Putting European lampreys into perspective: A global-scale multilocus phylogeny with a proposal for a generic structure of the Petromyzontidae
Contributing authors: André Levy ([email protected]), Jasna Vukić ([email protected]), Radek Šanda ([email protected]), Boris A. Levin ([email protected], [email protected]) Jörg Freyhof ([email protected]), Matthias Geiger ([email protected]), Lukáš Choleva ([email protected]), Sara M. Francisco ([email protected]), Joana I. Robalo ([email protected])
Abstract
enPrevious studies on the phylogenetic relationships between lamprey species relied either on a low number of morphological characters related to the feeding apparatus, or on a low number of molecular mitochondrial DNA markers. Here, we apply a multilocus approach to assess the phylogenetic relationships of northern hemisphere lampreys, with a special emphasis on the 17 European species. The study comprises two mitochondrial (cytochrome c oxidase subunit 1 gene—DNA barcodes, and cytochrome b gene) and two nuclear (internal transcribed spacers I and II) markers to investigate species' phylogenetic affinities. The phylogeny obtained with mitochondrial markers revealed a clear and highly supported separation of all northern hemisphere lampreys. Among those, our multilocus results show several polyphyletic genera, stressing the need for a taxonomic revision in a near future. Lampetra morii (Berg, 1931) from East Asia, often included in Eudontomyzon, is placed in the genus Lethenteron. Lampetra richardsoni Vladykov & Follett, 1965 and Entosphenus hubbsi (Vladykov & Kott, 1976) should be placed in a new genus, as well as the southern populations of Lethenteron camtschaticum (Tilesius, 1811) and Lethenteron reissneri (Dybowski, 1869). Considering European species, our results argue for a taxonomic revision of Eudontomyzon, with emphasis on Eudontomyzon vladykovi Oliva & Zanandrea, 1959.
Resumen
esEstudios anteriores sobre las relaciones filogenéticas entre especies de lampreas, se basan en un escaso número de caracteres morfológicos relacionados con las estructuras de alimentación, o en un bajo número de marcadores moleculares de ADN mitocondrial. Aquí aplicamos un enfoque multilocus para evaluar las relaciones filogenéticas de las lampreas del hemisferio norte, con especial énfasis en 17 especies europeas. Este estudio comprende dos marcadores mitocondriales (citocromo oxidasa c subunidad 1 —ADN barcodes, y citocromo oxidasa b) y dos marcadores nucleares (espaciadores transcritos internos I y II (ITS) ) para investigar la afinidad filogenética de las especies. La filogenia obtenida con losmarcadores mitocondriales reveló una separación clara y altamente apoyada de todas las lampreas del hemisferio norte. Entre ellas, nuestros resultados multilocus muestran varios géneros polifiléticos, lo que resalta la necesidad de una revisión taxonómica en el futuro. Lampetra morii (Berg, 1931) de Asia oriental, a menudo incluida en Eudontomyzon, se sitúa en el género Lethenteron. Lampetra richardsoni Vladykov & Follett, 1965 y Entosphenus hubbsi (Vladykov & Kott, 1976) deben colocarse en un nuevo género, así como las poblaciones meridionales de Lethenteron camtschaticum (Tilesius, 1811) y Lethenteron reissneri (Dybowski, 1869). Considerando las especies europeas, nuestros resultados abogan por una revisión taxonómica de Eudontomyzon, con énfasis en Eudontomyzon vladykovi Oliva & Zanandrea, 1959.
1 INTRODUCTION
Lampreys are an ancient vertebrate group, with a general anatomy conserved for at least 360 million years (Gess et al., 2006). This group's life cycle includes a filter feeding larval phase that lasts for several years, while buried in freshwater sediment (e.g., Potter et al., 2015; Renaud, 2011). After this growth period, metamorphosis takes place and individuals acquire teeth on the oral disk, functional eyes and, in certain species, osmoregulatory ability, allowing them to enter marine waters. In some species, juveniles migrate to the sea or to a different freshwater body, and a trophic phase begins. The animals then feed by parasitism or predation for some time, while growing and sexually maturing. After this period, they migrate back to the rivers, where they spawn and end their semelparous life (e.g., Potter et al., 2015; Renaud, 2011). This life cycle is usually considered the ancestral state in lampreys (Docker, 2009). Other lampreys lack a trophic phase and spawning follows metamorphosis in the freshwater body where larval growth took place. These species are called non-trophic and, together with their ancestral trophic species, form species pairs or species complexes. Several genera include one trophic and one or several non-trophic species. In this situation, the non-trophic species are called satellite species (Vladykov, & Kott, 1979). When the non-trophic satellite species evolved recently, one expects a similar morphology between trophic and non-trophic species. This phenomenon has been observed in many lamprey genera (Potter et al., 2015). However, when divergence is old, the non-trophic species may present a morphology quite distinct from the ancestor, as the non-functional feeding apparatus could have degenerated (Docker, 2009). Therefore, traditional morphology-based phylogenetic analyses have only considered the relationships between trophic species, assuming these as proxies for their respective satellite ones (Gill et al., 2003).
Lampreys occupy wide geographic areas, displaying an anti-tropical distribution (Renaud, 2011). They are currently restricted to the regions above 20° northern and southern latitudes (Renaud, 2011). In the southern hemisphere, there are two families of lampreys: Geotriidae, with two predatory species (Riva-Rossi et al., 2020); and Mordaciidae, with three species, two of which are trophic. In the northern hemisphere, the family Petromyzontidae contains approximately 40 species, 15 of which with a trophic life cycle (Potter et al., 2015).
A morphology-based phylogeny of trophic lamprey species was published by Gill et al. (2003) using several characters: mouth structure, number and type of teeth and oral laminas, number and shape of velar tentacles, eye position, relative position of dorsal fins, and position of the cloaca relative to the dorsal fins. This work revealed a basal polytomy, failing to resolve the relationship between the families Geotriidae, Mordaciidae, and Petromyzontidae (Figure 1 left). The latter was shown to be monophyletic and its basal dichotomy revealed two sister groups, one grouping Ichthyomyzon and Petromyzon (both North American genera, but the anadromous Petromyzon also occurs in Europe) and the other one clustering the remaining northern hemisphere lamprey species. In the latter group, the most basal genus was Caspiomyzon (confined to the Caspian Sea), followed by Tetrapleurodon (a Mexican Pacific genus) and Entosphenus (a Pacific genus, present in both, North America and Asia). The remaining species belong to three distinct genera: Lampetra (a West Palearctic and North American genus), its sister genus Eudontomyzon (European with one Asian species), and Lethenteron (Europe, Arctic, and Asia).
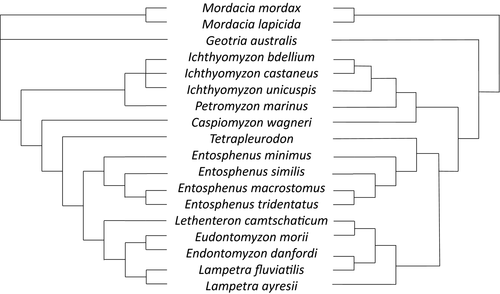
The phylogenetic relationships between the aforementioned trophic species were also assessed in molecular studies. Using the mitochondrial cytochrome b gene, Potter et al. (2015) revealed a somewhat different topology: the basal polytomy was resolved, with Mordaciidae as the most ancient family and sister to the Geotriidae + Petromyzontidae group (Figure 1 right); contrasting results were also found for within the group Lethenteron + Eudontomyzon + Lampetra, with the cytb phylogeny raising questions about the taxonomic validity/congruence of the genus Eudontomyzon and Lampetra.
The contrasting tree topologies obtained with morphological and molecular data may be related to methodological limitations. Morphological studies rely on few characters, mostly associated with the feeding apparatus (Docker et al., 1999), likely to reflect homoplasy. Lampreys are thus still a challenge for taxonomists and a good example for cryptic taxa exhibiting only few characters that can be measured or counted (no fin rays, no scales, and no ossified structures). Furthermore, lampreys show strong allometric growth after metamorphosis (Krappe, 2004) limiting morphometric studies to individuals in exactly the same phase of life. Krappe (2004) reports growth of the disk and shrinkage of the body and tail between 5%–15% after metamorphosis for different body proportions in Lampetra planeri . Therefore, the traditional taxonomy in lampreys is mainly based on their dentition and number of myomeres. On the other hand, molecular studies conducted so far used only mitochondrial genes (Potter et al., 2015). Hence, a comprehensive study with a broad database, including non-trophic species and additional molecular markers (with both nuclear and mitochondrial genes), is still lacking, despite its relevance in clarifying the phylogenetic relationships among the different families of lampreys.
Here, we apply a multilocus approach to assess the phylogenetic relationships of northern hemisphere lampreys, with a special emphasis on European species. This study comprises mitochondrial marker sequences (cytochrome c oxidase subunit 1 gene [COI]—DNA barcodes, and cytochrome b gene [cytb]) and nuclear markers (internal transcribed spacers I and II [ITS-1 and ITS-2]), the spacer DNA regions situated between the nuclear ribosomal RNA genes. A total of 17 European species were investigated. This is the first work to include mitochondrial and nuclear markers in a wide number of lamprey species with trophic and non-trophic lifecycles, shedding light into taxonomical/genetic discordances found in previous studies and providing insight into a fully comprehensive phylogeny of the group.
2 MATERIALS AND METHODS
DNA sequences were obtained from 37 individuals from 17 described and one putative undescribed European species (Barbieri et al., 2015) (Table 1). Lampreys were caught by electrofishing or collected by hand net following local laws and regulations. Specimens, mainly larvae, were attributed to each species based on their sampling location and preserved in ethanol 96º for molecular analyses. Whenever possible, samples were taken from individuals near the species-type locality. Given the diversity of type localities attributed to Eudontomyzon vladykovi, individuals from two sampling points were included in this study. Two samples of the Mexican Tetrapleurodon geminis , were also included for topological stability. Sample tissue was deposited in ISPA-IU Genetics laboratory.
| Taxon and distribution | Sampling point | Specimen voucher | GenBank Accession numbers | |||
|---|---|---|---|---|---|---|
| COI | Cyt b | ITS-1 | ITS-2 | |||
|
Lampetra fluviatilis (Linnaeus, 1758) EUR |
Neva River, Russia | LFLUST9 | MW917226 | MW925737 | MW937338 | |
| LFLUST25 | MW937304 | |||||
|
Lampetra planeri (Bloch, 1784) EUR |
Rhine River, Germany | LPLAOB10 | MW925739 | MW937339 | ||
| LPLAOB16 | MW925740 | MW937306 | ||||
| KM373659 gb | ||||||
| Lampetra auremensis Mateus, Alves, Quintella & Almeida, 2013 EUR | Ribeira do Olival, Portugal | LTJN35 | MW917223 | MW925734 | MW937298 | MW937331 |
| Lampetra alavariensis Mateus, Alves, Quintella & Almeida, 2013 EUR | Vouga River, Portugal | LPVG8 | MW917221 | MW925732 | MW937296 | MW937329 |
| LPVG9 | MW917222 | MW925733 | MW937297 | MW937330 | ||
| Lampetra lusitanica Mateus, Alves, Quintella & Almeida, 2013EUR | Sado River, Portugal | LPSAD12 | MW917227 | MW925738 | ||
| LPSAD2 | MW937305 | MW937338 | ||||
| Lampetra zanandreai (Vladykov, 1955)EUR | Po River, Italy | LAMPZ25 | MW925730 | MW937295 | ||
| LAMPZ26 | MW925731 | |||||
| KJ554015 gb | ||||||
| Lampetra soljani Tutman, Freyhof, Dulčić, Glamuzina & Geiger, 2017EUR | Krupa River, Bosnia and Herzegovina | EUKR1 | MW925741 | MW937326 | ||
| EUKR2 | MW925742 | MW937294 | MW937327 | |||
| EUKR3 | MW925743 | MW937328 | ||||
| KJ554074gb, KJ553990gb, KJ553874gb, KJ553819gb, KJ553778gb, KJ553756gb, KJ553665gb | ||||||
| Lampetra lanceolata Kux & Steiner, 1972 EUR | Iyidere River, Turkey | LLANC1 | MW917211 | MW925720 | MW937316 | |
| LLANC2 | MW917212 | MW925721 | MW937286 | MW937317 | ||
| Petromyzon marinus Linnaeus, 1758 EUR; ENA | Guadiana River, Portugal | PMG1 | MW937307 | MW937340 | ||
| NC_001626 gb | NC_001626 gb | |||||
| Lampetra ninae (Naseka, Tuniyev & Renaud, 2009) EUR | Kakhatskali River, Georgia | LENI3 | MW917224 | MW925735 | MW937301 | MW937334 |
| LENI4 | MW917225 | MW925736 | MW937302 | MW937335 | ||
| Tskaltsminda Creek, Georgia | LENI5 | MW937303 | MW937336 | |||
| LENI1 | MW937299 | MW937332 | ||||
| LENI2 | MW937300 | MW937333 | ||||
| Eudontomyzon danfordi Regan, 1911EUR | Mureş River, Romania | EUDA10 | MW917206 | MW925714 | MW937282 | MW937311 |
| EUDA8 | MW925715 | |||||
| Eudontomyzon mariae (Berg, 1931) EUR | Don River, Russia | EUMA33 | MW917213 | MW925722 | MW937287 | MW937318 |
| EUMA34 | MW917214 | MW925723 | MW937288 | MW937319 | ||
| Eudontomyzon vladykovi Oliva & Zanandrea, 1959 EUR | Sava River, Danube, Croatia | EUVL4 | MW917220 | MW925729 | MW937293 | MW937325 |
| Eudontomyzon vladykovi Oliva & Zanandrea, 1959 EUR | Drava River, Croatia | EUVL2 | MW917219 | MW925728 | MW937292 | MW937324 |
| Eudontomyzon stankokaramani Karaman, 1974 EUR | Ohrid Lake, Macedonia | EUST1 | MW917217 | MW925726 | MW937291 | MW937322 |
| EUST2 | MW917218 | MW925727 | MW937323 | |||
| Eudontomyzon sp. Almopaios EUR | Aridaios River, Greece | EU1 | MW917215 | MW925724 | MW937289 | MW937320 |
| EU2 | MW917216 | MW925725 | MW937290 | MW937321 | ||
| Caspiomyzon wagneri (Kessler, 1870) EUR; AS | Kura River, Azerbaijan | CWK1 | MW917204 | MW925712, MW925713 | MW937280, MW937281 | MW937309, MW937310 |
| CWK2 | MW917205 | |||||
| Caspiomyzon hellenicus (Vladykov, Renaud, Kott & Economidis, 1982) EUR | Prosotsani, Strymon drainage, Greece | EUHE1 | MW937315 | |||
| EUHE3 | MW917209 | MW925718 | MW937314 | |||
| EUHE4 | MW917210 | MW925719 | MW937285 | |||
| Caspiomyzon graecus (Renaud & Economidis, 2010) EUR | Kembi spring, Greece | EUGR3 | MW917207 | MW925716 | MW937283 | MW937312 |
| EUGR4 | MW917208 | MW925717 | MW937284 | MW937313 | ||
| Lethenteron camstchaticum (Tilesius, 1811)EUR; AS; ENA; WNA | KF701113gb, KC353468gb, KJ866208gb, KJ866209gb | KF701113gb, KC353468gb, KJ866208gb, KJ866209gb | ||||
| Tetrapleurodon geminis Álvarez, 1964 WNA | Celio River, Mexico | LMEX3827 | MW937308 | MW937341 | ||
| LMEX3826 | MW937342 | |||||
| JN028424gb, JN028425gb | GQ206187 gb | |||||
| Entosphenus hubbsi (Vladykov & Kott, 1976) WNA | HQ55730gb, JN025327gb | GQ206150 gb | ||||
| Entosphenus lethophagus(Hubbs, 1971) WNA | HQ579097gb, KX389870gb | GQ206153 gb | ||||
| Entosphenus similis Vladykov & Kott, 1979WNA | JN025330gb, JN025331gb | GQ206156 gb | ||||
| Entosphenus tridentatus(Richardson, 1836) WNA; AS | KF918874gb, KF929845gb | GQ206157gb, GU120749gb, GU120750gb, GU120753gb, GU120798gb, GU120810gb | ||||
| Ichthyomyzon bdellium(Jordan, 1885)ENA | JN026861gb, JN026859gb | GQ206166 gb | ||||
| Ichthyomyzon castaneus Girard, 1858ENA | EU524088gb, EU524087gb, JN026867gb | GQ206168 gb | ||||
| Ichthyomyzon fossor Reighard & Cummins, 1916 ENA | KM267716 gb | KM267716 gb | ||||
| Ichthyomyzon gagei Hubbs & Trautman, 1937ENA | JN026886gb, JN026888gb, HQ557147gb, JN026892gb, JN026884gb | GQ206169 gb | ||||
| Ichthyomyzon unicuspisHubbs & Trautman, 1937 ENA | KM267717 gb | KM267717 gb | ||||
| Ichthyomyzon greeleyi Hubbs & Trautman, 1937 ENA | JN026897gb, HQ557146gb | GQ206167 gb | ||||
| Lampetra ayresii(Günther, 1870) ENA | JQ354155 gb | GQ206174gb, GU120807gb, GU120867gb | ||||
| Lampetra aepypyera (Abbott, 1860)ENA | KP742974 gb | KP742974 gb | ||||
| Lampetra richardsoniVladykov & Follett, 1965 WNA | JN026960 gb | GQ206177gb, GU120728gb, GU120758gb, GU120802gb | ||||
| Lethenteron kessleri(Anikin, 1905)AS | AB198748gb–AB198752gb, JN027078gb | GQ206183gb, KJ684774gb, KJ684724gb–KJ684731gb | ||||
| Lethenteron alaskenseVladykov & Kott, 1978 WNA | JN027060gb–JN027062gb | GQ206178 gb | ||||
| Lethenteron appendix (DeKay, 1842) ENA | KM267719 gb | KM267719 gb | ||||
| Lethenteron reissneri(Dybowski, 1869) AS | KC353466gb, AB565771gb | KC353466gb, AB565771gb | ||||
| Eudontomyzon morii(Berg, 1931)AS | KM267718 gb | KM267718 gb | ||||
| Geotria australis Gray, 1851 AUS; SA | KT185629 gb | KT185629 gb | ||||
| Geotria macrostoma(Burmeister, 1868) SA | MT478622gb, MT478624gb, MT478625gb, MT478632gb, MT478636gb, MT478642gb–MT478644gb | MT478645gb–MT478652gb | ||||
| Mordacia praecoxPotter, 1968 AUS | KJ669535 gb | GQ206186 gb | ||||
| Mordacia lapicida(Gray, 1851) SA | JN027251 gb | GQ206185 gb | ||||
Total DNA was obtained using REDExtract-N-Amp kit (Sigma-Aldrich) following the manufacturer's instructions. Amplifications were conducted in 20 µl total-reaction volume with 10 µl of REDExtract-N-ampl PCR reaction mix (Sigma–Aldrich), 0.8 µl of each primer (10 µM), 4.4 µl of sigma-water, and 4 µl of template DNA. COI was amplified using the primers COI-F1(5′-TCA ACC ACC CAC AAA GAC ATT GGC AC-3′ (Ivanova et al., 2007) and COI-2R (5′-ACT TCA GGG TGA CCG AAG AAT CAG AA-3′; adapted from Ward et al., 2005). The amplicon size was 698 bp (alignment size 604 bp). Cyt b was amplified using the primers Cytb494L (3′-AGC CTT CTC TTC AGT TAT ACA CAT TTG TCG-3′) and Phe1612H (5′-CTT CAG TGC TCT GCT TTA ATG-3′) (Lang et al., 2009). The amplicon size was 1187 bp (alignment size 894 bp). ITS-1 was amplified using the primers Kp2 (5′-AAA AAG CTT CCG TAG GTG AAC CTG CG-3′) and 5.8S (5′-AGC TTG CTG CGT TCT TCA TCG A-3′; Phillips et al., 1995). The amplicon size was 433 bp (alignment size 340 bp). ITS-2 was amplified using the primers 5.8SR (5′-CTA CGC CTG TCT GAG TGT C-3′) and 28S (5′-ATA TGC TTA AAT TCA GCG GG-3′) (Phillips et al., 1995). The amplicon size was 393 bp (alignment size 293 bp). PCR followed usual conditions, with 55°C as annealing temperature for all fragments. Sequencing and purification were performed in Macrogen Europe.
Chromatograms were inspected and edited using CodonCode Aligner (CodonCode Corporation, www.codoncode.com). GenBank accession numbers of obtained sequences (from MW917204 to MW917227, MW925712 to MW925743, MW937280 to MW937308, and MW937309 to MW937342) are listed in Table 1 and alignments presented in supplementary material. Whenever published sequences were available, they were added to the dataset and used in the analyses (Table 1—a total of 65 COI sequences available from 28 species and 55 cyt b sequences available from 25 species).
The mitochondrial database was extended by including cytb and COI sequences for 23 additional taxa available in GenBank: Entosphenus hubbsi, Entosphenus lethophagus, Entosphenus similis, Entosphenus tridentatus, Eudontomyzon morii, Geotria australis, Geotria macrostoma, Ichthyomyzon bdellium, Ichthyomyzon castanaeus, Ichthyomyzon fossor, Ichthyomyzon gagei, Ichthyomyzon unicuspis, Ichthyomyzon greeleyi, Lampetra ayresii, Lampetra aepypyera, Lampetra richardsoni, Lethenteron camtschaticum, Lethenteron kessleri, Lethenteron alaskense, Lethenteron appendix, Lethenteron reissneri, Mordacia praecox, and Mordacia lapicida ( see Table 1 for details). Sequences were selected using the following criteria: when mitogenomes were available, their cytb and COI were selected from the same individual; when mitogenomes were not available, only longer sequences (>500 bp) were used; when several longer sequences were available, all haplotypes were used; sequences with ambiguities were discarded whenever other sequences were available. We concatenated sequences from the same individual, whenever possible.
Maximum Composite Likelihood genetic distances between sequences and net distances between taxa were estimated using Mega v.7 (Kumar et al., 2016). Phylogenetic analyses were performed on all markers independently (see Alignments S1–S3 for European species and S4 and S5 for all lamprey species), on a mitochondrial alignment (concatenating cytb and COI—see alignments S6 for European species and S7 for all lamprey species), and on a full concatenation of all markers (Alignment S8 for European species). Given their physical proximity, ITS-1 and ITS-2 were considered a single marker. Maximum parsimony-based (MP) phylogenetic relationships were estimated using PAUP (Swofford, 2001), with 100 heuristic searches using random additions of sequences and implementing the TBR algorithm. Branch support values for each node were tested by bootstrap analysis, with 1000 resamplings (Felsenstein, 1985). Maximum likelihood (ML) phylogenetic trees were inferred using RAxML BlackBox (Stamatakis et al., 2008). Branch support was tested by 100 rapid bootstrap inferences. Bayesian analysis (BA) was performed using MrBayes 3.2.6 (Ronquist et al., 2012) with two independent runs of four simultaneous Markov Monte Carlo chains (MCMC) for four million generations (sampling every 100 generations). Separate partitions were used for each mitochondrial protein-coding gene, while also partitioning the first and second codon positions from third codon positions, each with independent GTR models.
In addition, we also estimated the phylogeny of European species and their divergence times using BEAST v. 1.8.4 (Drummond et al., 2012), with two separate site and clock models, one for both mitochondrial and another for both nuclear markers. A lognormal relaxed clock was assumed for both regions. We applied an a priori rate of 0.01 substitutions/site/MY for the mitochondrial markers, as previously used for lampreys by Docker et al. (1999) and consistent with Kuraku and Kuratani (2006), while clock rates for the nuclear markers were left with a uniform prior, due to the lack of any suitable rate estimate. We used a Yule speciation prior for both partitions and ran four independent analyses for one billion generations, logging parameters every 10,000 generations. We used a burn-in of 10%, combined the four analyses using LogCombiner v.1.10.4. (Drummond et al., 2012), and checked for convergence of all parameters by inspecting Effective Sample Size (ESS) values, using Tracer v.1.7.2. Convergence was considered acceptable when ESS values were >300. The maximum clade credibility tree was constructed with TreeAnnotator v.1.10.4 (Drummond et al., 2012).
3 RESULTS
The phylogeny obtained using the mitochondrial dataset (895 bp cytb + 604 bp COI) revealed a clear separation of northern hemisphere lampreys from southern ones with high support (Figure 2 and Figures S1 and S2). Northern hemisphere lampreys formed two basal clades. The first comprised Caspiomyzon wagneri and its two Greek satellite species, Caspiomyzon graecus and Caspiomyzon hellenicus, together with their sister group Petromyzon + Ichthyomyzon (T clade in Figure 2). In the second basal clade, neither Lethenteron, Lampetra nor Eudontomyzon were found to be monophyletic. The representatives of the genus Lethenteron appeared in two subclades (clades U and V in Figure 2), and the species Le. camtschaticum in both: one clustering most of the species of this genus (V clade in Figure 2) and a second in a basal position (U clade in Figure 2). The representatives of the genus Lampetra appeared in four clades, one of them comprising all European Lampetra species (Z clade in Figure 2). Lampetra richardsoni and La. ayresii and La. aepytera were excluded from this clade and recovered as basal to subclades comprising the majority of Eudontomyzon and Lampetra species. All Eudontomyzon species clustered in a monophyletic group X clade in Figure 2, except Eu. morii, which clusters with Lethenteron and Lampetra of the V clade in Figure 2.
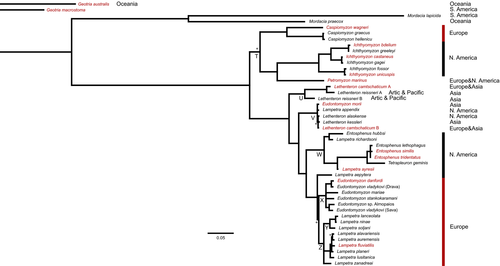
The phylogenetic relationships among European lamprey species inferred with the concatenated dataset (2151 bp long, from COI, cytb, ITS-1, and ITS-2) also recovered well-supported subclades for Eudontomyzon and Lampetra (Figure 3). In contrast, C. graecus and C. hellenicus did not cluster with C. wagneri, and Petromyzon marinus had a different position from the mitochondrial-based tree (cf. Figures 2 and 3).
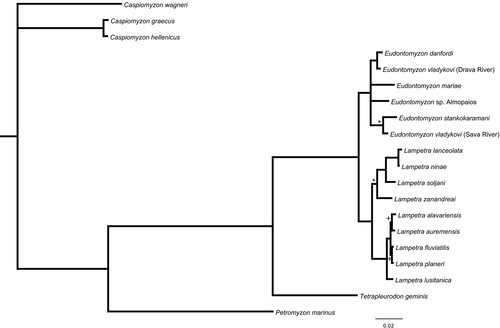
Within Lampetra clade, the different analyses yielded a well-supported subclade including Lampetra zanandreai, Lampetra lanceolata, Lampetra soljani, and Lampetra ninae. This subclade is grouped with the other European Lampetra, when using both the complete and the mitochondrial datasets (Figures 2 and 3), but not with the concatenated nuclear markers alone (Figures S3–S5). The genetic distances between these clades are presented in Table 2.
| Eudontomyzon | “Strict” European Lampetraa | Lampetra zanandreai + Lampetra lanceolata + Lampetra ninae | “Broad” European Lampetrab | Caspiomyzon hellenicus + C. graecus | |
|---|---|---|---|---|---|
| Eudontomyzon |
1.144 ± 0.301% 2.522 ± 0.400% 0.0814 ± 0.075% 0.1936 ± 0.1794% |
||||
| “Strict” European Lampetraa |
2.145 ± 0.757% 3.067 ± 0.571% 0.666 ± 0.494% 0.10820 ± 0.608% |
0.359 ± 0.185% 0.680 ± 0.172% 0.001 ± 0.007% 0.162 ± 0.152% |
|||
| Lampetra zanandreai + Lampetra lanceolata + Lampetra ninae |
1.758 ± 0.637% 2.531 ± 0.518% 0.384 ± 0.330% 0.428 ± 0.348% |
1.125 ± 0.459% 2.139 ± 0.442% 1.081 ± 0.657% 1.478 ± 0.704% |
0.826 ± 0.291% 2.245 ± 0.416% 0.724 ± 0.380% 0.000 ± 0.000% |
||
| “Broad” European Lampetrab |
1.618 ± 0.609% 2.234 ± 0.447% 0.241 ± 0.130% 0.404 ± 0.223% |
1.215 ± 0.369% 2.656 ± 0.413% 0.922 ± 0.393% 0.865 ± 0.455% |
|||
| Caspiomyzon hellenicus + C. graecus |
8.800 ± 2.657% 21.307 ± 2.822% 12.073 ± 55.999% 9.659 ± 3.834% |
9.764 ± 2.929% 23.55 ± 3.249% 12.85 ± 50.00% 11.15 ± 29.74% |
9.135 ± 2.792% 22.29 ± 3.107% 12.91 ± 52.52% 10.42 ± 29.74% |
9.081 ± 2.747% 22.40 ± 2.966% 12.58 ± 56.86% 10.04 ± 4.211% |
0.601 ± 0.377% 0.564 ± 0.246% 0.000 ± 0.000% 0.000 ± 0.000% |
- Estimates were calculated using Mega v.7. The “strict” European Lampetra clade includes Lampetra fluviatilis, Lampetra planeri, Lampetra alavariensis, Lampetra auremensis, and Lampetra lusitanica. The “broad” European Lampetra clade also includes Lampetra zanandreai, Lampetra lanceolata, and Lampetra ninae.
- a Includes La. fluviatilis, La. planeri, La. alavariensis, La. auremensis, and La. lusitanica.
- b Includes La. fluviatilis, La. planeri, La. alavariensis, La. auremensis, La. lusitanica, La. zanandreai, La. lanceolata, and La. ninae.
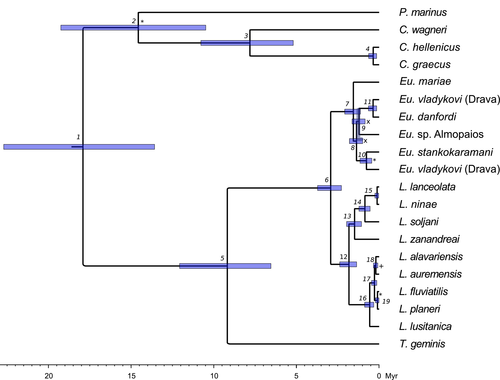
According to the calibrated species tree (Figure 4), Eudontomyzon and Lampetra separated from each other around three mya. The group comprising P. marinus, C. wagneri, and C. graecus and C. hellenicus separated from the rest of analyzed species around 16 mya. Separation of C. graecus and C. hellenicus from C. wagneri took place around 7 mya (Figure 4, Table S1). The mean estimated ITS clock was 0.0034 substitutions/site/MY (95% HPD interval of 2.22 × 10−3 to 4.73 × 10−3).
4 DISCUSSION
4.1 Inferences from the mitochondrial dataset
In this comprehensive lamprey phylogenetic study, the monophyly of northern hemisphere lampreys is clearly confirmed (Figure 2). One of our most striking results was the polyphyletic nature of four northern hemisphere genera of lampreys, resulting in the need for future taxonomic revision of Lethenteron, Entosphenus, Lampetra, and Eudontomyzon. The general topology of the phylogenetic tree resulting from the concatenated mitochondrial data is similar to that found by Lang et al. (2009) and Potter at al. (2015) using only the cytb gene. This was expected, as we used the same or similar sequences as used in those studies. Nevertheless, the inclusion of mitochondrial COI data provided better support for their conclusions and some differences concerning genus relationships.
The genus Lethenteron appeared in two well-separated clades. Lethenteron camtschaticum was polyphyletic when using the concatenated mitochondrial dataset (Figure 2). Considering that the type locality of this genus-type species (Le. appendix) is in USA, we argue that the taxa in clade V (northern populations of Le. camtschaticum, Le. appendix, Le. alaskense, Le. kessleri and “Eu.” morii) should be considered Lethenteron, while the southern populations of Le. camtschaticum and Le. reissneri (clade U) should be assigned to a new genus. In the work of Lang et al. (2009), Le. reissneri was shown to belong to Lethenteron. However, the samples included in that study were from the northern area of distribution of the species (in Russia), while those in our study (taken from complete mitochondrial genomes) are from the southern area (Japan). Lang et al. (2009) also included two undescribed Lethenteron: Le. sp. N, collected in Japan, grouped with other Lethenteron species; and Le. sp. S, sampled in South Korea, shown to belong to a new undescribed genus. The phylogenetic position of this new genus is similar to what was found in the present study for Le. camtschaticum and Le. reissneri, both from Japan. Both studies strongly suggest that Lethenteron is a northern genus, comprising species from Eastern and Western North America basins, Artic basins, and northwest Pacific drainages (including “Eu.” morii, which is here proposed to be a species of Lethenteron). Conversely, a new genus should be described to include southern Japan and South Korea lampreys.
Our mitochondrial results also revealed the polyphyletic nature of the genus Entosphenus: a clade comprising the type species En. tridentatus, En. lethophagus, and En. similis, and another clade, clustering En. hubbsi and La. richardsoni. These phylogenetic relationships had already been suggested by Lang et al. (2009) using only cytb. However, the two studies yielded distinct results concerning La. ayresii: in Lang et al. (2009), this species is sister to La. richardsoni, while in the present work, it is closer to the genus Entosphenus than to the La. richardsoni and En. hubbsi clade. Therefore, we suggest the description of a new genus encompassing En. hubbsi and La. richardsoni. The taxonomic clarification of La. ayresii requires further work using nuclear markers and / or genomic data. For the time being, we exclude La. richardsoni and also La. ayresii from Lampetra and encourage the description of a new genus for these species.
The present work corroborated additional conclusions of previous studies such as the suggestion of Lang et al. (2009) to only include the European species in the genus Lampetra, moving Lampetra richarsoni, La. ayresii, and Lampetra aepyptera to new genera. This later species, although close to European Lampetra, was already considered Okelbergia aepyptera by Lang et al. (2009).
4.2 Inferences from the extended dataset (mitochondrial + nuclear markers)
Concerning the West Paleartic species, the present phylogenetic analysis with the extended dataset revealed the monophyly of Eudontomyzon and Lampetra, clustering them as sister genera (Figure 3). A similar result was reported both by our mitochondrial data (Figure 2) and by Lang et al. (2009). The inclusion of La. ninae in the European Lampetra clade had very good support when combining all fragments (Figure 3), and it is in agreement with Li (2014) and Tutman et al. (2017).
The genus Lampetra probably originated in the Lower Pleistocene (2.38–1.36 My; node 12 in Figure 4 and Table S1), an epoch characterized by oscillations between glacial and interglacial conditions. In glacial periods, a migratory form of Lampetra might have colonized southern rivers, whose temperatures were suitable for breeding and reproduction. Although presently Lampetra fluviatilis is restricted to western Mediterranean basins (Freyhof, 2011), it could have been an abundant species in the Eastern Mediterranean and Black Sea in glacial periods, when the sea and river temperatures were lower. During interglacials, the distribution of the migratory Lampetra was probably restricted to northern locations, with more favorable temperatures, similar to present day conditions. Under such environmental conditions, the non-migratory southern populations, confined to freshwater systems, isolated from other populations and with smaller population sizes, could have diverged from their migratory ancestor by genetic drift. This process might have led to the formation of these relict species, confined to southern locations.
The sister–species relationship of C. hellenicus and C. graecus was previously reported by Lang et al. (2009) and is confirmed here with the mitochondrial and combined datasets (Figures 2 and 3). The combined mitochondrial and nuclear analysis did not confirm their cluster with C. wagneri, as suggested in previous studies and our extended mitochondrial analysis. However, as it does not contradict this relation, we support the two Greek species should be placed in Caspiomyzon.
Eudontomyzon is a European monophyletic genus and diverged from Lampetra in the upper Pliocene (between 3.5 and 2.3 My; node 6 in Table S1). It is characterized by the absence of an extant anadromous species: Eudontomyzon danfordi is the trophic species of this group, but is potamodromous (Talabishka et al., 2012). The colonization patterns for this genus, assuming the absence of any other anadromous species, could have followed the evolution of the main European freshwater bodies during the Plio-Pleistocene. Our multilocus approach reveals the existence of several groups within Eudontomyzon, which seem to correspond to geographic affinities: Eu. danfordi, the predatory species, is the sister species of Eu. vladykovi which is widespread in the Danube drainage; and Eudontomyzon stankokaramani, contrary to the results in Lang et al. (2009), does not occupy a basal position in this genus, but is instead the sister group of Eu. sp., which might be an undescribed species from the Sava river. These results argue for a taxonomic revision of this genus, with emphasis on Eu. vladykovi. Also the Greek Eudontomyzon sp. Almopaios might represent an undescribed species. Its inclusion in this genus has high support (Figures 2 and 3). However, the specific status of this population should be further analyzed, as divergence from its sister species/population is very low (0.45% for cytb and 0.8% for COI).
This study represents the most comprehensive work done so far on the phylogeny of European lampreys using mitochondrial and nuclear markers. Our results not only corroborate previous studies, they also raise several new pertinent questions and suggest taxonomic changes that should be addressed in the future. The inclusion of non-trophic species in this study enables us to clarify some taxonomic uncertainties, especially important when the divergence between them and their trophic species is old.
ACKNOWLEDGEMENTS
This study was funded by Fundação para a Ciencia e Tecnologia (FCT) Portugal, through the strategic projects MARE/UIDB/MAR/04292/2020 and MARE/UIDP/MAR/04292/2020 granted to MARE (MARE-ISPA), by a grant of Russian Foundation for Basic Research, no. 19-04-00719 (Boris Levin), by the Ministry of Culture of the Czech Republic (DKRVO 2019-2023/6.IV.c National Museum, 00023272) (Radek Šanda), by institutional resources of the Ministry of Education, Youth, and Sports of the Czech Republic (Jasna Vukić), by the Czech Academy of Sciences grant no. RVO 67985904 (Lukáš Choleva), and by the SYNTHESYS Project http://www.synthesys.info/ which is financed by European Community Research Infrastructure Action under the FP7 Integrating Activities Programme (CZ-TAF-5437). We want to thank Bella Japoshvili, Manos Koutrakis, Stamatis Zogaris, Spase Shumka, Nikola Hristovski, Antun Delić, and Ivan Bogut for their help in specimens’ collection and Karen Avellaneda for her help in spanish translation.




