos-da-Paz, 1998; Albert & Fink, 1996; Alda et al., 2019; Alves-Gomes, 1998; Alves-Gomes et al., 1995; Fink & Fink, 1981; Hulen et al., 2005; Lundberg & Mago-Leccia, 1986; Mago-Leccia, 1978; Tagliacollo et al., 2016a; Triques, 1993). Thus, by examining most of its species diversity and using characters of external anatomy, osteology, myology, and neuroanatomy we tested the phylogenetic relationships in Eigenmanniinae and redifined its classification.
2 MATERIAL AND METHODS
2.1 Institution abbreviations
AMNH—American Museum of Natural History, New York, USA; ANSP—The Academy of Natural Science of Drexel University, Philadelphia, USA; CAS—California Academy of Science, San Francisco, USA; FMNH—Field Museum of Natural History, Chicago; IAvH—Instituto Alexander von Humboldt, Bogotá, Colombia; INPA—Instituto Nacional de Pesquisas da Amazônia, Manaus, Brazil; MCP—Museu de Ciência e Tecnologia da Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil; MCZ—Museum of Comparative Zoology, Cambridge, USA; MNHN—Museum National d'Histoire Naturelle, Paris, France; MNRJ—Museu Nacional da Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; MPEG—Museu Paraense Emílio Goeldi, Belém, Brazil; MZUSP—Museu de Zoologia da Universidade de São Paulo, São Paulo, Brazil; NUP—Núcleo de Pesquisa em Limnologia, Ictiologia e Aquicultura, Maringá, Brazil; UFRGS—Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; and USNM—National Museum of Natural History, Smithsonian Institution, Washington DC, USA.
2.2 Taxonomic sampling and outgroup comparisons
In total, 731 specimens were examined, 509 fixed in alcohol, 152 cleared and counterstained, 56 prepared for musculature and neuroanatomy studies, 10 scanned via computerized tomography, and four radiographed. We examined morphological characters of 37 ingroup species (out of 46) representing 80% of the valid species of Eigenmanniinae (Table 1), plus eight outgroups Sternopygus astrabes Mago-Leccia, 1994 and S. macrurus (Bloch & Schneider, 1801) (Sternopygidae); Apteronotus albifrons (Linnaeus, 1766) and Sternarchorhamphus muelleri (Steindachner 1881) (Apteronotidae); Gymnotus aff. carapo Linnaeus, 1758 and Gymnotus coropinae Hoedeman, 1952 (Gymnotidae); Brachyhypopomus brevirostris (Steindachner, 1868) and Microsternarchus bilineatus Fernández-Yépez, 1968 (Hypopomidae).
2.3 Anatomical preparations
Specimens were cleared and counterstained for cartilage and bone using the method described by Taylor and Van Dyke (1985), and dissected according to Weitzman (1974) during osteological character analysis. For musculature and neuroanatomy analysis, specimens were cleared and counterstained following Taylor and Van Dyke (1985) and Springer and Johnson (2000), with modifications described in Datovo and Bockmann (2010). In addition, data were also complemented by available information from the literature (Lundberg & Mago-Leccia, 1986; Mago-Leccia, 1978). Details of the osteology of “Eigenmannia” goajira were examined only via 2D radiographs with Kevex, PXS10-16W 130kVp 6 Micron Spot MicroFocus X-Ray Source with end window, Varian PaxScan 4030R Std. GadOx DRZ-Plus Screen, and VIVA k.03 Software. Furthermore, specimens of “E.” goajira” and Distocyclus guchereauae were unavailable for musculature and neuroanatomy preparation.
2.4 Anatomical terminology
Osteological terminology follows Weitzman (1962) with summarized modifications by Vari (1995), applied in studies of Gymnotiformes (e.g., de Santana & Vari, 2010): posterior ceratohyal instead of epihyal and anterior ceratohyal instead of ceratohyal posterior, as proposed by Nelson (1969). We used mesethmoid rather than ethmoid as proposed by Fink and Fink (1981, 1996); infraorbitals 1 + 2 following Mago-Leccia (1978) rather than lachrymal which was utilized by de la Hoz and Chardon (1984); and endopterygoid rather than mesopterygoid as proposed by Fink and Fink (1996) and later on applied in Gymnotiformes (e.g., Albert, 2001; de Santana & Vari, 2010). Terminology on cephalic lateral line system follows Pastana et al. (2019), which includes all cranial bones associated with canal tubules and pores of this system. Sternopygidae presents enlarged infraorbital canals which homology is not clear. Considering that infraorbitals are dermal ossification, and ossification in canal segments is autogenous (Pastana et al., 2019), elements in Sternopygidae could be a fusion of infraorbitals and its associated canal segments, or mainly compounded by canal segments and infraorbitals would be completely or partially absent. The resolution of such homologies is out of the aims of the present paper; thus, we keep the name infraorbitals for those elements, which is traditionally used in anatomical nomenclature for Gymnotiformes (e.g., Albert, 2001; Peixoto et al., 2015). Homology of postcleithral elements follows de Santana and Vari (2010). Myological nomenclature follows Winterbottom (1974), except for the adductor mandibulae, which was based on Datovo and Vari (2013, 2014). Terms “origin” and “insertion” are used to reflect the stationary attachment site of a muscular component and the point of attachment that moves from muscle contraction, respectively (Winterbottom, 1974). Neuroanatomical nomenclature follows Meek and Nieuwenhuys (1998) and Maler et al. (1991). Nomenclature of the nerve R-Avn (“recurrent ramus of anteroventral part of anterior lateral line nerve”) follows Carr et al. (1982) and Vischer et al. (1989). Ramus mandibularis trigeminus (RMT) follows Freihofer (1978). The homology and nomenclature of dark stripes along the body of species follows Peixoto et al. (2015) and Peixoto and Wosiacki (2016).
2.5 Image preparation
Specimens were scanned on a 300 kV μ-focus X-ray source microcomputed tomography Phoenix v|tome|x m microfocus (General Electric Company). To improve image resolution a multiscan of the whole specimens was produced based on three individual scans. Reconstruction of raw data was performed using the system-supplied software phoenix datos|x reconstruction v. 2.3.0 (General Electric Measurement and Control Solutions). Three-dimensional visualization as well as the analysis of the reconstructed data was performed using VGStudio MAX 2.2.3 64 bit (Volume Graphics GmbH).
Images of osteological, myology, and neuroanatomical preparations were obtained with stereomicroscope Zeiss Discovery.V20 coupled with digital camera Axiocam 506 color. Multifocal images were obtained separately and combined with Combine ZP (Hadley, 2009). Illustrations for neuroanatomical characters were compiled in only two plates in order to optimize the comparative approach and understanding of brain complex morphology.
2.6 Counts and measurements
All counts previously used as diagnostic features within Eigenmanniinae species were included as quantitative characters (e.g., Peixoto et al., 2015; Vari et al., 2012). The scale counts follow Peixoto et al. (2015). The number of precaudal vertebrae includes the four vertebrae of the Weberian apparatus, in addition to all the vertebrae without fully developed hemal spines. Transitional vertebrae are recognized by the absence of directly associated pleural ribs, not bearing fully developed hemal spines (Campos-da-Paz & Queiroz, 2017). The gape of the mouth was previously delimited considering the position of the posterior terminus of gape and the nasal capsule (Albert, 2001; Albert & Campos-da-Paz, 1998), or eye diameter (Albert & Fink, 1996). Because the position of the posterior naris varies along the anterior portion of neurocranium, and the eye diameter varies within Gymnotiformes, especially in the cave knifefish Eigenmannia vicentespelaea, the extension of the gape was compared to the width of the mouth. The length of infraorbital 1 + 2 was measured from its anterior portion to the posterior limit as proposed by Peixoto et al. (2015).
2.7 Phylogenetic methods
Character data for phylogenetic analysis were obtained through exhaustive comparative analysis of the external morphology, osteology, dorsolateral head muscles, and neuroanatomy of all taxa analyzed herein (except for Archolaemus orientalis and Rhabdolichops zareti, not analyzed for neuroanatomy; and Distocyclus guchereauae and “Eigenmannia” goajira, not analyzed for both dorsolateral head muscles and neuroanatomy). Autapomorphic characters historically proposed to define monotypic genera (e.g., Distocyclus, Japigny) were included in the analysis in order to provide a complete definition of these genera. A matrix with 45 taxa (eight outgroups and 37 ingroup) and 144 characters (see Examined Material, Table 1, Tables S2–S6) was assembled in Notepad++ 7.5.1 (Ho, 2019). Gymnotus aff. carapo was chosen as the rooting point, since it is considered a generalized species of Gymnotidae, which is considered sister group of all the remaining Gymnotiformes according to phylogenies from different data sources (e.g., Albert, 2001; Tagliacollo et al., 2016a).
The matrix was submitted to a maximum parsimony analysis under TNT 1.5 (“Tree Analysis using New Technology”—Goloboff & Catalano, 2016). “Traditional search” (= heuristic search; RAS + TBR, “random addition sequences” and “tree-bisection reconnection”). It ran with 10,000 replicates and saving 100 trees per replication, aiming the best score hitting 20 times (Goloboff et al., 2008).
When two or more MPTs are used to produce strict consensus, the consensus is longer (= more steps) than fundamental trees; thus, character optimizations are inappropriate (Goloboff et al., 2008). Therefore, only common changes to all MPTs were used to clades diagnosis using TNT commands (apo[;). Consistency (CI) and Retention (RI) indices (Farris, 1969, 1989) of MPTs and all characters were calculated using TNT scripts (“wstats.run”; “stats.run”). Characters with different fits between the MPTs are expressed as minimum and maximum values for each index separated by a slash. Relative Bremer support (rbs; Goloboff & Farris, 2001) was calculated using TBR algorithm in TNT (5000 replicates) from suboptimal trees with up to 10 steps longer.
Quantitative characters (e.g., number of pectoral-fin rays, number of anal-fin rays, and number of pleural ribs) were analyzed as continuous, without discretization (Catalano et al., 2010; Goloboff & Catalano, 2016; Goloboff et al., 2006). In characters descriptions, continuous character states are exposed as ranges of minimum and maximum observed values for each taxa. These values were normalized in a range that corresponds from 0 (minimum value) to 1 (maximum value; for observed values and their normalizations see Table S2–S4) for analyses, following the methodology synthesized and justified in Ferrer et al. (2014). The range of the observed values for all taxa were acquired directly from specimens analyzed herein, and compiled from the literature in order to apply the largest range known for each count [Archolaemus species: Vari et al. (2012); Distocyclus species: Dutra et al., (2014), Meunier et al., (2014); Eigenmannia species: Peixoto et al. (2015), Peixoto and Wosiacki (2016), Peixoto and Waltz (2017), Campos-da-Paz and Queiroz (2017), Dutra et al. (2017), Dutra et al. (2018); Japigny: Meunier et al. (2011); Rhabdolichops species: Lundberg and Mago-Leccia (1986), Correa et al., (2006); Brachyhypopomus brevirostris: Crampton et al. (2016); Gymnotus coropinae: Crampton and Albert (2003); Sternarchorhamphus muelleri: Campos-da-Paz (1995)].
Missing data are exposed in the matrix as “?”, inapplicable as “-”, and polymorphic characters as “0&1”. All multistate characters were treated as non-additive.
3 RESULTS
3.1 Character description
The characters described and discussed below are arranged by morphological complexes following whenever possible overall an anterior to posterior pattern. Each character entry includes the name of the character, description of character states and, the consistency and retention indices on the final phylogenetic hypothesis. In the Neuroanatomy section, numbers after each structure name correspond to those indicated in Figure 45. Unutilized characters that present great variations were not included in the analysis. Explanations for inapplicability of each character are discussed in Data S1.
3.1.1 Quantitative characters
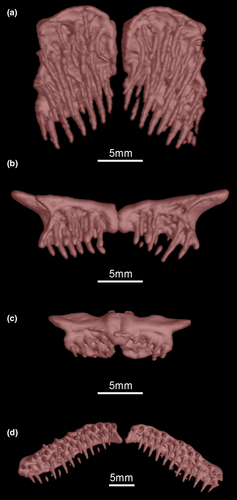
- 1. Number of premaxillary teeth: (0) minimum eight; (1) maximum 75 (CI = 0.201, RI = 0.372).
- 2. Number of teeth rows on premaxilla: (0) minimum one; (1) maximum nine (CI = 0.286, RI = 0.535).
- 3. Number of dentary teeth: (0) minimum one; (1) maximum 88 (CI = 0.247, RI = 0.505).
- 6. Number of gill rakers on first branchial arch: (0) minimum seven; (1) maximum 35 (CI = 0.469, RI = 0.446).
- 7. Number of teeth on upper pharyngeal plate: (0) minimum five; (1) maximum 38 (CI = 0.250, RI = 0.484).
- 8. Number of teeth on lower pharyngeal plate: (0) minimum six; (1) maximum 24 (CI = 0.197, RI = 0.361).
- 9. Number of pectoral-fin rays: (0) minimum 11; (1) maximum 24 (Hulen et al., 2005, char. 56; CI = 0.290, RI = 0.600).
- 11. Number of precaudal vertebrae: (0) minimum 11; (1) maximum 44 (Albert, 2001, char. 205–206; Albert & Campos-da-Paz, 1998, char. 206–207; Albert & Fink, 1996, char. 40–41; Correa et al., 2006, char. 34; Hulen et al., 2005, char. 62; Lundberg & Mago-Leccia, 1986, char. 5 in part; Mago-Leccia, 1978; CI = 0.624, RI = 0.739).
- 13. Number of pleural ribs: (0) minimum three; (1) maximum 37 (Albert, 2001, char. 209; Albert & Campos-da-Paz, 1998, char. 210; Albert & Fink, 1996, char. 42; Correa et al., 2006, char. 35; Hulen et al., 2005, char. 60; Lundberg & Mago-Leccia, 1986, char. 5 in part and 13; CI = 0.499, RI = 0.613).
- 14. Number of displaced hemal spines: (0) minimum one; (1) maximum five (Albert, 2001, char. 191; Albert & Campos-da-Paz, 1998, char. 192; Correa et al., 2006, char. 40; Lundberg & Mago-Leccia, 1986, char. 21; CI = 0.250, RI = 0.250).
- 15. Number of lateral line scales: (0) minimum 77; (1) maximum 225 (CI = 0.450, RI = 0.602).
- 16. Number of scale rows above lateral line: (0) minimum four; (1) maximum 25 (CI = 0.324, RI = 0.521).
The deepness of the electric organ can be expressed by the number of electrocytes organized over the end of anal-fin base (Lundberg & Mago-Leccia, 1986: 67). When present, rows of visible electrocytes over the end of anal-fin base range from one to nine in Rhabdolichops. The codification in all the other analyzed species is uncertain because electrocytes are not externally visible.
3.1.2 Mouth
- 18. Position of mouth: (0) superior (Figure 3a); (1) terminal (Figure 3b); (2) subterminal (Figure 3c; Albert, 2001, char. 21; Albert & Campos-da-Paz, 1998, char. 21–22; Correa et al., 2006, char. 7; Lundberg & Mago-Leccia, 1986, char. 26 and 28; Tagliacollo et al., 2016b; char. 8; CI = 0.222; RI = 0.611).
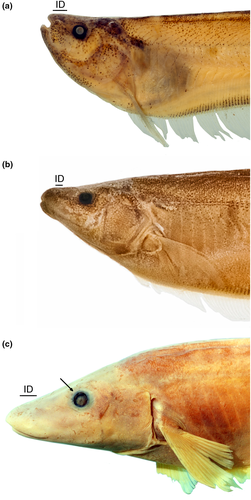
- 19. Extent of mouth gape: (0) shorter than its width (Figure 3a–b); (1) longer than its width (Figure 3c; Albert, 2001, char. 19; Albert & Campos-da-Paz, 1998, char. 19–20; Albert & Fink, 1996, char. 3; Hulen et al., 2005, char. 14; Tagliacollo et al., 2016b, char. 6–7; Vari et al., 2012; CI = 0.500; RI = 0.800).
- 20. Upper lip: (0) without teeth; (1) with teeth (CI = 1.000; RI = AUT).
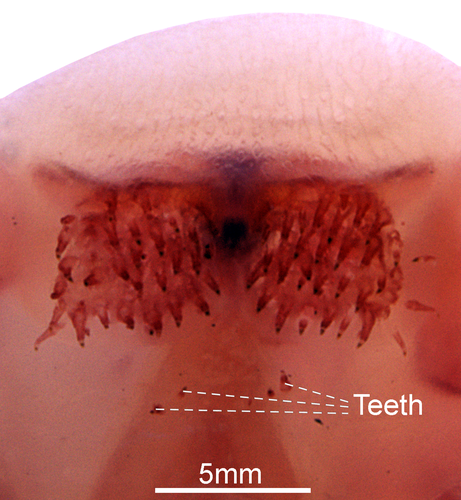
A toothed oral valve presents teeth on the soft tissue located at the anterior portion of the vomer.
3.1.3 Nares
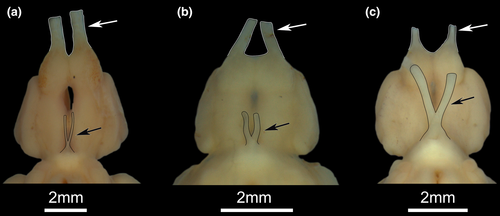
Short internarial distance is a reflect of the anterior position of posterior nostril, and the horizontally oriented nasal bone that shifts anterior nostril to a more posterior position (Dutra et al., 2014), a condition present only in D. conirostris (state 1—Figure 3d).
3.1.4 Orbital region
- 23. Orbital rim: (0) eye subcutaneous, attached to orbital rim; (1) orbital rim free (Figure 3c; Albert, 2001, char. 104; Albert & Campos-da-Paz, 1998, char. 105; Albert & Fink, 1996, char. 14; Correa et al., 2006, char. 6; Fink & Fink, 1981; Hulen et al., 2005, char. 16; Korringa, 1970; Lundberg & Mago-Leccia, 1986, char. 16; Mago-Leccia & Zaret, 1978; Vari et al., 2012; CI = 0.500; RI = 0.857).
3.1.5 Upper jaw
- 24. Relative length of premaxilla: (0) twice or less as long as its width (Figure 5a–c); (1) four times or more as long as its width (Figure 5d; Albert, 2001, char. 25 in part; Albert & Campos-da-Paz, 1998, char. 26; Lundberg & Mago-Leccia, 1986, char. 20 in part; Mago-Leccia, 1978; Tagliacollo et al., 2016b, char. 39; CI = 1.000; RI = 1.000).
- 26. Anterolateral process on premaxilla: (0) absent (Figure 5a,d); (1) present (Figure 5b–c; CI = 0.250; RI = 0.883).
- 27. Teeth on both jaws in adults: (0) present; (1) absent. (Albert, 2001, char. 22; Albert & Campos-da-Paz, 1998, char. 24; Tagliacollo et al., 2016b, char. 36; Triques, 1993, char. 23 and 24; CI = 1.000, RI = 1.000).
- 28. Shape of teeth: (0) conical; (1) villiform (Albert, 2001, char. 24; Albert & Fink, 1996, char. 9; Correa et al., 2006: char. 9; Hulen et al., 2005, char. 34; Lundberg & Mago-Leccia, 1986, char. 2; Mago-Leccia, 1978; Mago-Leccia & Zaret, 1978; Tagliacollo et al., 2016b, char. 37; CI = 0.500, RI = 0.500).
- 29. Attachment area of anterior row of premaxillary teeth: (0) teeth firmly, but not fully ankilosed, attached to ventral surface of premaxilla; (1) teeth attached to ventral surface of premaxilla only by their anterobasal margin (Dutra et al., 2018; Fink, 1981; Vari et al., 2012; CI = 0.500; RI = 0.857).
- 30. Relative width of descending blade of maxilla: (0) narrower than its dorsal most portion (Figure 6b–d); (1) as wide as its anterior most portion (Figure 6a; Albert, 2001, char. 30 in part; Albert & Campos-da-Paz, 1998, char. 37; Correa et al., 2006, char. 11; Hulen et al., 2005, char. 36; Lundberg & Mago-Leccia, 1986, char. 20 in part; CI = 1.000; RI = 1.000).
Rhabdolichops caviceps, R. eastwardi, R. electrogrammus, R. troscheli, R. zareti, and Sternopygus lack the anterior hook-like process on maxilla. Such process is present in small (less than 75 mm) Rhabdolichops species and reduces with growth (Lundberg & Mago-Leccia, 1986). Considering this ontogenetic variation, this character was codified only in adult specimens.
3.1.6 Lower jaw
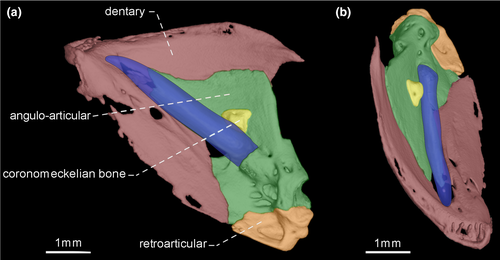
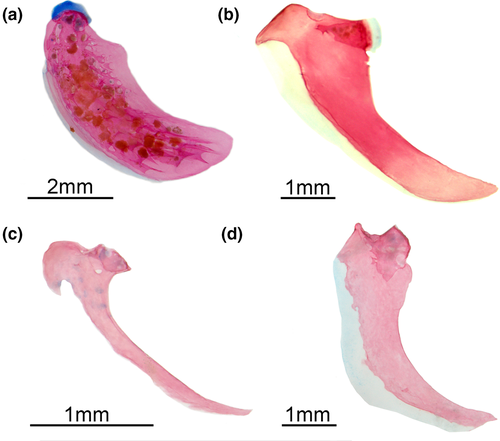
- 33. Size of teeth along dentigerous surface of dentary: (0) similar in size along dentigerous surface (Figures 7-10); (1) increasing in size along dentigerous surface (Figure 11; Peixoto et al., 2015; de Santana & Vari, 2013; CI = 0.500; RI = 0.750).
- 34. Shape of dentary teeth: (0) medially curved (Figures 8-11); (1) straight (Figure 7; CI = 0.143; RI = 0.400).
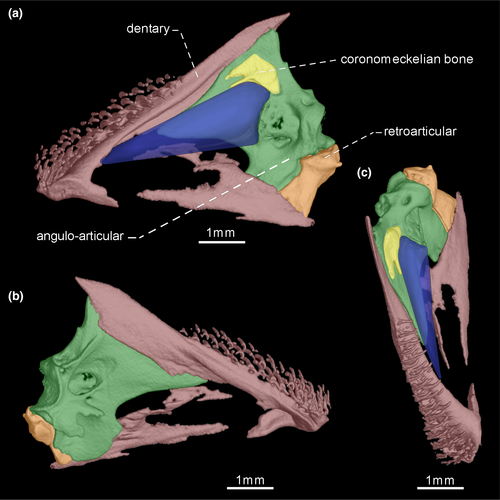
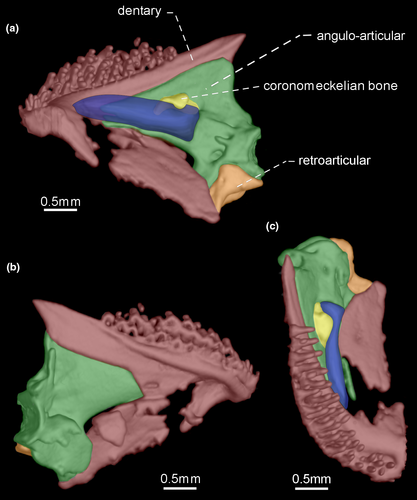
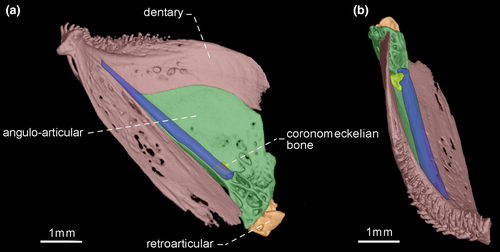
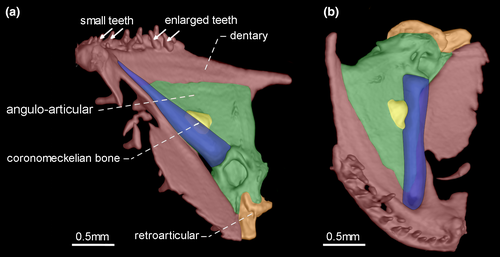
- 36. Orientation of coronoid process: (0) ventrally curved in its posterior end (Figures 7 and 10); (1) nearly straight (Figures 8, 9 and 11; CI = 0.167; IR = 0.545).
- 37. Composition of coronoid process tip: (0) ossified; (1) cartilaginous (Vari et al., 2012; CI = 1.000; RI = 1.000).
- 38. Form of Meckel's cartilage: (0) rectangular shaped, its anterior portion approximately as wide as posterior portion (Figures 7, 9-11); (1) triangular-shaped, its anterior portion approximately half as wide as posterior portion (Figure 8; CI = 1.000; RI = 1.000).
- 39. Relative length of coronomeckelian bone: (0) coronomeckelian bone corresponds to 45% or more of length of Meckel's cartilage (Figures 8, 9); (1) coronomeckelian bone corresponds to 30% or less of length of Meckel's cartilage (Figures 7, 10 and 11; Peixoto et al., 2015; Vari et al., 2012; CI = 0.200; RI = 0.692).
Snout elongation in Gymnotiformes is not homologous within the order, since different bones are involved in the elongation process (e.g., de Santana & Vari, 2010: 267–670). One of the bones may be involved to the snout elongation is the dentary. The relative length of dentary is a ratio between the dentary length and dentary depth, in which dentary length corresponds to the distance between symphysis and coronoid process, whereas the lower jaw depth corresponds to distance between dentary rim and ventral margin of retroarticular.
3.1.7 Suspensorium
- 43. Ventral surface of endopterygoid: (0) edentulous (Figures 12, 13); (1) toothed (Figure 14; Albert, 2001, char. 133; Albert & Campos-da-Paz, 1998, char. 134 in part; Albert & Fink, 1996, char 22; Correa et al., 2006, char. 16; Dutra et al., 2014; Hulen et al., 2005, char. 42; Lundberg & Mago-Leccia, 1986, char. 25; Mago-Leccia & Zaret, 1978; Tagliacollo et al., 2016b, char. 125; CI = 0.250–0.333; RI = 0.700–0.800).
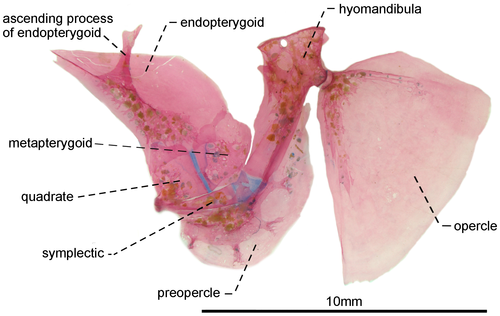
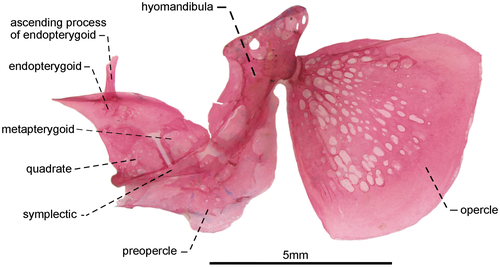
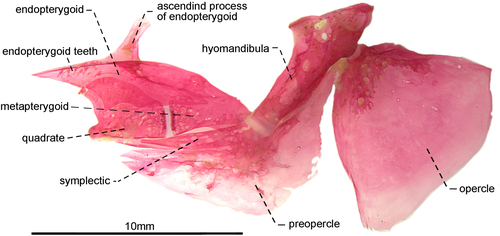
- 46. Relative length of symplectic: (0) one to two thirds as long as hyomandibula (Figures 12, 13); (1) as long as hyomandibula (Figure 14; Albert, 2001, char. 137; Albert & Campos-da-Paz, 1998, char. 138; Tagliacollo et al., 2016b, char. 129; CI = 0.500; RI = 0.667).
- 47. Orientation of hyomandibula: (0) main axis of hyomandibula forming an angle of nearly 40° in relation to its dorsal margin (Figure 12): (1) main axis of hyomandibula forming an angle of nearly 60° in relation to its dorsal margin (Figures 13, 14; Albert, 2001, char. 138; Tagliacollo et al., 2016b, char. 130; CI = 0.200; RI = 0.556).
- 48. Shape of posterodorsal portion of hyomandibula: (0) extending posteriorly to condyle that receives opercular socket (Figures 13, 14); (1) ending at same level of condyle that receives opercular socket (Figure 12; CI = 0.500; RI = 0.857).
- 49. Foramen on posterodorsal margin of hyomandibula: (0) present (Figure 15a); (1) absent (Figure 15b; CI = 0.333; RI = 0.714).
The recurrent ramus of the anteroventral part of the anterior lateral line nerve (R-Avn) extends through the postotic foramen of the neurocranium. In most of analyzed Gymnotiformes, this ramus passes through the foramen in the posterodorsal margin of the hyomandibula, before extending to the body. In contrast, the posterodorsal foramen of hyomandibula is absent in R. caviceps, R. electrogrammus, R. eastwardi, R. troscheli, R. zareti, Sternarchorhamphus, and Sternopygus, and the R-Avn passes next to the hyomandibula.
3.1.8 Cephalic lateral line system
- 52. Shape of nasal: (0) slender and tubular (see Peixoto et al., 2019: fig. 4b); (1) enlarged and half-pipe shaped (Figures 17-20; Albert, 2001, char. 67; Albert & Campos-da-Paz, 1998, char. 68; Triques, 1993, char. 22; CI = 1.000; RI = 1.000).
- 53. Shape of supraorbital canal: (0) slender and tubular; (1) enlarged and half-pipe shaped (Figures 17-20; Triques, 1993, char. 5; CI = 1.000; RI = 1.000).
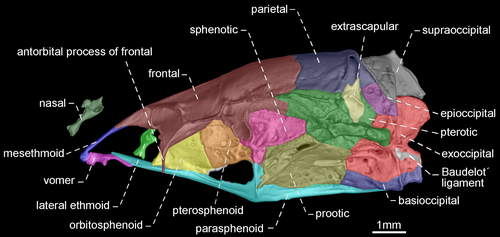
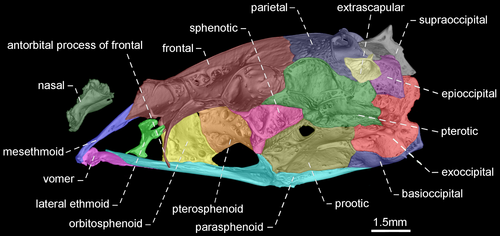
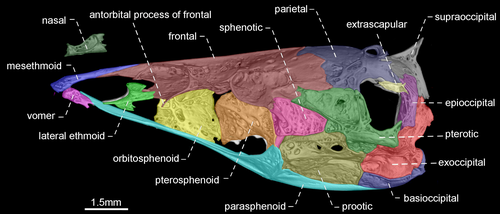
- 54. Shape of antorbital and infraorbitals 1–4: (0) slender and tubular; (1) enlarged and half-pipe shaped (Figure 21—Albert, 2001: char. 87; Albert & Campos-da-Paz, 1998, char. 88; Albert & Fink, 1996, char. 17; Correa et al., 2006, char. 12; Lundberg & Mago-Leccia, 1986: char. 1; Mago-Leccia, 1978; Mago-Leccia & Zaret, 1978; Triques, 1993: char. 21; Tagliacollo et al., 2016b, char. 97; CI = 1.000; RI = 1.000).
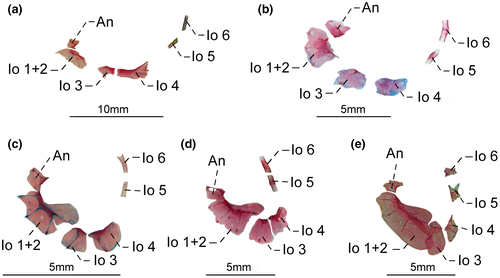
- 57. Association between infraorbital 1 + 2 and infraorbital 3 (Figure 21a–d): (0) not in contact; (1) contacting each other (Figure 21e; CI = 1.000; RI = 1.000).
- 58. Shape of infraorbitals 5 and 6: (0) slender and tubular (Figure 21a–d); (1) enlarged and half-pipe shaped (Figure 21e; Correa et al., 2006, char. 13; Lundberg & Mago-Leccia, 1986, char. 19 in part; CI = 1.000; RI = 1.000).
- 59. Site of connection between infraorbital and supraorbital canals: (0) anterior to sphenotic process; (1) on sphenotic process (Correa et al., 2006, char. 15; Lundberg & Mago-Leccia, 1986, char. 19 in part; CI = 0.333; CI = 0.800).
- 60. Shape of otic canal: (0) slender and tubular (Figures 17, 18); (1) enlarged and half-pipe shaped (Figures 19, 20; CI = 0.500; RI = 0.875).
- 61. Association between extrascapular and neurocranium: (0) extrascapular at joint of parietal, pterotic and epioccipital bones (Figures 17-19); (1) extrascapular at posterodorsal border of posttemporal fossa (Figure 20; Albert, 2001, char. 170; Albert & Campos-da-Paz, 1998, char. 171; Correa et al., 2006, char. 20; Lundberg & Mago-Leccia, 1986, char. 18 in part; CI = 0.500; RI = 0.833).
3.1.9 Hyoid Arch
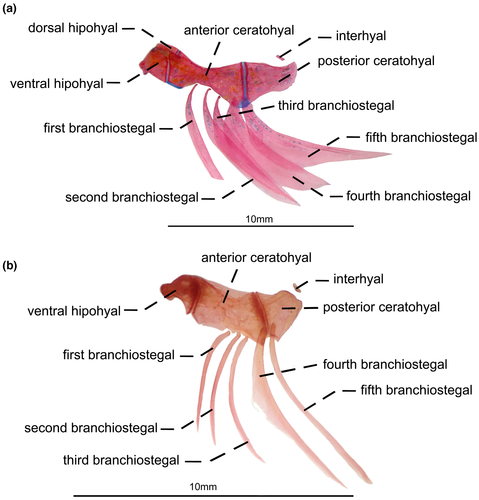
- 67. Shape of second branchiostegal: (0) thin (Figure 23a); (1) spatuladed (Figure 23b; Albert, 2001, char. 145 in part; Albert & Campos-da-Paz, 1998, char. 146 in part; Tagliacollo et al., 2016b; char. 140 in part; CI = 0.333; RI = 0.600).
- 68. Shape of third branchiostegal: (0) spatulated (Figure 23b); (1) thin (Figure 22a; Albert, 2001, char. 145 in part; Albert & Campos-da-Paz, 1998, char. 146 in part; Tagliacollo et al., 2016b; char. 140 in part; CI = 0.167; RI = 0.167).
- 69. Shape of fifth branchiostegal: (0) spatulated (Figure 23b); (1) thin (Figure 23a; Albert, 2001, char. 145 in part, Tagliacollo et al., 2016b; char. 140 in part; CI = 0.333; RI = 0.000).
3.1.10 Branchial arches
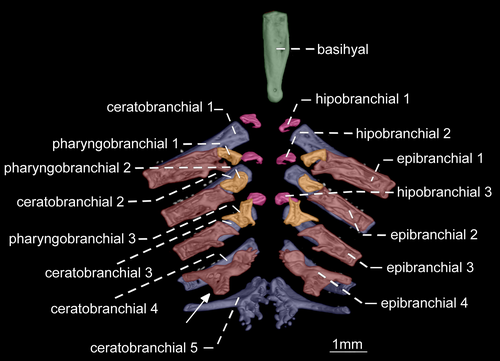
- 70. Form of gill rakers: (0) short and unossified; (1) long and ossified (Figure 24; Albert, 2001; char. 147; Albert & Campos-da-Paz, 1998, char. 148; Albert & Fink, 1996, char. 34; Correa et al., 2006, char. 21 and 23; Lundberg & Mago-Leccia, 1986, char. 24 in part; Fink & Fink, 1981; Mago-Leccia, 1978; Mago-Leccia & Zaret, 1978; CI = 1.000; RI = 1.000).
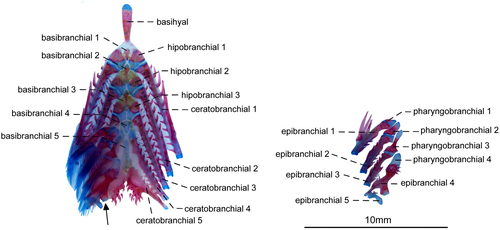
The presence of elongate and well-ossified gill rakers is related to the planktivorous feeding habits in fishes (Mago-Leccia & Zaret, 1978). In most Gymnotiformes, the gill rakers are short and unossified (see Hilton et al., 2007: fig. 14). However, elongate and ossified gill rakers are present in R. caviceps, R. eastwardi, R. electrogrammus, R. troscheli, and R. zareti. Mago-Leccia & Zaret (1978) initially proposed this unique gill morphology as a primitive condition within the order. Later, Fink and Fink (1981: 309) and Lundberg and Mago-Leccia (1986: 64), however, argued that presence of well-developed gill rakers is, in fact, an apomorphy within Gymnotiformes. Thus, this apomorphic condition was proposed as a synapomorphy for Rhabdolichops by several authors (e.g., Lundberg & Mago-Leccia, 1986). In spite of that, after the descriptions of R. lundbergi and R. nigrimans, in which the gill rakers are short and unossified, the presence of long and ossified gill rakers was restricted to a less inclusive clade in Rhabdolichops (Correa et al., 2006).
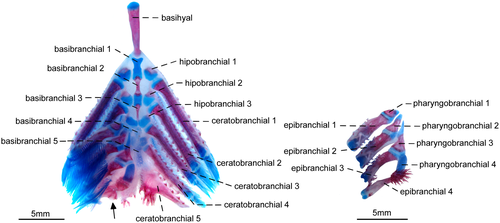
3.1.11 Neurocranium
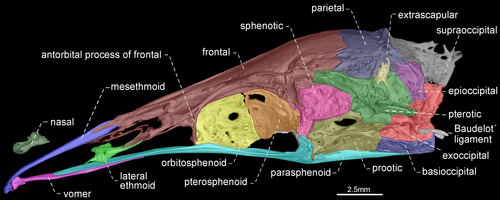
- 74. Relative length of mesethmoid: (0) 1.5 times as long as nasal length (Figures 18-20); (1) twice as long as nasal length; (2) three times as long as nasal length (Figure 17; Albert, 2001, char. 53; Albert & Campos-da-Paz, 1998, char. 54; Tagliacollo et al., 2016b, char. 61; CI = 0.222; RI = 0.682).

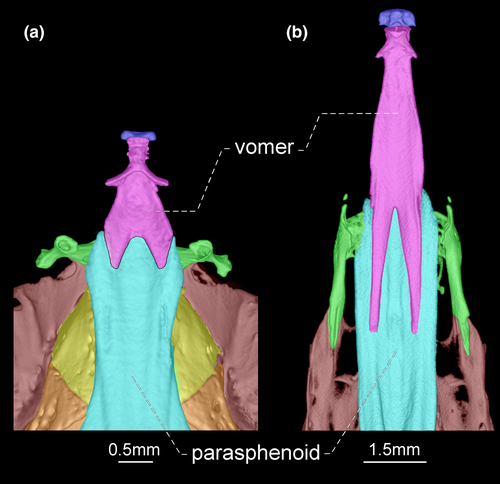
- 79. Antorbital process of frontal: (0) absent; (1) present (Figures 17-20; Aguilera, 1986; Albert, 2001, char. 69; Albert & Campos-da-Paz, 1998, char. 70; Albert & Fink, 1996, char. 20; Hulen et al., 2005, char. 27; Tagliacollo et al., 2016b, char. 77; Triques, 1993; char. 3; CI = 1.000; RI = 1.000).
- 80. Degree of elongation of antorbital portion of parasphenoid: (0) shorter than its orbital portion (Figures 18-20); (1) longer than its orbital portion (Figure 17; CI = 0.333; RI = 0.600).
- 83. Posttemporal fossa: (0) absent (Figures 17-19); (1) present (Figure 20; Albert, 2001, char. 81; Albert & Campos-da-Paz, 1998, char. 82; Albert & Fink, 1996, char. 25; Correa et al., 2006, char. 19; Fink & Fink, 1981, char. 12; Lundberg & Mago-Leccia, 1986, char. 18 in part; Mago-Leccia, 1978; Tagliacollo et al., 2016b, char. 88; CI = 0.500; RI = 0.833).
In most Gymnotiformes, the parietal, epioccipital, and pterotic bones are in contact; consequently, the posttemporal fossa is absent. However, the parietal, epioccipital and pterotic bones do not contact their mutual margins, and therefore, the posttemporal fossa is visible in Rhabdolichops caviceps, R. eastwardi, R. electrogrammus, R. troscheli, R. zareti, and Sternopygus.
3.1.12 Pectoral fin and girdle
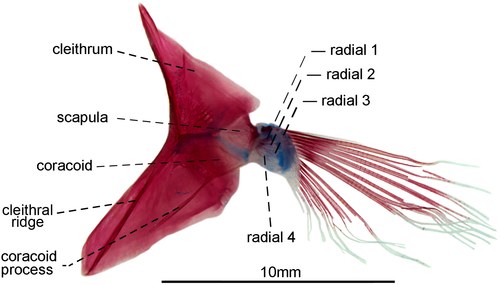
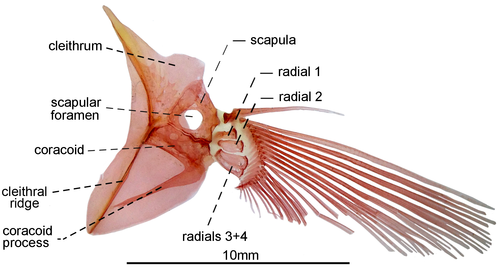
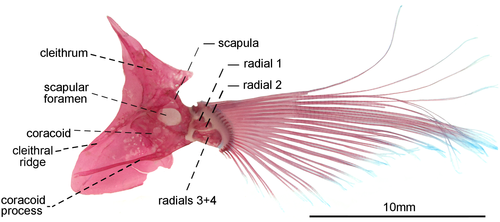
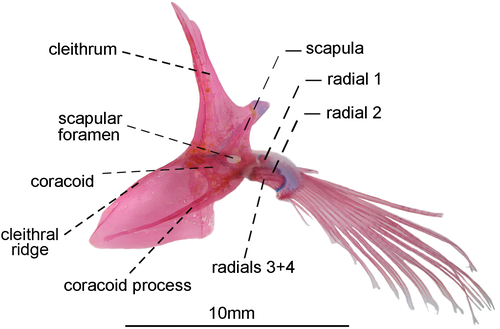
- 85. Association between anteroventral process of coracoid and cleithral ridge: (0) anterior process of coracoid short, not contacting the cleithral ridge on cleithrum anterior most portion (Figures 29, 30); (1) anteroventral process of coracoid long, contacting the cleithral ridge on cleithrum anterior most portion (state 0—Figures 31, 32; Albert, 2001, char. 175; Vari et al., 2012) (CI = 1.000; RI = 1.000).
- 87. Postcleithrum 1: (0) present; (1) absent (Hulen et al., 2005; char. 61; Lundberg & Mago-Leccia, 1986, char. 11 in part; CI = 0.500; RI = 0.667).
- 88. Shape of postcleithrum 1: (0) thin and discoid; (1) robust with posterior margin straight (Albert, 2001, char. 171; Lundberg & Mago-Leccia, 1986, char. 11 in part; CI = 1.000; RI = AUT).
- 89. Association between supracleithrum and posttemporal: (0) not fused (Figure 33a); (1) fused (Figure 33b; Albert, 2001, char. 169; Albert & Campos-da-Paz, 1998, char. 170; Correa et al., 2006, char. 24; Fink & Fink, 1981, char. 95 in part; Hulen et al., 2005, char. 51; Lundberg & Mago-Leccia, 1986, char. 10; Mago-Leccia, 1978; Mago-Leccia & Zaret, 1978; Tagliacollo et al., 2016b, char. 163; CI = 1.000; RI = 1.000).

- 90. Baudelot's ligament: (0) unossified (Figures 19, 20); (1) partially ossified (Figures 17, 18; Fink & Fink, 1981, char. 95 in part; CI = 0.333; RI = 0.833).
- 91. Position of scapular foramen: (0) between scapula and coracoid (Figure 29); (1) entirely included within the scapula (Figures 30-32; Albert, 2001, char. 173; Albert & Campos-da-Paz, 1998, char. 174; Albert & Fink, 1996, char. 26; Correa et al., 2006, char. 27; Hulen et al., 2005, char. 49; Lundberg & Mago-Leccia, 1986, char. 9; Mago-Leccia, 1978; Mago-Leccia & Zaret, 1978; Tagliacollo et al., 2016b, char. 164; CI = 1.000; RI = 1.000).
- 92. Mesocoracoid: (0) present; (1) absent (Figures 29-32; Albert, 2001, char. 174; Albert & Campos-da-Paz, 1998, char. 175; Fink & Fink, 1981; Lundberg & Mago-Leccia, 1986, char. 12; Mago-Leccia & Zaret, 1978; Tagliacollo et al., 2016b, char. 165; Triques, 1993, char. 46; CI = 1.000; RI = 1.000).
- 93. Association between proximal radials 3 and 4: (0) not fused (Figure 29); (1) fused (Figures 31, 32; Albert, 2001, char. 176; Albert & Fink, 1996, char. 28; Correa et al., 2006: char. 37; Hulen et al., 2005, char. 54; Lundberg & Mago-Leccia, 1986, char. 15; Mago-Leccia, 1978; Mago-Leccia & Zaret, 1978; Meunier et al., 2011; Tagliacollo et al., 2016b, char. 167; Triques, 1993, char. 47; Vari et al., 2012; I = 1.000; RI = 1.000).
- 94. Pectoral-fin length: (0) pectoral-fin tip not beyond abdominal cavity (Figure 34a–c); (1) pectoral-fin tip extending beyond abdominal cavity (Figure 34d; modified from Albert, 2001, char. 177; Albert & Campos-da-Paz, 1998, char. 178; Albert & Fink, 1996, char. 29; Correa et al., 2006, char. 28; Hulen et al., 2005, char. 55; Tagliacollo et al., 2016b, char. 168; CI = 0.333, RI = 0.750).
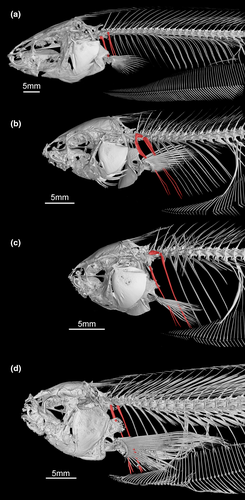
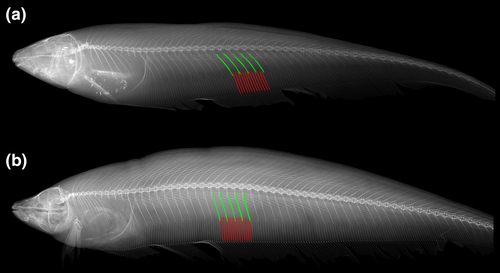
The pectoral-fin length was previously determined based in relation to head length (Correa et al., 2006). Because of the large variation of the snout length in Eigenmanniinae, pectoral fin elongation was instead estimated in function of the abdominal cavity length that does not vary as much within Eigenmanniinae, and could be easily estimated through X-ray images of specimens.
3.1.13 Weberian complex
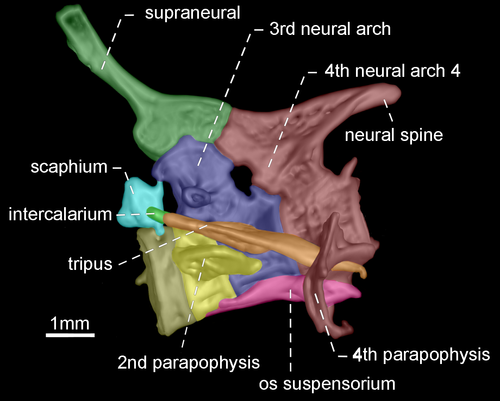
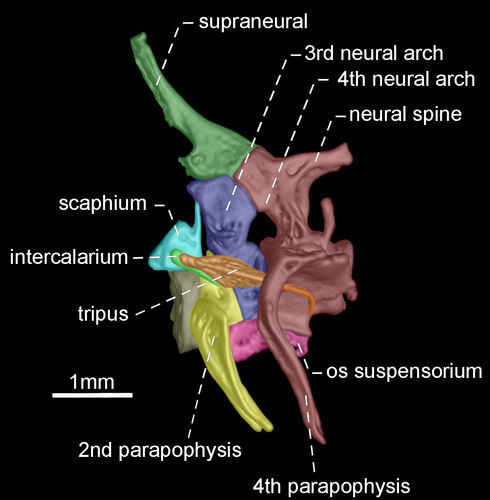
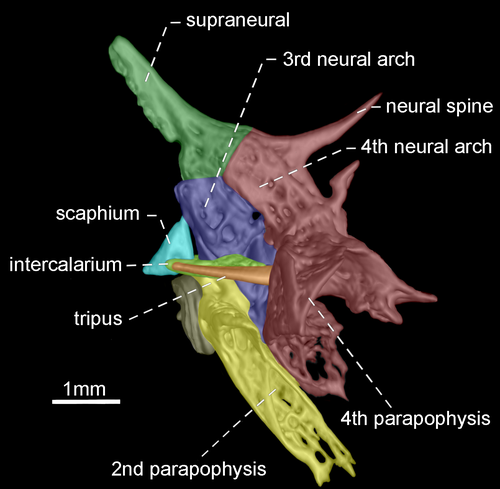
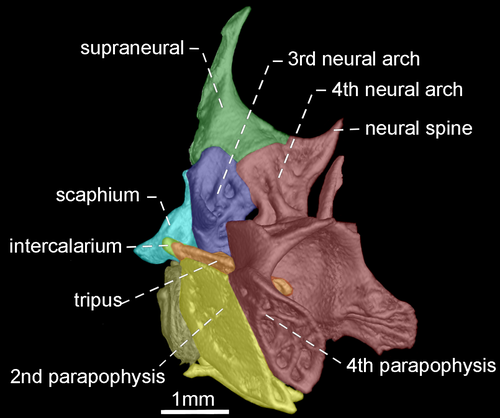
- 96. Association between parapophyses of second and fourth vertebrae: (0) distinctly separated (Figures 35, 36); (1) in contact (Figures 37, 38; Albert, 2001, char. 181; Correa et al., 2006, char. 32; Fink & Fink, 1981, char. 88 in part; Lundberg & Mago-Leccia, 1986, char. 8; Vari et al., 2012; CI = 0.500, RI = 0.889).
- 97. Shape of neural spine of fourth vertebra of Weberian complex: (0) long spine-shaped (Figure 37); (1) tiny spine-shaped (Figure 38); (2) nearly rectangular (Figures 35, 36; CI = 0.200; RI = 0.467).
The neural spine of fourth vertebra of Weberian complex when long and spine-shaped, it is almost as long as neural spine of fifth vertebra. In contrast, a tiny and spine-shaped neural spine of fourth vertebra is distinctly smaller than neural the spine of fifth vertebra. A third condition occurs when neural spine of fourth vertebra of Weberian complex is nearly rectangular with distal portion horizontally oriented.
3.1.14 Axial skeleton
- 98. Relative length of two anteriormost post-Weberian ribs: (0) approximately 80% as long as abdominal cavity depth (Figure 34a–b); (1) approximately as long as abdominal cavity depth (Figure 34c–d; Albert, 2001, char. 210; Albert & Fink, 1996, char. 43; Albert & Campos-da-Paz, 1998, char. 211; Correa et al., 2006, char. 36; Hulen et al., 2005, char. 59; Lundberg & Mago-Leccia, 1986, chars. 6 and 14; CI = 0.200, RI = 0.333).
- 99. Relative size of hemal spine of 26th to 30th vertebrae (modified from Albert, 2001, char. 198): (0) shorter than its associated pterygiophore (Figure 39a); (1) longer than its associated pterygiophore (Figure 39b; CI = 0.200, RI = 0.765).
3.1.15 Intermuscular bones
- 100. Rami arrangement of epineurals at 7–9th vertebrae: (0) all rami in same direction (Figure 40a); (1) rami arranged in several directions (Figure 40b; Albert, 2001, char. 184 in part; Albert & Campos-da-Paz, 1998, char. 185 in part; Albert & Fink, 1996, char. 38; Correa et al., 2006, char. 33 in part; Hulen et al., 2005, char. 57 in part; Lundberg & Mago-Leccia, 1986, char. 7 in part; CI = 0.250, RI = 0.800).
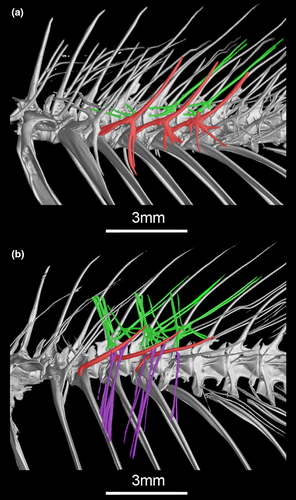
The intermuscular bones extend along body length in Gymnotiformes and are variably present in other fishes (de Santana & Vari, 2010). The highly branched intermuscular bones were proposed as a synapomorphy for Eigenmanniinae (Lundberg & Mago-Leccia, 1986). However, the ramification of the three series of intermuscular bones (epineurals, epicentrals, and “epipleurals”) vary independently in the subfamily.
- 101. Shape of epicentrals at 7–9th vertebrae: (0) unbranched (Figure 40b); (1) branched (Figure 40a; Albert, 2001, char. 184 in part; Albert & Campos-da-Paz, 1998, char. 185 in part; Albert & Fink, 1996, char. 37 in part; Correa et al., 2006, char. 33 in part; Hulen et al., 2005, char. 57 in part; Lundberg & Mago-Leccia, 1986, char. 7 in part; CI = 0.333, RI = 0.882).
- 102. “Epipleurals” at 7–9th vertebrae: (0) absent (Figure 40a); (1) present (Figure 40b; Albert, 2001, char. 184 in part; Albert & Campos-da-Paz, 1998, char. 185 in part; Albert & Fink, 1996, char. 37 in part; Correa et al., 2006, char. 33 in part; Hulen et al., 2005, char. 57 in part; Lundberg & Mago-Leccia, 1986, char. 7 in part; CI = 0.500, RI = 0.941).
Lundberg and Mago-Leccia (1986) indicated the presence of epipleurals in some Sternopygidae members as the ossified structure that links rib to epicentrals. Such definition contrast to Gemballa and Britz (1998), whose described the epipleurlas as fiber bundle that ossify into epipleural originates on the rib or the dorsal part of the hemal arch and runs posterodorsally to the integument. Considering the inconclusive homology of these structures located between epicentrals and pleural ribs, we called they here as “epipleurals”. The “epipleurals” are included in the figure depicting intermuscular bones in R. electrogrammus (Lundberg & Mago-Leccia, 1986: fig. 6D), but none of our analyzed specimens of R. electrogrammus possess any “epipleural” bone. Consequently, the character was codified as polymorphic for this taxon.
3.1.16 Unpaired fins
- 103. Branched anal-fin rays: (0) present along all the fin except for anterior 6 to 60 fin rays; (1) only 10 to 20 branched anal-fin rays present at midfin (Albert, 2001, char. 197; Albert & Campos-da-Paz, 1998, char. 198; Albert & Fink, 1996, char. 49; Hulen et al., 2005, char. 65; CI = 1.000, RI = 1.000).
3.1.17 Pigmentation
- 105. Vertical bands on body: (0) absent (Figure 1a–c,e); (1) present (see Meunier et al., 2011: fig. 2; Vari et al., 2012; CI = 0.500, RI = 0.000).
- 106. Lateral line stripe: (0) absent (Figure 1d–e); (1) present (Figure 1a–c; Albert, 2001, char. 8 in part; Albert & Campos-da-Paz, 1998, char. 8 in part; Peixoto et al., 2015; Tagliacollo et al., 2016b, char. 20 in part; Vari et al., 2012; CI = 0.250, RI = 0.857).
- 110. Coloration along distal margin of anal fin: (0) fin hyaline to dark but lacking a distinct band along the fin distal portions (Figure 1a,c-e); (1) distal margin dark (see Hilton & Cox-Fernandes, 2017: fig. 1d and 2; Correa et al., 2006, char. 2; CI = 0.250, RI = 0.400).
- 111. Humeral spot: (0) absent; (1) present (see Albert & Fink, 1996: fig. 7; Albert & Fink, 1996, char. 35; Hulen et al., 2005, char. 4; CI = 1.000, RI = 1.000).
3.1.18 Miscellaneous
- 112. Scales on anterodorsal portion of body: (0) present; (1) absent (Albert, 2001, char. 15; Albert & Campos-da-Paz, 1998, char. 15; Correa et al., 2006, char. 4; Lundberg & Mago-Leccia, 1986, char. 23; Mago-Leccia, 1978; Tagliacollo et al., 2016b, char. 30) (CI = 0.500, RI = 0.857).
- 113. Dorsal filament: (0) absent; (1) present (Albert, 2001, char. 192; Albert & Campos-da-Paz, 1998, char. 193; Tagliacollo et al., 2016b, char. 183; CI = 1.000, RI = 1.000).
The electric organ in Gymnotiformes comprises a series of electrocytes on the ventral portion of body. Lundberg and Mago-Leccia (1986) proposed the presence of visible rectangular electric organ subunits (electrocytes) above the end of anal-fin base, as a synapomorphy for a clade that includes: R. caviceps, R. eastwardi, R. stewardi, and R. troscheli. Further up, the authors recognized the presence of these visible electrocytes in other species of the genus (R. electrogrammus and R. zareti). However, the exposition areas of electrocytes are less visible in these species (as present in character 17).
The shape of these electrocytes also presents a puzzling variation in Eigenmannia. As discussed by Lundberg and Mago-Leccia (1986) and Albert (2001), the shape of these electrocytes in Eigenmannia is somehow different from those present in Rhabdolichops [e.g., Lundberg and Mago-Leccia (1986) described the electrocytes in Eigenmannia as hexagonal shaped]. However, although recognize the potential information in the electrocytes morphology, we did not prepare specimens to properly address this information (e.g., via histological preparation—see Giora & Carvalho, 2018). Therefore, the potential variation in electrocytes shape was not explored in the present analysis. Consequently, this character refers only to the presence of visible rectangular shaped electrocytes above the end of anal-fin base. This character is polymorphic in R. electrogrammus (see Lundberg & Mago-Leccia, 1986: Table 1).
3.1.19 Myology
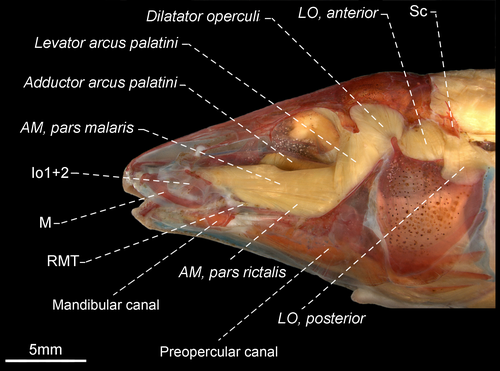
- 115. Attachment of adductor mandibulae: (0) pars malaris not inserted to infraorbital 1 + 2; (1) pars malaris inserted to infraorbital 1 + 2 (see Peixoto & Ohara, 2019: fig. 11; modified from Albert, 2001: char. 45; Albert & Campos-da-Paz, 1998: char. 46; Albert et al., 2005: char. 82; Peixoto & Ohara, 2019; Tagliacollo et al., 2016b: char. 57; CI = 1.000, RI = 1.000).
- 116. Topographical relationship among adductor mandibulae, pars malaris and adductor mandibulae, pars stegalis: (0) pars stegalis partially located medially to pars malaris (see Peixoto & Ohara, 2019: fig. 11); (1) pars stegalis fully located medially to pars malaris (Figure 16; CI = 1.000, RI = 1.000).
- 117. Origin of the adductor mandibulae, pars stegalis: (0) including sphenotic; (1) not including sphenotic (Peixoto & Ohara, 2019; CI = 0.500, RI = 0.875).
- 118. Association between pterosphenoid and origin of the adductor mandibulae, pars stegalis: (0) not including the pterosphenoid; (1) including the pterosphenoid (CI = 0.333, RI = 0.833).
- 119. Topographical relationship among adductor mandibulae, pars stegalis, and adductor arcus palatini: (0) pars stegalis fully lateral to adductor arcus palatini (see Peixoto & Ohara, 2019: fig. 13B); (1) pars stegalis lateral only to posterior portion of adductor arcus palatini (Figure 41); (2) pars stegalis lateral only to region near insertion of the adductor arcus palatini (Figure 16; CI = 0.500, RI = 0.818).
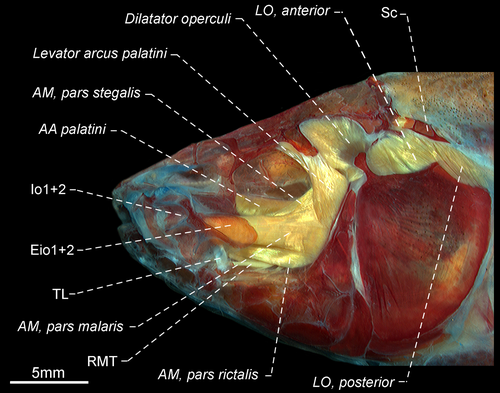
- 120. Adductor mandibulae, segmentum mandibularis: (0) absent; (1) present (CI = 0.250, RI = 0.571).
- 121. Segmentum mandibularis extension in relation to Meckel's cartilage: (0) contact between segmentum mandibularis and Meckel's cartilage occurring up to 80% of dorsal margin of the cartilage (Figure 42a); (1) contact between segmentum mandibularis and Meckel's cartilage occurring more than 95% of dorsal margin of the cartilage (Figure 42b; CI = 1.000, RI = 1.000).
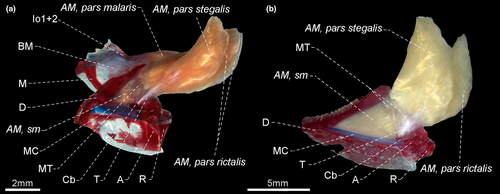
- 122. Insertion of segmentum mandibularis: (0) including angulo-articular and dentary (Figure 42a–b); (1) restricted to angulo-articular (CI = 0.333, RI = 0.000).
- 123. Origin of the adductor arcus palatini (Figure 43): (0) not including orbitosphenoid; (1) including orbitosphenoid (CI = 0.500, RI = 0.000).
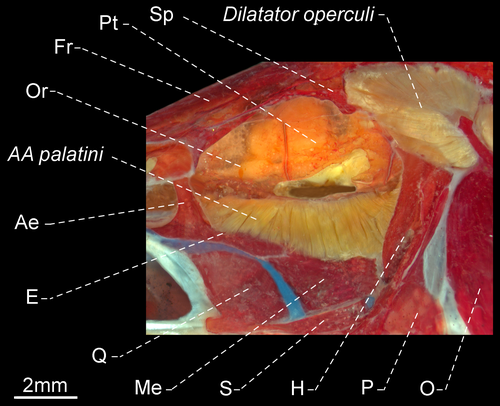
- 124. Adductor arcus palatini: (0) insertion occurring at base of endopterygoid, not including ascending process; (1) insertion occurring at base of endopterygoid and including ascending process (Figure 43; CI = 0.500, RI = 0.000).
- 125. Origin of levator arcus palatini: (0) including frontal; (1) not including frontal (CI = 0.167, RI = 0.737).
- 126. Association between pterosphenoid and origin of levator arcus palatini: (0) not including pterosphenoid; (1) including pterosphenoid (CI = 0.250, RI = 0.750).
- 127. Origin of levator arcus palatini: (0) width as broad as or broader than at its insertion; (1) narrower than its insertion (CI = 0.250–0.333, RI = 0.700–0.800).
- 129. Levator arcus palatini: (0) wider at origin; (1) origin and insertion with similar width (see Peixoto & Ohara, 2019: fig. 13B); (2) wider at insertion (Figures 15 and 40; CI = 1.000, RI = 1.000).
- 130. Relationship among R-Avn nerve and levator operculi anterior (Peixoto & Ohara, 2019): (0) R-Avn nerve passing laterally to levator operculi anterior (see Peixoto & Ohara, 2019: fig. 13B); (1) R-Avn nerve passing medially to levator operculi anterior (Figures 16 and 41; CI = 0.500, RI = 0.889).
- 131. Origin of levator operculi: (0) not including hyomandibula; (1) including hyomandibula (CI = 0.500 RI = 0.889).
- 132. Muscle forming posteroventral limit of muscular hiatus between first and second rib (Dutra et al., 2015, char. 3): (0) obliquus inferioris (see Dutra et al., 2015: fig. 1); (1) obliquus superioris (see Dutra et al., 2015: fig. 2E–F for comparable condition in Steatogenys; CI = 0.200, RI = 0.636).
The pseudotympanum is formed by the reduction of the hypaxialis muscle in the body wall lateral to the anterior portion of the swim bladder. This structure is present in all Gymnotiformes, and some informative characters were recently pointed out by Dutra et al. (2015). The delimitation of the hiatus h3 posteroventral margin varies due to the degree of reduction of the obliquus superioris in that area exposing or not the obliquus inferioris.
3.1.20 Neuroanatomy
The lobus vagi is compounded by two rod-shaped somewhat curved structures (Figure 44). Such lobe is located in the dorsal portion of the rhombencephalon and positioned posterior to the corpus cerebelli (ventral to posterior portion of it, in some species). The posterior portion of the lobus vagi has a tip-shaped ending, whereas its anterior portion can be convergent or parallel, without contact between its counterparts (Figure 44). In the convergent condition, the lobus vagi is somewhat horseshoe-shaped, resulting in a greater proximity between the anterior portion of its counterparts. In the parallel condition, such lobe is somewhat U-shaped, resulting in a parallel position between its counterparts.

- 137. Topographical relationship among corpus cerebelli (#6), tectum mesencephali (#7) and telencephalon (#14): (0) corpus cerebelli positioned above posterior portion of telencephalon (Figure 45a); (1) corpus cerebelli positioned dorsal to anterior margin of tectum mesencephali (Figure 45d–e); (2) corpus cerebelli positioned dorsal to anterior portion of tectum mesencephali (Figure 45f–g); (3) corpus cerebelli positioned dorsal to half length of tectum mesencephali (Figure 44b; CI = 0.600, RI = 0.818).
- 140. Position of valvula cerebelli (#5) in relation to tectum mesencephali (#7) in lateral view: (0) covering posterior portion of tectum mesencephali (Figure 45e–g); (1) covering posteromedial portion tectum mesencephali (Figure 45b); (2) covering hole tectum mesencephali (Figure 45c; CI = 1.000, RI = 1.000).
- 143. Position of telencephalon (#14): (0) above posterior portion of bulbus olfactorius; (1) above bulbus olfactorius. (CI = 0.200, RI = 0.600).
The nervus opticus connects the ventral portions of the tectum mesencephali to the retina in the eyes, whereas the nervus olfactorius connects the bulbus olfactorius to the olfactory organ.
3.2 Phylogenetic reconstruction
The analysis of the phylogenetic relationships among the species of Eigenmanniinae, based on 144 morphological characters of 45 taxa (37 representing species of Eigenmanniinae and eight outgroups representing other gymnotiform groups—Tables S5–S6, Data S2), resulted in two most parsimonious trees, with 389.152 score, and CI of 0.399 and RI of 0.754 (Figure 47).
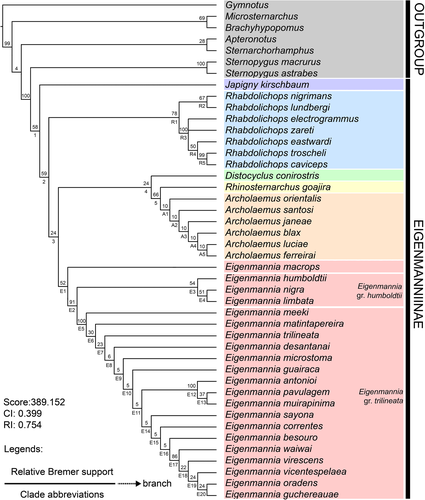
3.2.1 Monophyly of Eigenmanniinae Mago Leccia, 1978 (Clade 1)
Eigenmanninae Mago-Leccia, 1978: 14 (type genus: Eigenmannia Jordan & Evermann, 1896). Albert & Campos-da-Paz, 1998: 439 (phylogeny). Albert, 2001: 71 (phylogeny). Tagliacollo et al., 2006a: 31 (phylogeny).
Eigenmanniidae Alves-Gomes et al., 1995: 312 (phylogeny).
Eigenmanniinae Dutra et al., 2014: 346 (position of Distocyclus). Peixoto & Ohara, 2019:18 (discussion on myology).
Included genera
Archolaemus, Distocyclus, Eigenmannia, Japigny, Rhabdolichops, and Rhinosternarchus gen. nov.
Phylogenetic diagnosis
Eigenmanniinae is supported by the following unambiguous synapomorphies: (1) decrease of number of teeth in upper pharyngeal plate (char. 7, 13–15>12); (2) increase of number of pectoral-fin rays (char. 9, 15–17>18–20); (3) decrease of number of precaudal vertebrae, 14 or 15 (char. 11, 17–18>14–15); (4) decrease of number of pleural ribs (char. 13, 12–13>8); (5) site of connection between infraorbital and supraorbital canals on sphenotic process (char. 59, 0>1); (6) free urohyal blade as long as urohyal ridge (char. 64, 0>1); (7) supracleithrum and posttemporal fused (char. 89, 0>1); (8) scapular foramen entirely included within scapula (char. 91, 0>1). (9) parapophysis of second vertebra ventrally curved (char. 95, 0>1); (10) epicentrals at 7–9th vertebrae branched (char. 101, 0>1); (11) presence of anal-fin base stripe (char. 109, 0>1); (12) origin of the adductor mandibulae, pars stegalis not including the sphenotic (char. 117, 0>1); (13) Adductor mandibulae, pars stegalis lateral only to posterior portion of adductor arcus palatini (char. 119, 0>1); (14) origin of the levator arcus palatini narrower than its insertion (char. 127, 0>1); (15) R-Avn nerve passing medially to levator operculi anterior (char. 130, 0>1); (16) origin of the levator operculi including the hyomandibula (char. 131, 0>1); (17) Telencephalon located above bulbus olfactorius (char. 143, 0>1).
3.2.2 Japigny Meunier et al., 2011
Japigny Meunier et al., 2011: 48 [type species: Japigny kirschbaum Meunier et al., 2011; type by original designation (also monotypic). Gender feminine].
Included species
Japigny kirschbaum Meunier et al., 2011.
Phylogenetic diagnosis
Japigny is diagnosed by fourteen autapomorphies: (1) decrease of number of endopterygoid teeth (char. 5, 7–11>4); (2) decrease of number of anal-fin rays (char. 10, 216–217>132–164); (3) increase of number of displaced hemal spines (char. 14, 3>4); (4) mouth subterminal (char. 18, 1>2); (5) presence of teeth on oral valve (char. 21, 0>1); (6) dentary teeth attached on a dorsolateral flange of this bone (char. 35, 0>1); (7) third branchiostegal slender (char. 68, 0>1); (8) fifth branchiostegal slender (char. 69, 0>1); (9) basibranchial 3 unossified (char. 73, 1>0); (10) rami arrangement of epineurals at 7–9th in several directions (char. 100, 0>1); (11) presence of vertical bands on body (char. 105, 0>1); (12) Segmentum mandibularis insertion restricted to angulo-articular (char. 122, 0>1); (13) origin of the adductor arcus palatini including the orbitosphenoid (char. 123, 0>1); and (14) Adductor arcus palatini insertion on endopterygoid occurring at the base of the endopterygoid, and including the ascending process (char. 124, 0>1).
Taxonomic diagnosis
In addition to the aforementioned characters under the phylogenetic diagnosis, Japigny differs from Archolaemus by having the eye covered by skin (versus free orbital rim), and anteriormost row of the premaxilla teeth completely attached to the ventral surface of the premaxilla (versus teeth attached only along their anterobasal margins). It differs from Distocyclus by the presence of endopterygoid teeth (versus absence), mouth subterminal (versus terminal), and dentary teeth row extending posteriorly beyond anterior limit of Meckel’s cartilage (versus limited to anterior portion of dentary). Japigny can be distinguished from “E.” goajira by having the mouth subterminal (versus terminal), the snout elongate with the upper jaw distinctly longer than the lower jaw (versus the snout pointed but short turning it about equally developed as the dentary), and 132–164 (versus 259–263) anal-fin rays. It can be diagnosed from Rhabdolichops by the presence scales on anterodorsal portion of body (versus absence of such scales), the tip of the pectoral fin never extending beyond (versus extending beyond) the end of body cavity and the absence (versus presence) of visible rows of electrocytes above the end of anal-fin base.
3.2.3 Clade 2
Included genera
Archolaemus, Distocyclus, Eigenmannia, Rhabdolichops and Rhinosternarchus.
Phylogenetic diagnosis
The Clade 2 is diagnosed by nine unambiguous synapomorphies: (1) coronomeckelian bone corresponding to 20% or less of the length of Meckel’s cartilage (char. 39, 0>1); (2) anterior process of the coracoid contacting the main crest of cleithrum on its anterior portion (char. 85, 0>1); (3) parapophyses of the second and fourth vertebrae in contact (char. 96, 0>1); (4) hemal spine of 26th to 30th vertebrae longer than its associated pterygiophore (char. 99, 0>1); (5) origin of the adductor mandibulae, pars stegalis not including the pterosphenoid (char. 118, 1>0); (6) orientation of the anterolateral fibers of main axis of the levator arcus palatini approximately straight in relation to the vertical arm of the preopercle (char. 128, 0>1); (7) posterior portion of the corpus cerebelli extending to the anterior margin of the lobus vagi (char. 136, 0>1); (8) corpus cerebelli positioned dorsal to the anterior portion of the tectum mesencephali (char. 137, 1>2); and (9) Valvula cerebelli comma-shaped in dorsal view (char. 139, 0>2).
3.2.4 Rhabdolichops Eigenmann & Allen, 1942
Rhabdolichops Eigenmann & Allen, 1942: 316 (type species: Rhabdolichops longicaudatus Eigenmann & Allen, 1942; type by monotypy. Gender masculine).
Included species
Rhabdolichops caviceps (Fernández-Yépez 1968), R. eastwardi Lundberg & Mago-Leccia, 1986, R. electrogrammus Lundberg & Mago-Leccia, 1986, R. jegui Keith & Meunier, 2000, R. lundbergi Correa et al., 2006, R. navalha Correa et al., 2006, R. nigrimans Correa et al., 2006, R. stewarti Lundberg & Mago-Leccia, 1986, R. troscheli (Kaup, 1856), and R. zareti Lundberg & Mago-Leccia, 1986.
Phylogenetic diagnosis
Rhabdolichops is diagnosed by twelve unambiguous synapomorphies: (1) increase of number of gill rakers on first branchial arch (char. 6: 11>12–14); (2) decrease of number of precaudal vertebrae (char. 11: 14>12–13); (3) length of premaxilla corresponding to four times as long as its width (char. 24: 0>1); (4) absence of an anterolateral process on premaxilla (char. 26: 1>0); (5) posterodorsal margin of hyomandibula ending at same level of condyle that receives the opercle socket (char. 48: 0>1); (6) depth of the posterodorsal laminar expansion of infraorbital 1+2 half or less as long as infraorbital 1+2 length (char. 56: 1>0); (7) otic canal enlarged and half-pipe shaped (char. 60: 0>1); (8) extrascapular enlarged and half-pipe shaped (char. 62: 0>1); (9) urohyal ridge triangular (char. 63: 1>2); (10) mesethmoid 1.5 times as long as nasal length (char. 74: 1>0); (11) tip of pectoral fin surpassing the end of abdominal cavity (char. 94: 0>1); and (12) scales on anterodorsal portion of body absent (char. 112: 0>1).
Taxonomic diagnosis
In addition to the aforementioned characters under the phylogenetic diagnosis, Rhabdolichops differs from Archolaemus by having the eye covered by skin (versus orbital rim free). It differs from Distocyclus by having the snout rounded (versus conical), the internarial distance equal to or at least twice the diameter of the nostril, and the dentary teeth row extending posteriorly beyond the anterior limit of Meckel’s cartilage (versus limited to dentary anterior portion). Rhabdolichops further differs from Japigny by the absence (versus presence) of eight dark, vertical bands along the body, and the absence (versus presence) of teeth associated with the oral valve. It further differs from Rhinosternarchus by having a round (versus subconical) snout.
3.2.5 Clade 3
Included genera
Archolaemus, Distocyclus, Eigenmannia and Rhinosternarchus.
Phylogenetic diagnosis
The Clade 3 is diagnosed by five unambiguous synapomorphies: (1) increase of number of dentary teeth (char. 3: 24–40>23); (2) increase of number of teeth in lower pharyngeal plate (char. 8: 9>10–11); (3) Baudelot’ ligament partially ossified (char. 90: 0>1); (4) neural spine of four vertebra of weberian apparatus long spine-shaped (char. 97: 2>0); and (5) Levator arcus palatini wider at insertion (char. 126: 1>2).
3.2.6 Eigenmannia Jordan & Evermann, 1896
Eigenmannia Jordan & Evermann, 1896: 340 (type species: Sternopygus humboldtii Steindachner, 1878; type by being a replacement name of Cryptops Eigenmann, 1894, preoccupied by Cryptops Leach 1814 in Myriopoda, Cryptops Schoenherr, 1823 and Cryptops Solier 1851 in Coleoptera. Gender feminine).
Included species
Eigenmannia antonioi Peixoto et al., 2015, E. besouro Peixoto & Wosiacki, 2016, E. camposi Herrera-Collazos et al., 2020, E. correntes Campos-da-Paz & Queiroz, 2017, E. desantanai Peixoto et al., 2015, E. dutrai Peixoto et al., 2021, E. guchereauae (Meunier et al., 2014), E. guairaca Peixoto et al., 2015, E. humboldtii (Steindachner, 1878), E. limbata (Schreiner & Miranda-Ribeiro, 1903), E. loretana Waltz & Albert, 2018, E. macrops (Boulenger, 1897), E. magoi Herrera-Collazos et al., 2020, E. matintapereira Peixoto et al., 2015, E. meeki Dutra et al., 2017, E. microstoma (Reinhardt, 1852), E. muirapinima Peixoto et al., 2015, E. nigra Mago-Leccia, 1994, E. oradens Dutra et al., 2018, E. pavulagem Peixoto et al., 2015, E. sayona Peixoto & Waltz, 2017, E. sirius Peixoto & Ohara, 2019, E. trilineata López & Castello, 1966, E. vicentespelaea Triques, 1996, E. virescens (Valenciennes, 1936), E. waiwai Peixoto et al., 2015, and E. zenuensis Herrera-Collazos et al., 2020.
Phylogenetic diagnosis
Eigenmannia is diagnosed by four unambiguous synapomorphies: (1) increase in number of premaxillary teeth, 30–37 (char. 1: 21–25>30–37); (2) increase in number of teeth rows on premaxilla (char. 2: 4>5); (3) presence of “epipleurals” at 7–9th vertebrae (char. 102: 0>1); and (4) Nervus opticus thicker than nervus olfactorius (char. 144: 0>1).
Taxonomic diagnosis
In addition to the aforementioned characters under the phylogenetic diagnosis, Eigenmannia differs from Archolaemus by having the eye covered by skin (versus free orbital rim), and the teeth of premaxilla anteriormost teeth row completely attached to ventral surface of premaxilla (versus attached only along their anterobasal margins—also present in E. guchereauae and E. oradens). It can be distinguished from Distocyclus by the presence (versus absence) of endopterygoid teeth, and the dentary teeth row extending posteriorly beyond anterior limit of Meckel’s cartilage (versus limited to anterior portion of dentary). Eigenmannia is diagnosed from Japigny by not having teeth associated with the oral valve (versus teeth present), and the absence (versus presence) of eight dark, vertical bands along the body. It can be also diagnosed from Rhabdolichops by the presence scales on anterodorsal portion of body (versus absence of such scales), the tip of pectoral fin never extending beyond the end of body cavity (versus extending beyond). Eigenmannia can be further distinguished from Rhinosternarchus by having a round or pointed (versus subconical—see Dutra et al., 2014) snout.
3.2.7 Clade 4
Included genera
Archolaemus, Distocyclus and Rhinosternarchus.
Phylogenetic diagnosis
The clade 4 is diagnosed by five unambiguous synapomorphies: (1) absence of the posterodorsal laminar expansion in Infraorbital 1+2 (char. 55: 1>0); (2) mesethmoid length corresponds to at least three times of length of nasal bone (char. 73: 1>2); (3) posterodorsal process of lateral ethmoid longer than main axis of ethmoid lateral (char. 75: 1>0); (4) posterior margin of the ELL extends to posterior limit of the lobus vagi (char. 131: 2>1); and (5) posterior portion of the corpus cerebelli surpassing the posterior portion of the lobus vagi (char. 133: 2>3).
3.2.8 Distocyclus Mago-Leccia, 1978
Distocyclus Mago-Leccia, 1978: 267 (type species: Eigenmannia conirostris Eigenmann & Allen, 1942; type by original designation. Gender masculine).
Included species
Distocyclus conirostris (Eigenmann & Allen, 1942).
Phylogenetic diagnosis
Distocyclus is diagnosed by eleven autapomorphies: (1) decrease of number of premaxillary teeth, 14–19 (char. 1: 20–25>14–19); (2) decrease of number of teeth rows on premaxilla (char. 2: 4>3); (3) decrease of number of dentary teeth (char. 3: 23>1–6); (4) decrease of number of teeth rows on dentary (char. 4: 2–3>1); (5) internarial distance equivalent to diameter of posterior nostril (char. 22: 0>1); (6) teeth row of dentary not reaching anterior limit of Meckel’s cartilage (char. 32: 0>1); (7) coronoid process ventrally curved (char. 36: 1>0); (8) retroarticular limiting the ventralmost margin of lower jaw (char. 41: 1>0); (9) endopterygoid edentulous (char. 43: 1>0); (10) urohyal ridge triangular (char. 63: 1>2); and (11) distal margin of anal fin dark (char. 110: 0>1).
Taxonomic diagnosis
Dutra et al. (2014) provided a taxonomic diagnosis of Distocyclus based on external characters.
3.2.9 Clade 5
Included genera
Archolaemus and Rhinosternarchus.
Phylogenetic diagnosis
The clade 5 is diagnosed by three unambiguous synapomorphies: (1) premaxillary bone compact, longitudinal length approximately equal to its transverse width (char. 25: 0>1); (2) absence of anterolateral process on the premaxillary bone (char. 26: 1>0); and (3) hemal spine of 26th to 30th vertebrae longer than its associated pterygiophore (char. 99: 1>0).
3.2.10 Rhinosternarchus gen. nov.
urn:lsid:zoobank.org:act:B43E0006-FE9E-40DC-8753-A394A89B5BE9.
Type species. Eigenmannia goajira Schultz, 1949, by monotypy.
Included species
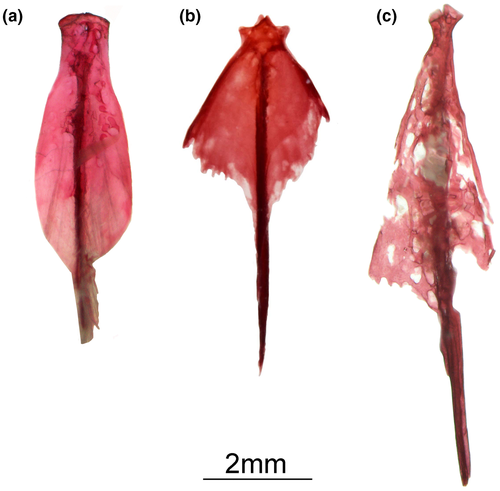
Rhinosternarchus goajira (Schultz, 1949; Figure 48).

Phylogenetic diagnosis
Rhinosternarchus is diagnosed by seven autapomorphies: (1) decrease of number of endopterygoid teeth (char. 5: 5–8>3–4); (2) increase of number of anal-fin rays (char. 10: 216–217>259–263); (3) decrease of number of pleural ribs (char. 13: 8–9>6); (4) decrease of number of displaced hemal spines (char. 14: 3>2); (5) anterior portion of endopterygoid approximately twice its width at ascending process (char. 44: 0>1); (6) antorbital portion of the parasphenoid longer than its orbital portion (char. 80: 0>1); and (7) anal-fin base stripe absent (char. 109: 1>0).
Taxonomic diagnosis
In addition to the aforementioned characters under the phylogenetic diagnosis, Rhinosternarchus can be diagnosed from Archolaemus by possessing the mouth terminal (versus subterminal), and the eye covered by skin (versus with a free orbital rim). It differs from Distocyclus by having a subconical (versus conical) snout, the internarial distance equal to at least twice the diameter (versus equivalent to a diameter of the posterior nostril), presence (versus absence) of teeth on the endopterygoid, and dentary with four rows (versus a single row of teeth). Rhinosternarchus is diagnosed from Eigenmannia by having a subconical (versus rounded or pointed) snout. It is diagnosed from Japigny by having the mouth terminal (versus subterminal), teeth associated with the oral valve absent (versus teeth present), and the absence (versus presence) of eight dark, vertical bands along the body. Rhinosternarchus also differs from Rhabdolichops by having a subconical (versus round) snout, the presence scales on the anterodorsal portion of body (versus absence of such scales), and the tip of pectoral fin never extending beyond (versus extending beyond) end of the body cavity.
Etymology
The genus name is from the Greek “rhino,” meaning nose, in reference to the elongated snout, and “sternarchus,” a commonly scientific suffix used in Gymnotiformes nomenclature, from the Greek “sternon,” chest, and “archus,” rectum, an allusion to the anterior position of the anus, a common feature in the order.
3.2.11 Archolaemus Korringa, 1970
Archolaemus Korringa, 1970: 267 [type species: Archolaemus blax Korringa, 1970; type by original designation (also monotypic). Gender masculine].
Included species
Archolaemus blax Korringa, 1970, A. ferreirai Vari et al., 2012, A. janeae Vari et al., 2012, A. luciae Vari et al., 2012, A. orientalis Stewart, Vari et al., 2012, A. santosi Vari et al., 2012.
Phylogenetic diagnosis
Archolaemus is diagnosed by five unambiguous synapomorphies: (1) decrease of number of anal-fin rays (char. 10: 216–217>205–213); (2) increase of number of precaudal vertebrae, 15 (char. 11: 14>15); (3) increase of number of scale rows above lateral line (char. 16: 13>14–15); (4) mouth subterminal (char. 18: 1>2); and (5) free orbital rim (char. 23: 0>1).
Taxonomic diagnosis
In addition to the aforementioned characters under the phylogenetic diagnosis, Archolaemus differs from all the other Eigenmanniinae genera by the teeth of the premaxilla anterior most row attached only along their anterobasal margins (versus anteriormost teeth completely attached to the ventral surface of the premaxilla in all Eigenmanniinae, except E. guchereauae and E. oradens). It further differs from Distocyclus by the presence of endopterygoid teeth (versus absence), and the dentary teeth row extending posteriorly beyond anterior limit of Meckel’s cartilage (versus limited to anterior portion of dentary). Archolaemus is also diagnosed from Japigny by the absence (versus presence) of eight dark, vertical bands along the body, and the absence (versus presence) of teeth associated with the oral valve. It can be also diagnosed from Rhabdolichops by the presence (versus absence) of scales on anterodorsal portion of body, the tip of pectoral fin never extending beyond the end of body cavity (versus pectoral-fin tip beyond that point), and presence of visible rows of electrocytes above end of anal-fin base (versus absence).
3.3 Key to the genera of Eigenmanniinae based on external morphology
- 1a. Eye subcutaneous without a free orbital rim (Figure 3a–b)… ………………………2
- 1b. Free orbital rim (Figure 3c)…………………………………………………Archolaemus
- 2a. Presence of eight dark, vertical bands along the body (Figure 1d)……Japigny
- 2b. Absence of eight dark, vertical bands along the body (Figure 1a–c,e)…3
- 3a. Presence of scales on the anterodorsal portion of body……4
- 3b. Absence of scales on the anterodorsal portion of body………Rhabdolichops
- 4a. Conical snout (Figures 1b and 3b); internarial distance equivalent to diameter of posterior nostril……………………………………………………………………Distocyclus
- 4b. Subconical snout (Figures 1a, 3c and 47); internarial distance corresponds to at least two diameters of posterior nostril……………………………Rhinosternarchus
- 4c. Rounded (Figures 1c,e and 3a) or pointed (Figure 1d) snout; internarial distance corresponds to at least two diameters of posterior nostril…Eigenmannia
4 DISCUSSION
Eigenmanniinae was proposed by Mago-Leccia (1978) to include Eigenmannia, Archolaemus, and Distocyclus. Those three genera shared the scapular foramen entirely included within the scapula, the fusion of the post-temporal and the supracleithrum into a single ossification, and by having 11–15 precaudal vertebrae (Table S1). Subsequently, Rhabdolichops and Japigny were included in Eigenmanniinae (Albert, 2001; Albert & Campos-da-Paz, 1998; Fink & Fink, 1981; Mago-Leccia, 1994; Maldonado-Ocampo, 2011; Meunier et al., 2011; Tagliacollo et al., 2016a; Vari et al., 2012). In the present study, we recovered all synapomorphies proposed by Mago-Leccia (1978), as well as all three putative synapomorphies based on musculature proposed by Peixoto and Ohara (2019). Additionally, eleven new synapomorphies were found to support the monophyly of the subfamily (see in Phylogenetic diagnosis of Eigenmanniinae).
Japigny was previously proposed as the sister group of all the other Eigenmanniinae by molecular and morphological datasets (Maldonado-Ocampo, 2011; Vari et al., 2012). Vari et al. (2012) presented three characters shared by all Eigenmanniinae except Japigny (Clade 2), to justify their hypothesis of Japigny as the sister group of all other members of the subfamily (Table S7). Among them, the parapophyses of the second and fourth vertebrae in contact was recovered here as a synapomorphy of Clade 2, while the retroarticular included in the socket to receive the quadrate condyle can be ambiguously optimized due its presence in Japigny and Sternopygus. The parapophysis of the fourth vertebra curved was not utilized in this study. Reasons are discussed in Data S1 (U22). Later, Tagliacollo et al. (2016a) recovered Japigny intermingled within Eigenmannia. The species J. kirschbaum was then recently proposed as a member of Eigenmannia by Waltz and Albert (2017) based in the Tagliacollo’ et al. (2016a) hypothesis, according to which this species was recovered as sister group of E. macrops by sharing a subterminal mouth. Tagliacollo et al. (2016a), however, highlighted that the inclusion of Japigny in their total evidence analysis was based only on morphological characters, suggesting that result could be influenced by the absence of molecular data for this species. Yet, it is important to note that the characters proposed by Vari et al. (2012) to consider Japigny as sister group of the remaining Eigenmanniinae were not included in the analysis by Tagliacollo et al. (2016a). Thus, considering Japigny’s position as the sister group of all Eigenmanniinae as recovered herein, we believe that location of Japigny in Tagliacollo et al. (2016a) was also influenced by the absence of informative morphological characters for this species rather than just by the missing data of molecular traits only.
Several authors recognized the genus Rhabdolichops as monophyletic based on both morphology (Correa et al., 2006; Lundberg & Mago-Leccia, 1986) and molecules (Tagliacollo et al., 2016a). On the contrary, Maldonado-Ocampo (2011) rejected that hypothesis and emphasized that R. lundbergi Correa et al., 2006 and R. nigrimans Correa et al., 2006 more closely related to Eigenmannia than others Rhabdolichops. The monophyly of Rhabdolichops was recovered herein contrasting with Maldonado-Ocampo (2011) hypothesis, who considered these two species as sister group of E. humboldtii + E. limbata and suggested that R. lundbergi and R. nigrimans should be placed to Eigenmannia. Thirteen synapomorphies supported the monophyly of Rhabdolichops, and the clade R. lundbergi + R. nigrimans (Clade R2) was recovered as the sister group of the other species of the genus as proposed by Correa et al. (2006). Most of the synapomorphies proposed by Lundberg and Mago-Leccia (1986) for Rhabdolichops, support a less inclusive group within the genus (Clade R3), which includes all species except R. lundbergi and R. nigrimans, as discussed by Correa et al. (2006). These last two species were only described in 2006 and were not available for the study by Lundberg and Mago-Leccia (1986). Correa et al. (2006) defined Rhabdolichops based on six synapomorphies: (1) scales absent above lateral line on anterior portion of body without scales above lateral line; (2) premaxilla elongate; (3) extrascapular characterized as an independent ossification; (4) two prootic foramina; (5) gill rakers long and bony; and (6) gill rakers ossified (Table S8). Among the proposed synapomorphies, the absence of scales above the lateral line on anterior portion of body, and an elongate premaxilla were recovered as synapomorphies for the genus herein, and the remaining characters as synapomorphies for a less inclusive group (clade R3). Lundberg and Mago-Leccia (1986) and Correa et al. (2006) proposed R. electrogrammus as sister group of all remaining species for clade R3. We did not recover this hypothesis herein; instead, we recovered an unresolved trichotomy with R. electrogrammus, R. zareti, and Clade R4 (R. eastwardi + R. caviceps + R. troscheli).
The monophyly of Clade 3 (Archolaemus +Distocyclus + Eigenmannia +Rhinosternarchus), which is recovered herein, was previously recovered by both morphological (Mago-Leccia, 1978), and molecular datasets (Alda et al., 2019; Alves-Gomes, 1995, 1998; Maldonado-Ocampo, 2011). Mago-Leccia’s (1978) proposed four characters to support the monophyly of this clade: (1) gill rakers cartilaginous without osseous bases; (2) branchial opening reduced or only moderately so; (3) mouth reduced; and (4) variable position of the anus. However, none of these characters supported Clade 3 in the present study. The gill rakers in most Gymnotiformes are unossified, except in some Rhabdolichops species that present elongated and osseous gill rakers, a clear derived condition within members of the genus (char. 70, state 1, see topic 3.2.4.2 for Rhabdolichops phylogenetic diagnosis). On the contrary, the size of the branchial opening did not present a conspicuous variation across the analyzed specimens. The mouth reduced, and the variable position of the anus was not utilized in this study. Reasons are discussed in Data S1 (U4 and U8).
The monophyly of Eigenmannia is obtained based on morphological characters for the first time herein. We studied over 80% of the valid species (21 of the 26 valid species—Table 1)—that represents the most comprehensive analysis yet done. The monophyly of Eigenmannia was recovered based on the four aforementioned synapomorphies (see Phylogenetic diagnosis of Eigenmannia), of which only one is unique for the genus, nervus opticus thicker than the nervus olfactorius. Mago-Leccia (1994) provided two diagnostic features for Eigenmannia: (1) five branchiostegal, two anterior slender, last three broad; (2) anterior intermusculars highly branched (Table S9). We evaluated the shape of each branchiostegal, and each intermuscular bone series independently. The shape branchiostegal were not recovered as a synapomorphy for Eigenmannia, but the presence of “epipleurals” at 7–9th vertebrae is recovered as a synapomorphy for the genus.
The two groups of species recognized by Peixoto and Ohara (2019) are recovered as monophyletic: E. humboldtii species group and E. trilineata species group. Also, E. macrops do not belong to any of them. Eigenmannia macrops was recovered as the sister group of the other congeners. Waltz and Albert (2017, 2018) included this species in the so-called Eigenmannia macrops species group, a suggestion not followed herein because it is not necessary to name a group to include a single species (Peixoto & Ohara, 2019). Waltz and Albert (2017) proposed Eigenmannia humboldtii species group (Clade E3) to include E. humboldtii, E. limbata, and E. nigra. It was diagnosed by the presence of distal margin of anal fin black, largest body sizes of the species of the genus (>45 cm TL), and by a relatively deep body in mature individuals (body depth >11% TL). Characters based on the total length are problematic, as the specimens are frequently damaged or regenerated in the posterior portion of the body, as discussed by Mago-Leccia et al. (1985; see Peixoto & Ohara, 2019 for additional comments on E. humboldtii species group). Thus, these characters were not included in the present study. Five synapomorphies recovered the monophyly of this group (clade E3): (1) number of premaxillary teeth, 38–54; (2) number of teeth on lower pharyngeal plate; (3) number of anal-fin rays, 226; (4) distal margin of anal fin dark (char. 110: 0>1); and (5) origin of the levator arcus palatini not including the frontal (char. 125: 1>0). This group is proposed as sister group of clade E5 (the E. trilineata species group). Recently, Waltz and Albert (2018) proposed Eigenmannia meeki as a member of the E. trilineata species group. Later on, Peixoto and Ohara (2019) removed E. meeki from this group due to the absence of the superior midlateral stripe. In the present study, we expanded the definition of Eigenmannia trilineata species group to include E. meeki, as proposed by Waltz and Albert (2018). This group, (Clade E5), is now defined by the presence of lateral line stripe (char. 106: 0>1). This character is absent in E. virescens; however, based on our comprehensive analysis, we considered the absence of this character in this species as a reversion of the condition present in Clade E5.
The monophyly of Clade 4 (Archolaemus + Distocyclus + Rhinosternarchus), or the close relationship between Archolaemus and Distocyclus—considering that Rhinosternarchus was not included in most previous studies—was proposed by Triques (1993), Maldonado-Ocampo (2011), Tagliacollo et al. (2016a), and Alda et al. (2019). Triques’ (1993) proposed the elongation of the cartilaginous palatine, and the sinuous anterior portion of fourth epibranchial as synapomorphies for this clade. Later, Clade 4 was additionally supported by Tagliacollo et al. (2016b) who provided six other synapomorphies: (1) preorbital length about one-third as long as total head length in mature specimens; (2) mesethmoid anterior portion horizontal in relation to ventral ethmoid; (3) fourth epibranchial with an elongate ascending process; (4) fifth epibranchial sinuous; (5) proximal surface of first DHS as broad as its descending blade; and (6) 200–299 anal-fin rays. The cartilaginous palatine is clearly more elongated in Archolaemus and Distocyclus than in the other members of Eigenmaniinae, however, considering the difficulties in establishing a precise way to evaluate this enlargement, this character was not included in the present analysis. Other elements responsible for the snout elongation have been coded as independent characters and some of them support these relationships. Characters on epibranchial shape (Tagliacollo et al., 2016b; Triques, 1993) and shape of first DHS (Tagliacollo et al., 2016b) were not included in the present analysis as discussed in Data S1 (U12, U14, and U21, respectively). The number of anal-fin rays was treated here as a quantitative character without discretization, which did not recover the relationships between Archolaemus and Distocyclus as sister groups, contrasting with the findings of Tagliacollo et al. (2016b).
Distocyclus was established to include the nominal species E. conirostris and E. goajira (Mago-Leccia, 1978). Albert and Fink (1996) recovered the close relationships between these species by the presence of a conical snout and a small nasal capsule (Table S10). Dutra et al. (2014), however, redefined both characters and restricted the genus to its type species, D. conirostris, not supporting Albert & Fink’ hypothesis. Meunier et al. (2014) described D. guchereauae and assigned it to Distocyclus based on the shape of the snout. However, the snout of this species differs in shape when compared with that of D. conirostris. Its snout is more triangular than conical as defined by Dutra et al. (2014). Distocyclus guchereauae also differs from D. conirostris by having more teeth on both jaws and the presence of endopterygoid teeth. In the present analysis, D. conirostris is the sister group of Archolaemus plus Rhinosternarchus, while D. guchereauae is the sister group of E. oradens and both members of a distal clade in Eigenmannia. Thus, D. guchereauae is allocated in Eigenmannia, and Distocyclus is again recognized as a monotypic genus.
In the present study, the nominal species “Eigenmannia” goajira was included in the analysis only using external characters and osteological features accessed via radiographs or available in the literature, representing 53.5% of the character list (77 of 144), and 46.5% missing data. In spite of that, as discussed by Dillman et al. (2015), the inclusion of taxa with large amount of missing data in morphological-based phylogenies has provided valuable results to recover the phylogenetic position of taxa with uncertain relationships. These authors, however, reinforced that additional analyses are necessary to determine the threshold of missing data that allows the recovery of a reasonable phylogenetic position. “Eigenmannia” goajira was recovered as the sister group of Archolaemus in the present analysis. Further, considering that Archolaemus have been historically diagnosed by the presence of a free orbital rim (e.g., Korringa, 1970; Vari et al., 2012—see Table S11), we opted to keep traditional characters used to recognize this genus and raise Rhinosternarchus to include “E.” goajira.
In the most recent revisionary study of Archolaemus, Vari et al. (2012) proposed four putative synapomorphies for the genus: (1) free orbital rim; (2) first row of teeth attached to premaxilla only by the anterobasal margin; (3) a pronounced gap between lower lip posterior margin and premaxilla anterior margin; and (4) upper lip ventral surface sponge-like with pores and fleshy raised papillae (Table S11). The presence of a free orbital rim was recovered as a synapomorphy for the genus in this study. The first row of mobile teeth mobile presented an ambiguous optimization due to the missing data in “E.” goajira. Other proposed characters were not included as discussed in the Data S1 (U2 and U3). The presence of the superior midlateral stripe was proposed as a synapomorphy for a more inclusive clade within Archolaemus by Vari et al. (2012); however, a more detailed examination of the type series of A. orientalis revealed the presence of a weak dusky to dark pigmentation on the same position. This character was recovered as a synapomorphy for the genus. The monophyly of clade A2 (Archolaemus except A. orientalis) was supported by 11 synapomorphies (see Data S3). Vari et al. (2012) proposed an unresolved trichotomy between A. santosi, A. blax plus A. janeae, and A. ferreirai plus A. luciae. In the present study, derived features define a series of successive sister groups (clades A2–A5) in which most of clades are supported by characters related to the elongation of snout. Vari et al. (2012) presented two putative synapomorphies to support the monophyly of A. janeae plus A. blax: (1) posterior ceratohyal approximately 1.5 times as long as ventral hypohyal; and (2) presence of a single row of teeth on the posterior portion of the dentary. The first character is also present in A. luciae and consequently represents a synapomorphy for clade A3 with a reversal in A. ferreirai. While the other character was treated as a quantitative character without discretization, it was not recovered as a synapomorphy for this clade. Thus, the hypothesis of monophyly of A. janeae plus A. blax was not corroborated; instead, A. janeae is proposed as sister group of A. blax + A. ferreirai + A. luciae. The presence of a coronomeckelian bone approximately 50% or more as long as Meckel's cartilage was presented as a synapomorphy for A. ferreirai plus A. luciae by Vari et al. (2012). This condition, however, is ambiguous due to the polymorphism in A. blax. The clade A5 (A. ferreirai + A. luciae) is supported herein by three additional synapomorphies (see Data S3).
Traditional morphological-based phylogenies on Neotropical fishes are mostly based on external anatomy and osteology (e.g., de Santana & Vari, 2010). Consequently, some anatomical complexes that are putative source of phylogenetic information, such as myology and neuroanatomy, are frequently overlooked [e.g., Mago-Leccia, 1978 (Sternopygidae); Triques, 1993 (Gymnotiformes); Buckup, 1998 (Characiformes); Birindelli, 2014 (Doradoidea—Siluriformes)]. Information on myology and neuroanatomy is available in Gymnotiformes literature (Aguilera, 1986; Albert, 2001; Albert et al., 1998; Dutra et al., 2015; de La Hoz & Chardon, 1984; Peixoto & Ohara, 2019) and then used in studies on the phylogenetic reconstruction of the group. Albert (2001) included 30 characters of sensorial and nervous complexes, representing approximately 12% of the total listed characters, being most of them also used by Tagliacollo et al. (2016b). On the contrary, characters derived from myology represent less than 0.2% of the entire universe explored of the morphological traits in cladistics studies in Gymnotiformes (Peixoto & Ohara, 2019). In the present study, 18 myological and 12 neuroanatomical characters were included, representing approximately 20% of the total of analyzed characters (12% and 8%, respectively). Informative characters in these anatomical complexes are represented by few intraspecific modifications, as discussed by Abrahão et al. (2018), and consequently, their contribution to the phylogenetic reconstruction are noticed in more inclusive taxonomic levels. For example, these characters represented six synapomorphies supporting the monophyly of Eigenmanniinae (five myological and one neuroanatomical).
Quantitative characters, such as number of anal-fin rays, have historically been employed in phylogenetic reconstructions of Gymnotiformes (e.g., Albert, 2001). However, in all previous studies, this information was treated through a discretization of range of variation, representing less than 0.5% of the entire universe explored in recent contributions (Albert, 2001; Tagliacollo et al., 2016a, 2016b). It is important to note that characters that were used as a diagnostic features within Eigenmanniinae species, such as the number of premaxilla and dentary teeth (e.g., Peixoto et al., 2015) were never addressed in a phylogenetic context. Consequently, information on quantitative characters was restricted to shallower clades. For instance, the variation in the number of precaudal vertebrae, which is historically recovered as a synapomorphy for Eigenmanniinae (Table S1), has never been properly explored to resolve relationships at the species level. In the present study, 17 quantitative characters without discretization were included for the first time in a phylogenetic study of Gymnotiformes, representing approximately 12% of the total of analyzed characters. As a result, quantitative characters have proven to be a valuable source of information for resolving relationships in all levels of inclusiveness. For comparison, the analysis excluding quantitative characters resulted in 310 most parsimonious trees [344 score, CI of 0.352 and RI of 0.707 (Figure S1)]. Where Eigenmannia's monophyly is not recovered and most relationships within Clade 2 are unsolved, except for the hypothesis of Rhinosternarchus as Archolaemus's sister group. Ferrer et al. (2014) found a similar scenario, in which analysis including quantitative characters without discretization resulted in a better-resolved topology.
Thus, the sum of poorly explored anatomical complexes (myology and neuroanatomy) and quantitative characters without discretization associated with morphological characters (external anatomy and osteology) traditionally employed in phylogenetic analyses resulted in a set of informative characters at all taxonomic levels within Eigenmanniinae, resulting in a robust hypothesis presented herein. Also, it provides a solid basis for future studies involving taxonomy and evolution of the group.
5 EXAMINED MATERIAL
5.1 Apteronotus albifrons
MCZ 52011, 1CS, 112.0 mm TL, Igarapé Paracuri near Icoaraci, Brazil. MPEG 2434, 2+1CS, Rio Goiapi, Marajó Island, Brazil. MPEG 23606, 1, 103.1 mm LEA, Igarapé Motosserra, tributary of Rio Cateté, Brazil. MPEG 23475, 2, 60.5–67.7 mm LEA, Rio Itacaiucas, Brazil. MZUSP 22251, 1MS of 28, 150.1 mm LEA, Rio Capim, Brazil. MZUSP 89044, 1MS of 3, 75.8 mm LEA, Rio Araguaia, Brazil.
5.2 Sternarchorhamphus muelleri
MPEG 1317, 7+1CS, Rio Pará, Brazil. MPEG 2107, 2, 57.4–61.3 mm, Rio Negro, Brazil. MPEG 3712, 2MS of 7, 335.1–335.4 mm LEA, Rio Goiapi, Ilha do Marajó, Brazil. USNM 228807, 7CS of 11, 162.0–326.0 mm TL, La Providencia, Venezuela. USNM 373030, 1MS of 5, 222.2 mm LEA, Rio Negro, Brazil.
5.3 Gymnotus aff. carapo
MPEG 2431, 22+1CS, 118.4–275.0 mm LEA, Rio Goiapi, Ilha do Marajó, Brazil. MPEG 3012, 1MS of 4, 232.2 mm LEA, Rio Turiaçu, Brazil. MZUSP 90618, 1MS of 4, 177.8 mm LEA, Córrego do Campo Bonito, São Paulo, Brazil. USNM 233582, 4CS, 107.0–138.0 mm TL, Venezuela. USNM 233853, 3CS, 56.0–83.0 mm TL, Venezuela.
5.4 Gymnotus coropinae
MPEG 21510, 1MS of 6, 112.5 mm LEA, Rio Araticum, Brazil. MPEG 25973, 2+2CS, 71.7–94.7 mm LEA, Igarapé Murum, Amazonas, Brazil. MZUSP 80142, 1MS of 32, 137.0 mm LEA, Rio Tiquié, Brazil.
5.5 Brachyhypopomus brevirostris
MPEG 980, 20+3CS, 58.5–131.1 mm LEA, Rio Tefé, Brazil. MPEG 2397, 2MS of 8, 65.9–71.2 mm LEA, Rio Apeú, Rio Amazonas basin, Brazil. MPEG 7295, 2MS of 6, 50.0–61.3 mm LEA, Igarapé Paraquequara, Rio Capim basin, Brazil. MZUSP 30047, 1MS of 12, 144.2 mm LEA, Rio Tefé, Brazil.
5.6 Microsternarchus bilineatus
MPEG 12757, 1MS of 3, 69.5 mm LEA, Rio Tapajós, Brazil. MPEG 17815, 45+3CS, 40.5–75.3 mm LEA, Igarapé Taiassuí, Guamá basin, Brazil.
5.7 Archolaemus blax
INPA 6424, 20+4CS, 118–270 mm TL, Rio Tocantins above Tucuruí Dam, Pará, Brazil. MNRJ 12158, 18+4CS of 93, 90.0–382.0 mm TL, Rio Bezerra, Rio Tocantins basin, Brazil. MZUSP 54080, 1CT of 3, 187.0 mm LEA, Serra da Mesa, Brazil. MZUSP 89304, 1MS, 101.5 mm LEA, Rio Tiquié, Brazil. MZUSP 89393, 1, 132.7 mm LEA, Rio Crixás-Açu, Brazil.
5.8 Archolaemus ferreirai
INPA 6422, 8+4CS paratypes, 131.0–269.0 mm TL; INPA 6496, 1MS de 11, 128.5 mm LEA; INPA 36379, 20+1CS paratype, 119.0–342.0 mm TL, Rio Mucajaí, Rio Branco basin, Brazil.
5.9 Archolaemus janeae
INPA 36380, 14+2CS paratypes, 136.0–225.0 mm TL, Rio Iriri, Rio Xingu basin, Brazil. MZUSP 97383, 1CS (178.8 mm LEAS) +1MS (171.0 mm LEA) of 10, Rio Jamaxim, Rio Tapajós basin, Brazil.
5.10 Archolaemus luciae
INPA 20964, 8+4CS paratypes, 106.0–200.0 mm TL, Rio Trombetas, Brazil. MPEG 23607, 1MS, 220.5 mm LEA, Rio Jamaxim, Rio Tapajós basin, Brazil.
5.11 Archolaemus orientalis
MPEG 21508, holotype, 156.0 mm TL; FMNH 94418, 1CS of 3 paratype; MPEG 21509, 1MS, 150.0 mm TL, Rio Paracatu, Rio São Francisco basin, Brazil.
5.12 Archolaemus santosi
INPA 36382, 6+3CS paratypes, 73.0–212.0 mm TL, Rio Jamari, Rio Madeira basin, Brazil. LIRP 13010, 1MS of 4, 171.5 mm LEA, Cachoeira de São Félix, Rio Madeira basin, Brazil.
5.13 Distocyclus conirostris
INPA 11482, 7+3CS of 32, 100.2–197.0 mm LEA, Rio Purus, Brazil. INPA 28879, 2CS of 19, 108.7–165.0 mm LEA, Rio Negro, Brazil. INPA 28915, 2CS of 11, 108.1–130.5 mm LEA, Rio Negro, Brazil. INPA 34018, 8+1CS, 132.0–174.5 mm LEA, Praia Grande above community of Carapanã, Rio Purus basin, Brazil. MPEG 20023, 2+1CS, 120.9–196.4 mm LEA, Rio Arari, Ilha do Marajó, Brazil. MPEG 20024, 2+1CS, 149.1–181.0 mm LEA, Rio Arari, Ilha do Marajó, Brazil. MZUSP 6551, 1CT of 4, Lago Manacapuru, Brazil. MZUSP 6982, 2+1CS, 156.2–166.0 mm LEA, Rio Madeira, Brazil. MZUSP 23316, 1MS of 3, 242.2 mm LEA, Rio Solimões, Amazonas, Brazil. MZUSP 124547, 2CS of 14, 113.4–1131.2 mm LEA, Rio Trombetas, Brazil. USNM 323935, 1xr, paratype, 187.8, Iquitos, Peru.
5.14 Eigenmannia antonioi
MPEG 10182, 12+3CS, 77.0–118.3 mm LEA, Rio Anapu at Floresta Nacional de Caxiuanã, Rio Amazonas basin, Brazil. MPEG 29487, 1MS of 11, 80.0 mm LEA, rio Anapu, Rio Amazonas basin, Brazil.
5.15 Eigenmannia besouro
MZUSP 57890, holotype, 91.9 mm LEA, Rio Grande, São Desidério, Brazil. MZUSP 83792, 6+1CS, paratypes, 55.8–68.8 mm LEA, Rio Preto, Brazil. MZUSP 98748, 1MS of 2, parátipo, 89.2 mm LEA, Rio São Francisco basin, Brazil. MZUSP 114281, 1 paratype, 80.6 mm LEA, Rio Veredinha, Brazil. MZUSP 119104, 5+1CS, paratypes, 69.6–106.1 mm LEA.
5.16 Eigenmannia correntes
MNRJ 46334, 6+1CS+1MS of 31 paratypes, 76.6–103.9 mm LEA; MNRJ 46335, 5 of 21 paratypes, 59.6–75.4 mm LEA, Córrego de Baixo, left margin tributary of rio Correntes.
5.17 Eigenmannia desantanai
NUP 3470, 11+2CS, 119.8–142.8 mm LEA, Rio Cuiabá, Rio Paraguai basin, Brazil. MZUSP 38169, 1MS of 4, 133.5 mm LEA, Rio Paraguai, Brazil.
5.18 Eigenmannia guairaca
LBP 9911, 1MS of 3, 107.4 mm TL, Rio Paraná basin, Brazil. NUP 6467, 8+2CS, 81.4–135.8 mm LEA, Riacho Água do Ó, upper Rio Paraná basin.
5.19 Eigenmannia guchereauae
MNHN 2003–0013, 1CS, 222.0 mm TL, Maroni drainage, French Guiana. MNHN 2003–0014, 1, 232.0 mm TL, Maroni drainage, French Guiana. MNHN 2003–0018, 2, 322.0–321.0 mm TL, Maroni drainage, French Guiana.
5.20 Eigenmannia humboldtii
FMNH 56812, 1MS of 7, 186.2 mm LEA, Puerto del Rico, Colombia. IAvH-P 6806, 1CS, 205.7 mm LEA, Rio Atrato, Colombia. IAvH-P 7415, 2, 240.9–270.1 mm LEA, Río Atrato, Colombia. IAvH-P 7822, 1, 312.0 mm LEA, Río Magdalena, Colombia. IAvH-P 7823, 1, 264.0 mm LEA, Río Magdalena, Colombia.
5.21 Eigenmannia limbata
MNRJ 1186, holotype, 324.0 mm LEA, Amazonas, Brazil. INPA 18281, 9+1CS, 160.3–219.0 mm LEA, Paraná Maiana, Amazonas, Brazil. INPA 28510, 2+1 CS, 222.2 mm LEA, Rio Caeté, tributary of Rio Purus, Brazil. MCP 28641, 1, Lago Pirapora, Acre, Brazil. MZUSP 75569, 1MS of 2, 160.0 mm LEA, Lago da Terra Preta, Rio Negro, Brazil. USNM 228861, 12+2CS, 133.6–271.9 mm LEA, Laguna on south side of Isla Isabela, Venezuela. USNM 305802, 10+2CS, 122.6–250.5 mm LEA, Río Matos, Beni, Bolivia.
5.22 Eigenmannia macrops
USNM 402672, 12+2 CS, 83.8–106.9 mm LEA; USNM 405266, 14+1CS+1MS, 6.5–16.6 mm LEA, Cuyuni River, Guyana.
5.23 Eigenmannia matintapereira
MZUSP 109618, 3+1CS, 79.7–143.6 mm LEA; MZUSP 109695, 5+1CS, 65.7–167.7 mm LEA, Rio Urubaxi, Rio Negro basin, Brazil. MZUSP 29979, 1MS, 113.0 mm LEA, Rio Negro, Brazil.
5.24 Eigenmannia meeki
USNM 293171, holotype, 235.7 mm LEA; MPEG 33912, 1+1 CS, 194.6–222.0 mm LEA; MZUSP 119018, 1+1 MS, 162.0–177.2 mm, Río Pucuro just above confluence with Río Tuíra, Panamá.
5.25 Eigenmannia microstoma
BMNH 1868.7.8.2–3, 2 syntypes, Lagoa Santa, Minas Gerais, Brazil. MCP 45216, 4+1CS+1MS, 57.7–91.6 mm LEA, Rio Pandeiros, tributary of Rio São Francisco, Brazil. MZUSP 24643, 1 (131.1 mm LEA) +1CS, Três Marias dam, Rio São Francisco basin, Brazil.
5.26 Eigenmannia muirapinima
MPEG 21777, 1+3CS, 84.6–98.5 mm LEA, Lago Jará, tributary of Rio Amazonas, Brazil. MPEG 29489, 11+2CS, 76.2–97.7 mm LEA; MZUSP 97577, 1MS of 48, 117.0 mm CFA, Rio Jamaxim, Brazil. MZUSP 116796, 1CT of 2 paratypes, 96.2 mm LEA, Igarapé Santo Antônio, tributary of Rio Amazonas, Brazil.
5.27 Eigenmannia nigra
AMNH 58642, 3 paratypes, Caño Urami, tributary of Rio Negro, Amazonas, Venezuela. ANSP 162130, 3 paratypes, 243.0–265.0 mm LEA, Río Casiquiare, Venezuela. CAS 54387, 3+1CS of 5, 139.2–166.9 mm LEA, Río Orinoco, Venezuela. INPA 9976, 3+1CS of 10, 149.7–223.1 mm LEA, Paraná Apara, Amazonas, Brazil. MPEG 2430, 1MS of 8, 154.1 mm LEA, Rio Goiapi, Ilha do Marajó, Brazil. MPEG 27121, 2MS of 5, 170.6–180.1 mm LEA, Rio Japurá, Brazil.
5.28 Eigenmannia oradens
ANSP 190768, holotype, xr, 121.6 mm LEA, Río Ventuari at Raudales Chipirito, Amazonas, Venezuela. ANSP 190912, 2xr, 62.6–101.1 mm LEA, Río Ventuari, Amazonas, Venezuela. ANSP 203212, 1, 76.7 mm LEA, Río Ventuari at Raudales Chipirito, Amazonas, Venezuela. MPEG 35287, 1+1CS, 94.7–111.1 mm LEA, Río Ventuari at Raudales Chipirito, Amazonas, Venezuela. MZUSP 122802, 1MS, 102.3 mm LEA, Río Ventuari at Raudales Chipirito, Amazonas, Venezuela.
5.29 Eigenmannia pavulagem
MPEG 9524, 3CS, 90.7–108.5 mm LEA, Igarapé Anuera-Grande, Rio Guamá basin, Brazil. MPEG 29490, 25+2CS, 26.2–176.6 mm LEA, Igarapé Paraquequara, Rio Guamá basin, Brazil. MPEG 7308, 1MS of 6, 90.9 mm LEA, Rio Capim, Brazil.
5.30 Eigenmannia sayona
MPEG 33926, 1MS paratype, 103.7 mm LEA; MZUSP 96497, holotype, 131.8 mm LEA, Río Parguaza, Cedeño, Venezuela; MZUSP 119711, paratypes, 6+2CS, 27.8–116.2 mm LEA, Rio Parguaza, Cedeño, Venezuela.
5.31 Eigenmannia trilineata
MZUSP 22616, 1CS, 142.9 mm LEA; MZUSP 111146, 1MS, 305.0 mm LEA, Río de La Plata, Argentina. UFRGS 5965, 7+3CS of 32, 75.1–115.0 mm LEA, Lagoa Negra, Rio Grande do Sul, Brazil.
5.32 Eigenmannia vicentespelaea
MZUSP 83461, 3+1CS, 108.0–164.5 mm LEA, Cave of São Vicente I, Rio Tocantins basin, Brazil. MZUSP 83463, 1CS, 118.9 mm LEA; MZUSP 83467, 1MS of 3, 115.9 mm LEA, Cave of São Vicente II, Rio Tocantins basin, Brazil.
5.33 Eigenmannia virescens
MCP 16797, 5+2CS, 143.1–184.0 mm LEA, Rio Ijuizinho, Rio Grande do Sul, Brazil. MZUSP 6319, 1MS of 2, 155.4 mm LEA, Rio Paraná, Paraná, Brazil.
5.34 Eigenmannia waiwai
INPA 37594, 31+2CS, 94.0–138.1 mm LEA, Rio Mapuera, Rio Trombetas basin, Brazil. INPA 37567, 3+1CS, 74.9–154.8 mm LEA, Cachoeira Porteira, Rio Trombetas, Brazil. MZUSP 15882, 1MS, 99.1 mm LEA, Rio Trombetas, Brazil.
5.35 Japigny kirschbaum
FMNH 50185, 3CS+1MS (137.2 mm LEA) of 16, Itabu Creek, New River drainage, head of Itabu Creek, Guyana. MZUSP 26517, 1CT, 98.7 mm LEA, New River, Guyana.
5.36 Rhabdolichops caviceps
INPA 20157, 7+2CS+1MS, 108.7–134.5 mm LEA, Paraná do Xiboquena, tributary of Rio Solimões, Brazil.
5.37 Rhabdolichops eastwardi
INPA 12361, 2CS of 41, Lago do Prato, Rio Negro, Amazonas, Brazil. MPEG 1189, 2CS, 115.1–127.8 mm LEA, Rio Goiapi, Ilha do Marajó, Brazil. MPEG 8148, 1MS of 5, 113.7 mm LEA, Rio Capim, Brazil. MZUSP 81178, 1MS, 188.3 mm LEA, Rio Tiquié, Brazil.
5.38 Rhabdolichops electrogrammus
INPA 28863, 7+2CS+1MS of 79, 96.8–101.5 mm LEA, Rio Negro, Brazil.
5.39 Rhabdolichops lundbergi
INPA 11406, 6+3CS+1MS of 111, 133.6–155.6 mm LEA, Rio Coari, tributary of Rio Solimões, Brazil. MZUSP 124441, 2CS (112.4–117.4 mm LEA) +1CT (145.0 mm LEA) of 8, Rio Amazonas, Brazil.
5.40 Rhabdolichops nigrimans
INPA 28862, 10+2CS+1MS, 97.3–132.0 mm LEA, Rio Negro, Brazil. MZUSP, 1CT, 125.2 mm LEA, Rio Negro, Brazil.
5.41 Rhabdolichops troscheli
MPEG 1174, 1, Rio Goiapi, Marajó island, Brazil. MPEG 2604, 9+2CS, 90.0–94.7 mm LEA, Rio Goiapi, Marajó island, Brazil. MPEG 2803, 1CS, 222.0 mm LEA, Rio Goiapi, Marajó island, Brazil. MPEG 8482, 1CS, 170.1 mm LEA, Tomé-Açu, Pará, Brazil. MZUSP 57704, 3CS (109.7–143.1 mm LEA) + 2MS (122.2–140.2 mm LEA) +1 CT (131.0 mm LEA) of 74, Rio Negro, Amazonas, Brazil.
5.42 Rhabdolichops zareti
ANSP 199233, 2+3CS of 79, 83.0–120.0 mm LEA, Rio Orinoco, Venezuela. CAS 57444, 1MS of 37, 88.9 mm LEA), Río Orinoco, La Providencia, Venezuela.
5.43 Rhinosternarchus goajira
USNM 121596, holotype of Eigenmannia goajira, xr, 377.0 mm LEA, Río Socuy, Venezuela. USNM 121596, 1xr, paratype of Eigenmannia goajira, 335.6 mm LEA, Río Socuy, Venezuela.
5.44 Sternopygus astrabes
INPA 30502, 2CS of 13, 79.0–98.0 mm LEA, Igarapé Tucumã, Parque Estadual do Rio Negro, Brazil. MZUSP 88795, 1MS of 2, 151.0 mm LEA, rio Preto da Eva, Amazonas, Brazil.
5.45 Sternopygus macrurus
INPA 4869, 4CS of 6, Paraná Janauacá, Lago Castanho, Amazonas, Brazil. INPA 16001, 1CS, Igarapé Mutum, Rio Urubu, Amazonas, Brazil. MZUSP 32215, 1CS (165.0 mm LEA) +1MS (212.6 mm LEA) +1CT (197.9 mm LEA) of 13, Rio Amapá, Brazil. USNM 394544, 1CS of 11, 161.1 mm LEA, Río Orinoco, Venezuela.
ACKNOWLEDGEMENTS
We thank B. Brown (AMNH); M. Sabaj and M. Arce (ANSP); J. Maclaine (BMNH); D. Catania (CAS); C. McMahan and S. Mochel (FMNH); C. Uribe (IAvH); L. Rapp Py-Daniel and R. de Oliveira (INPA); C. Lucena and Z. Lucena (MCP); K. Hartel (MCZ); P. Pruvost, A. Laurent, Z. Gabsi, and L. Duqye-Vélez (MNHN); M. Britto (MNRJ); W. Wosiacki (MPEG); C. Pavanelli (NUP); L. Malabarba (UFRGS); and L. Parenti (USNM) for the loan of specimens. I. Maschio (MPEG), S. Raredon, K. Murphy and J. Clayton (USNM), and O. Oyakawa and M. Gianetti (MZUSP) for help in locating study material and other assistance. The Laboratório Multiusuário de Processamento de Imagens de Microtomografia Computadorizada de Alta Resolução do Museu de Zoologia da Universidade de São Paulo assisted us with the generating and editing images of the Micro CT-Scan. This manuscript is part of the results of the Ph.D. Thesis of the first author, which was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for funding a visit to the Smithsonian Institution through the Programa Ciência sem Fronteiras (245310/2012-6). GMD and LAWP are supported by FAPESP (grant# 2018/09445-9 and 2018/05084-1). VPA is supported by CAPES (grant# 88887.318624/2019-00). This contribution was also supported by the Diversity and Evolution of Gymnotiformes Project (FAPESP/Smithsonian proc. 2016/19075-9). This paper is dedicated to the memory of Richard P. Vari.




