Life on the edge: Tadpoles of Cycloramphidae (Amphibia; Anura), anatomy, systematics, functional morphology, and comments on the evolution of semiterrestrial tadpoles
Contributing authors: Florencia Vera Candioti ([email protected]), Ariadne Fares Sabbag ([email protected]), Gustavo Colaço ([email protected]), Hélio Ricardo da Silva ([email protected]), Célio F. Baptista Haddad ([email protected]), Ana Maria Paulino Telles de Carvalho-e-Silva ([email protected]), Taran Grant ([email protected])
Abstract
enThe evolutionary success of anurans can be partially explained by the occurrence of free-living larvae. Tadpoles occupy several distict habitats, including the terrestrial environment. Semiterrestriality appears to have evolved seven times in anurans, and tadpoles of distantly related lineages have converged in a set of phenotypic characters, such as a depressed body, ventral mouth, massive, well-keratinized and laterally compressed jaw sheaths, low fins, and well-developed hind limbs. The semiterrestrial tadpoles of the South American family Cycloramphidae remain poorly studied. In this work, we perform a comparative analysis of the external and internal morphology of these larvae, we comment on the systematic and evolutionary implications for the family, and finally, we discuss the convergent evolution of semiterrestrial tadpoles in anurans. We studied the external, buccopharyngeal, and musculoskeletal morphology of semiterrestrial tadpoles of 14 species of Cycloramphidae. These tadpoles are highly modified and present several character-states associated with semiterrestrial life. Most of them are unique and restricted to the family, such as the novel configuration of the muscles subarcualis rectus I, rectus abdominis, and levator arcuum branchialium III. We propose 13 new synapomorphies for Cycloramphidae and one for Thoropa. The presence of similar, homoplastic, character-states in all semiterrestrial tadpoles of unrelated phylogenetic lineages seems to suggest that these character-states are adaptations for semiterrestriality.
Resumo
ptO sucesso evolutivo dos anuros pode ser parcialmente explicado pela ocorrência de larvas de vida livre. Os girinos ocupam vários habitats distintos, incluindo o ambiente terrestre. A semiterrestrialidade parece ter evoluído sete vezes em anuros, e girinos de linhagens distantemente relacionadas apresentam uma série de caracteres fenotípicos convergentes, como corpo deprimido, boca ventral, lâminas mandibulares maciças, fortemente queratinizadas e comprimidas lateralmente, nadadeiras baixas e membros posteriores desenvolvidos. Entretanto, os girinos semiterrestres da família sul-americana Cycloramphidae permanecem pouco estudados. Neste estudo, fazemos uma análise comparativa da morfologia externa e interna dessas larvas, comentamos as implicações sistemáticas e evolutivas para a família e, por fim, discutimos a evolução convergente de girinos semiterrestres em anuros. Estudamos a morfologia externa, bucofaríngea e musculoesquelética de girinos semiterrestres de 14 espécies de Cycloramphidae. Esses girinos são altamente modificados e apresentam vários estados de caráter associados à vida semiterrestre. A maioria deles são estados únicos e restritos à família, como a nova configuração dos músculos subarcualis rectus I, rectus abdominis e levato arcuum branchialium III. Propomos 13 novas sinapomorfias para Cycloramphidae e uma para Thoropa. A presença de estados de caráter homoplásticos semelhantes em todos os girinos semiterrestres de linhagens filogenéticas não relacionadas parece sugerir que esses estados de caráter são adaptações para a semiterrestrialidade.
1 INTRODUCTION
Anura comprises more than 7,000 species widely distributed on all continents except in Antarctica and several oceanic islands (Frost, 2020). The evolutionary success of anurans can be partially explained by the occurrence of free-living larvae, commonly known as tadpoles (Altig, 2006; Roelants et al., 2011), which allows frogs to exploit niches in both terrestrial and aquatic environments, reducing intraspecific competition. Tadpoles occupy habitats ranging from temporary ponds to phytotelmata in the canopy, dozens of meters above the ground, and in many cases, phenotypic characters can be associated with these habitats (Altig & Johnston, 1989; Altig & McDiarmid, 1999). The wide variety of forms and environments occupied can be explained by the hypothesis that morphological characters were selected independently in each developmental stage (Harris, 1999). As extreme examples, one can cite neustonic tadpoles with umbelliform oral discs that feed on the water surface (e.g., Dias et al., 2018, 2021; Grosjean et al., 2011), and fossorial tadpoles with reduced eyes, unpigmented skin, and vermiform bodies (e.g., Rada et al., 2019). Tadpoles can even occupy terrestrial environments (e.g., Menin & Rodrigues, 2013). Although some authors have reported that some suctorial tadpoles, such as Ascaphus Stejneger, 1899 (Noble, 1927), Nasikabatrachus Biju & Bossuyt, 2003 (Zachariah et al., 2012), and Meristogenys Yang, 1991 (Gan et al., 2015), can be found out of the water for short periods, very few species persist unsubmerged throughout their development.
Semiterrestrial tadpoles spend most of their life out (or partially out) of water, inhabiting wet rock faces and rivulets (Altig & Johnston, 1989). Semiterrestriality appears to have evolved seven times in anurans, occurring in the following taxa (a) Cycloramphus Tschudi, 1838 and Thoropa Cope, 1865 (Cycloramphidae, South America; Bokermann, 1965; Caramaschi & Sazima, 1984; Cocroft & Heyer, 1988; Heyer, 1983a, 1983b; Heyer & Crombie, 1979; Lima et al., 2010; Moura et al., 2019; Nunes-de-Almeida et al., 2016; Silva & Ouvernay, 2012; Verdade et al., 2019), (b) Arthroleptides Nieden, 1911 and Petropedetes Reichenow, 1874 (Petropedetidae, Africa; Barej et al., 2010; Channing et al., 2002, 2012; Drewes et al., 1989; Lawson, 1993), (c) Nothophryne Poynton, 1963 (Pyxicephalidae, Africa; Bitencourt-Silva et al., 2016; Conradie et al., 2018; Poynton & Broadley, 1985), (d) Ptychadena broadleyi Stevens, 1972 and Ptychadena mutinondoensis Channing & Willems, 2018 (Ptychadenidae, Africa; Channing & Willems, 2018; Stevens, 1972), (e) Sclerophrys perreti (Schiøtz, 1963) (Bufonidae, Africa; Channing et al., 2012; Onadeko et al., 2014; Schiøtz, 1963), (f) Nannophrys ceylonensis Günther, 1869 (Dicroglossidae, Asia; Kirstinghe, 1958), and (g) Indirana Laurent, 1986 and Walkerana Dahanukar et al., 2016 (Ranixalidae, Asia; Annandale, 1918; Gopalan et al., 2012; Modak et al., 2018; Padhye et al., 2014). The tadpoles of these distantly related lineages convergently evolved a set of phenotypic characters, such as a depressed body, ventral mouth, massive, well-keratinized and laterally compressed jaw sheaths, low fins, and strong, muscular, well-developed hind limbs (Altig & Johnston, 1989).
The semiterrestrial tadpoles of the family Cycloramphidae remain poorly studied. The family comprises the genera Cycloramphus, Thoropa, and Zachaenus Cope, 1866, with 36 recognized species endemic to eastern Brazil (Frost, 2020). Its monophyly is well supported (but see Grant et al., 2017), but its phylogenetic placement among other hyloid lineages is highly ambiguous (e.g., Frost et al., 2006; Grant et al., 2006; Jetz & Pyron, 2018; Pyron & Wiens, 2011). Among the species with known biology, two distinct reproductive modes have been reported: the two species of Zachaenus and the eight species of the Cycloramphus bolitoglossus and Cycloramphus eleutherodactylus groups breed independently from running water and develop endotrophic tadpoles (Heyer & Crombie, 1979; Lutz, 1944); the remaining Cycloramphus (Cycloramphus fuliginosus, Cycloramphus granulosus, and Cycloramphus ohausi groups, ca. 20 spp.) and all known Thoropa (6 spp.) are associated with saxicolous environments (Bokermann, 1965; Heyer, 1983a, 1983b; Lutz, 1944; Wassersug & Heyer, 1983).
External larval morphology has been described—although not always accurately or with properly defined characters—for approximately half of the species of Cycloramphidae, including both species of Zachaenus (Almeida-Silva et al., 2019; Lutz, 1944) and 10 of the 28 species of Cycloramphus (Heyer, 1983a, 1983b; Heyer & Crombie, 1979; Lima et al., 2010; Nunes-de-Almeida et al., 2016; Silva & Ouvernay, 2012; Verdade et al., 2019). In Thoropa, assignation of tadpoles described is beyond doubt in four of the six species (Bokermann, 1965; Caramaschi & Sazima, 1984; Cocroft & Heyer, 1988; Moura et al., 2019). The description of Thoropa miliaris (Spix, 1824) tadpoles by Bokermann (1965) was based on 12 specimens of unspecified origin, except for the illustrated individual (WCAB 13307; Bokermann, 1965: 531) from Cubatão, São Paulo state. On the basis of morphometric and morphological analysis, Feio et al. (2006) revalidated Thoropa taophora for populations of T. miliaris from São Paulo state, which was corroborated by molecular evidence (Fitzpatrick et al., 2009; Sabbag et al., 2018). Consequently, Bokermann's description corresponds to T. taophora (Miranda-Ribeiro, 1923) and the tadpole of T. miliaris is undescribed (but see Barth, 1956; Fatorelli et al., 2018; Lutz, 1954). A summary of published tadpole descriptions is shown in Table 1.
| Species | External morphology | Buccopharyngeal morphology | Chondrocranium morphology | Muscles |
|---|---|---|---|---|
| Cycloramphus bandeirensis | Verdade et al. (2019) | |||
| Cycloramphus boraceiensis | Heyer (1983a), this study | This study | This study | This study |
| Cycloramphus brasiliensis | Heyer (1983a), this study | This study | This study | This study |
| Cycloramphus fuliginosus | Heyer (1983a) | This study | This study | This study |
| Cycloramphus izecksohni (as Cycloramphus duseni) | Heyer (1983a) | Wassersug and Heyer (1983, 1988) | Wassersug and Heyer (1983, 1988) | |
| Cycloramphus lithomimeticus | Silva and Ouvernay (2012), this study | This study | This study | This study |
| Cycloramphus lutzorum | Lima et al. (2010), this study | This study | This study | This study |
| Cycloramphus rhyakonastes | Nunes-de-Almeida et al. (2016) | |||
| Cycloramphus stejnegeri | Heyer and Crombie (1979), Heyer(1983a) | Wassersug and Heyer (1983, 1988) | Lavilla (1991) | |
| Cycloramphus valae | Heyer (1983b) | |||
| Thoropa lutzi | Bokermann (1965) | This study | This study | This study |
| Thoropa megatympanum | Caramaschi and Sazima (1984) | This study | This study | This study |
| Thoropa miliaris | This study | This study | This study | This study |
| Thoropa petropolitana | Bokermann (1965), this study | Wassersug and Heyer (1983, 1988) | This study | This study |
| Thoropa saxatilis | Cocroft and Heyer (1988), this study | This study | This study | This study |
| Thoropa taophora | Bokermann (1965), this study | This study | This study | This study |
| Zachaenus carvalhoi | Almeida-Silva et al. (2019) | |||
| Zachaenus parvulus | Lutz (1944) |
The buccopharyngeal cavity has been described for four cycloramphid species, including T. miliaris (partially; Barth, 1956), Thoropa petropolitana (Wandolleck, 1907), T. taophora (as T. miliaris; Wassersug & Heyer, 1983, 1988), and Cycloramphus izecksohni Heyer, 1983b (as Cycloramphus duseni; Wassersug & Heyer, 1983). The only chondrocranium known for the entire family is that of the endotrophic Cycloramphus stejnegeri (Noble, 1924) (Lavilla, 1991). Information on larval muscles is limited (Barth, 1956), and data on visceral elements are lacking for all species.
Given our limited knowledge, lack of accurate descriptions, poorly defined characters, and almost complete absence of information on internal morphology, a detailed review of Cycloramphidae larvae is most needed. In this study, we first describe the tadpole of T. miliaris from its type locality and then perform a comparative analysis of the external morphology, buccopharyngeal anatomy, and musculoskeletal morphology of cycloramphid larvae. Finally, we comment on the systematic and evolutionary implications for the family, consider the possible functional significance of apomorphic character-states, and assess the convergent evolution of semiterrestrial tadpoles in anurans.
2 MATERIAL AND METHODS
2.1 Specimens
Examined tadpoles of T. miliaris were from the type locality, Praia Vermelha, Urca, Rio de Janeiro Municipality (22°57ʹ14.4ʺS, 43°9ʹ44.3ʺW). In addition to being the only species of Thoropa known at that locality, two individuals were raised to metamorphosis to confirm species identity. The description is based on 11 tadpoles in Gosner (1960) Stages 35–36. Tadpoles were measured to 0.1 mm precision with a digital caliper, following Altig and McDiarmid (1999) and Altig (2007): total length (TL), body length (BL), tail length (TAL), body width (BW), body height (BH), tail height (TH), nostril to snout distance (NSD), eye to snout distance (ESD), interorbital distance (IOD), eye to nostril distance (END), internarial distance (IND), oral disc width (ODW) and eye diameter (ED).
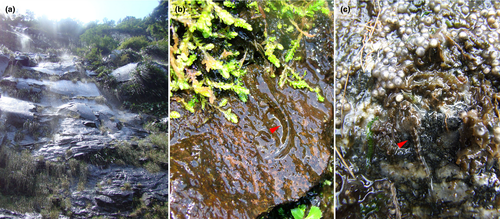
We also studied the external and internal larval morphology of other 13 cycloramphid species, namely, Thoropa lutzi Cochran, 1938, T. megatympanum Caramaschi & Sazima, 1984, T. petropolitana, T. saxatilis Cocrofr & Heyer, 1988, T. taophora, Cycloramphus boraceiensis Heyer, 1983a, C. brasiliensis (Steindachner, 1864), C. fuliginosus Tschudi, 1838, C. izecksohni Heyer, 1983b, C. lithomimeticus Silva & Ouvernay, 2012, C. lutzorum Heyer, 1983a, C. stejnegeri, and C. valae Heyer, 1983a. Figure 1 shows examples of common habitats of these tadpoles. Species assignment was based on comparison with the published tadpole descriptions and geographic distribution, given that species of Cycloramphidae are rarely syntopic (Frost, 2020; Sabbag et al., 2018). Additionally, we present behavioral observations on T. saxatilis based on fieldwork at Cachoeira das Pedras Brancas, Três Forquilhas, Rio Grande do Sul, Brazil (29º23.670ʹS, 50º02.551ʹW, 267 m).
To explore character evolution, we studied character-states in the closely related hyloid lineages Alsodidae, Batrachylidae, Ceratophryidae, Dendrobatoidea, Hylodidae, Rhinodermatidae, and Telmatobiidae, most of which (except Rhinodermatidae) possess aquatic tadpoles, in light of recent phylogenetic hypotheses (e.g., Grant et al., 2017; Jetz & Pyron, 2018); some data were taken from direct observation, but for most outgroup taxa character-states were obtained from the literature. For comparison, we also examined the semiterrestrial tadpoles of Arthroleptides martiensseni Nieden, 1911, Petropedetes perreti Amiet, 1973, and Petropedetes vulpiae Barej et al., 2010 (Petropedetidae) and Indirana beddomi (Günther, 1876) (Ranixalidae). The complete list of specimens examined is provided in Table 2; museum acronyms follow Sabaj (2016).
| Taxon | Collection | Locality | N | Stage | External morphology | Buccopharyngeal cavity | Muscles | Chondrocranium |
|---|---|---|---|---|---|---|---|---|
| Cycloramphidae | ||||||||
| Cycloramphus bandeirensis | Verdade et al. (2019) | |||||||
| Cycloramphus boraceiensis | CFBH 36944; RU-GIR 69; USNM 217933 | Angra dos Reis, RJ/XXX/Salesópolis, SP | 2, 8, 1 | 29–36, 34 | DIS | DIS | C&S | |
| Cycloramphus brasiliensis | RU-GIR 56 | Nova Friburgo, RJ | 5 | 30–32 | ✓ | DIS | DIS | C&S |
| Cycloramphus fuliginosus | CFBH 37886 | No information | 2 | 28 | ✓ | DIS | DIS | C&S |
| Cycloramphus izecksohni | Heyer (1983a) | |||||||
| Cycloramphus lithomimeticus | RU-GIR 73 | Itaguaé, RJ | 12 | 30–37 | ✓ | DIS | DIS | C&S |
| Cycloramphus lutzorum | LGE 10296 | Guaratuba, PR | 1 | |||||
| Cycloramphus stejnegeri | USNM 209370, | Teresópolis, RJ | 14 | 28–31 | ✓ | DIS | Lavilla (1991) | |
| Thoropa lutzi | MZUSP 79301 | Sumaré, RJ | 4 | 29 | ✓ | DIS | DIS | C&S |
| Thoropa megatympanum | UFMG 158 | Santana do Riacho, MG | 8 | 34–37 | ✓ | DIS; SEM | DIS; µCT | C&S; µCT |
| Thoropa miliaris | UNIRIO 4732 | Praia Vermelha, Urca, Rio de Janeiro, RJ | 20 | 34–37 | ✓ | DIS; SEM | DIS | C&S |
| Thoropa petropolitana | MZUSP 80015 | Petrópolis, RJ | 4 | 28–30 | ✓ | LIT | DIS | C&S |
| Thoropa taophora | CFBH 9016 | Picinguaba, Ubatuba, SP | 6 | 33–37 | ✓ | DIS; LIT | DIS | C&S |
| Thoropa saxatilis | MZUSP 64785 | Itambézinho, SC | 4 | 28 | ✓ | DIS | DIS | C&S |
| Other taxa | ||||||||
| Alsodes neuquensis | MACN 46544 | 2 | ✓ | Barrasso et al. (2016) | Barrasso et al. (2016) | Barrasso et al. (2016) | ||
| Arthroleptides martiensseni | USNM 316556 | Tanga, Tanzania | 2 | 40 | ✓ | |||
| Atelognathus patagonicus | Wassersug and Heyer (1988) | |||||||
| Batrachyla taeniata | KU 162051 | Lago Chapo, Llaquihue, Chile | 2 | 27–28 | ✓ | DIS | ||
| Ceratophrys cranwelli | ✓ | Vera Candioti (2007) | Vera Candioti (2007) | Vera Candioti (2007) | ||||
| Crossodactylus gaudichaudii | MNRJ 35073, 35087 | Floresta da Tijuca, Rio de Janeiro, RJ | 6 | 28–31 | ✓ | DIS | DIS | C&S |
| Eupsophus emiliopugini | Vera Candioti et al. (2011) | Vera Candioti et al. (2011) | Vera Candioti et al. (2011) | Vera Candioti et al. (2011) | ||||
| Hylodes phyllodes | MNRJ 35034 | Angra dos Reis, RJ | ✓ | DIS; SEM | DIS | C&S | ||
| Hylorina sylvatica | Cárdenas-Rojas et al. (2007) | Cárdenas-Rojas et al. (2007) | Cárdenas-Rojas et al. (2007) | Cárdenas-Rojas et al. (2007) | ||||
| Indirana beddomi | USNM 232370 | Kerala, India | 3 | 28, 31, 40 | ✓ | |||
| Insuetophrynus acarpicus | Rabanal and Formas (2009) | Rabanal and Formas (2009) | Rabanal and Formas (2009) | |||||
| Limnomedusa macroglossa | CFBH 40054 | 8 | 30–35 | ✓ | DIS; SEM | DIS | C&S | |
| Megaelosia goeldi | UNIRIO 2348 | Parnaso, Tersópolis, RJ | 2 | 25–26 | ✓ | DIS | DIS | C&S |
| Petropedetes perreti | ZMB 73738 | Mt. Manengouba, Cameroon | 8 | 38 | ✓ | DIS | DIS | C&S |
| Petropedetes vulpiae | ZMB 82846 | Ebo Forest, Cameroon | 2 | 37–38 | ✓ | |||
| Rheobates palmatus | ICN 9751, 55303, 55301 | Guayabetal, Colombia/ San Vicente de Chucurí, Colombia | 20 | 25–38 | ✓ | DIS; SEM | DIS | C&S |
| Rhinoderma darwinii | Lavilla (1987) | Wassersug and Heyer (1988) | Lavilla (1987) | |||||
| Telmatobius spp | Vera Candioti (2008) | Vera Candioti (2008) | Vera Candioti (2008) | |||||
Note
- Acronyms are in accordance with Sabaj (2016).
- Abbreviations: µCT, micro-computed tomography; C&S, clearing and staining; DIS, dissection; SEM, scanning electron microscopy.
2.2 Clearing and staining and dissection methods
After external morphology inspection, some specimens (see Table 2) were processed according to the clearing and double staining protocol of Dingerkus and Uhler (1977); the procedure was interrupted after the alcian blue step, and specimens were manually dissected for inspection of cranial muscles and the buccopharyngeal cavity (stained with methylene blue solution). After character description and illustration, the procedure was finished to study chondrocranial morphology.
2.3 Micro-CT
One tadpole of T. megatympanum was treated with Lugol's (I/I2) solution for 48 h, following Metscher (2009), washed in distilled water, and submitted to high-resolution micro-computed tomography (micro-CT) in 360° using a 40Kv X-ray beam in X-ray microscope Xradia 410 Versa by Carl Zeiss X-ray microscopy. Volumetric data were generated and explored in Amira 6.1 (Thermo Fischer Scientific).
2.4 Scanning electron microscopy
Some individuals were manually dissected according to Wassersug (1976) to expose the buccopharyngeal cavity and, following inspection using a stereoscopic microscope, submitted to the protocol of Alcalde and Blotto (2006) for scanning electron microscopy (SEM). We also applied SEM methods to the tail fins of two tadpoles of T. miliaris.
2.5 Histology
One whole tadpole of T. miliaris was decalcified (Dietrich & Fontaine, 1975), embedded in paraffin, and sectioned at 7–10 µm thickness. Serial slices were stained with toluidine blue and basic fuchsine and hematoxylin–eosin.
2.6 Terminology and character optimization
External morphology and oral disc terminology follow Altig and McDiarmid (1999) and Altig (2007), and that of the lateral line system is in accordance with Schlosser (2002). Terminology for the musculoskeletal system follows Haas (2003). Buccopharyngeal cavity terminology follows Wassersug (1976).
Transformation series (Grant & Kluge, 2004; Hennig, 1966) were recorded (see Data S1) and the character matrix (Data S2) was constructed in Mesquite V. 3.51 (Maddison & Maddison, 2018). Variable phenotypic characters were optimized on the phylogenetic hypothesis of Jetz and Pyron (2018), which is currently the most comprehensive hypothesis of anuran relationships in terms of the number of analyzed taxa; we also optimized our characters onto Sabbag et al.'s (2018) more detailed hypothesis of cycloramphid relationships for comparison (Figure S1). Parsimonious (Fitch, 1971) optimization was performed in TNT software (Goloboff & Catalano, 2016).
3 RESULTS
3.1 External morphology
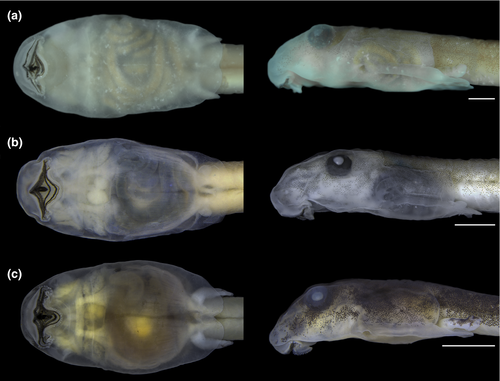
3.1.1 The tadpole of Thoropa miliaris (n = 11; Gosner Stages 35–36; Figures 2 and 3)
Body depressed (BW/BH = 1.3–2.0), elliptical in dorsal view, snout rounded (Figure 2a). Eyes dorsal, large (ED/BL = 0.08–0.2), directed anterolaterad; meniscus on upper iris present, rounded, covering part of the lens. Nares rounded, dorsal, directed anterolaterad; marginal rim present but fleshy projection absent. Nares closer to eyes than snout (NSD/END = 1.2–2.2). Internarial distance 50–75% of interorbital distance. Spiracle sinistral, tubular, lateral, ventral to midline of body, directed posterolaterad in dorsal view, dorsad at angle of 30–45° in lateral view; inner wall absent; opening round, equal to spiracle width, margin regular. Coiled gut with switchback point sinistral. Vent tube medial, short, conical, free of ventral fin, opening elliptical. Tail long (TAL/TL = 0.6–0.7); caudal muscles not reaching acute tail tip; dorsal fin reduced, very low, originating at midlength of tail; ventral fin forming a groove along the entire tail (Figure 3). Preorbital and dorsal lines of lateral line system visible in some specimens, remaining lines undetectable. Oral disc (Figure 2b) ventral, large (55%–80% of body width), not emarginated, bordered by single row of rounded, non-alternating marginal papillae; upper lip with large gap; submarginal papillae absent. Labial tooth row formula 2(2)/3(1); A1 and A2 lengths subequal; A2 interrupted with small gap; P1 and P2 lengths subequal, longer than P3. Jaw sheaths present, serrate, compressed laterally, entirely black; upper sheath U-shaped; lower sheath narrower, V-shaped.
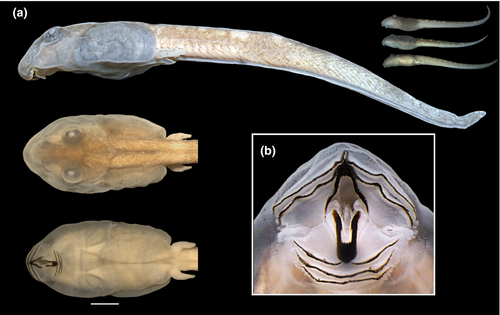
Measurements, given in mm as mean ± standard deviation (range): TL 17.8 ± 0.5 (16.9–19.0); BL 5.6 ± 0.3 (5.1–6.3); BH 1.8 ± 0.1 (1.6 – 2.1); BW 3.0 ± 0.2 (2.5–3.4); TH 1.5 ± 0.2 (1.0–1.9); TAL 12.2 ± 0.3 (11.5–12.7); ESD 1.8 ± 0.2 (1.0–2.2); NSD 1.0 ± 0.2 (0.50–1.5); ED 0.7 ± 0.1 (0.5–1.1); IOD 0.9 ± 0.1 (0.6–1.2); END 0.6 ± 0.1 (0.4–1.0); IND 0.6 ± 0.1 (0.5–0.9); ODW 2.0 ± 0.1 (1.8–2.2).
3.1.2 Comparison with other cycloramphid tadpoles (Figures 4-8)
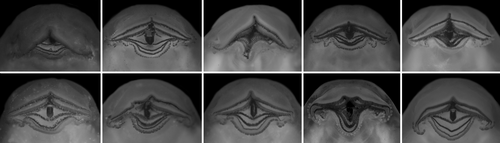
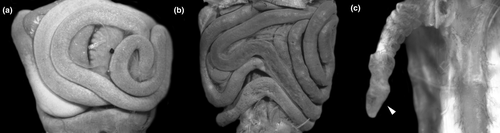
Tadpoles of Cycloramphidae share several external morphological characteristics. The body is elliptical in dorsal view and distinctively depressed, with a long tail and very low fins (Figures 4 and 6). A meniscus is present in the upper iris of all tadpoles examined, with minor variations in shape and extent of lens covering. For instance, it is large, rounded, and covers a large portion of the lens in C. boraceiensis and C. lithomimeticus; small, rounded, and covers a small portion of the lens in C. brasiliensis, C. fuliginosus, and T. lutzi; and large, rounded, and covers a part of lens in T. saxatilis and T. taophora. Nares are dorsal, rounded, and marginally rimmed. The spiracle is sinistral, tubular, lateral, ventral to midline of body, and directed posterodorsad; its inner wall is absent. The coiled gut, usually visible through the abdominal skin, has a sinistral switchback point (Figure 5). The vent tube is medial, short, conical, and free of the ventral fin. The oral discs are similar among species, being wide, unemarginated, and surrounded by a single row of marginal papillae interrupted by a dorsal gap (Figure 8). Jaw sheaths are fully keratinized and laterally compressed, and the tooth row formula is 2(2)/3(1) in most species, (2(2)/3 in Cycloramphus rhyakonastes Heyer, 1983a; Nunes-de-Almeida et al., 2016). Submarginal papillae are absent. Endotrophic tadpoles show reduction in some larval characters, such as the absence of a spiracle in Zachaenus and a lower labial tooth row formula in Zachaenus and C. stejnegeri, 1(1)/2(1–2) and 2(1–2)/2(1–2) respectively (Almeida-Silva et al., 2019; Heyer & Crombie, 1979).
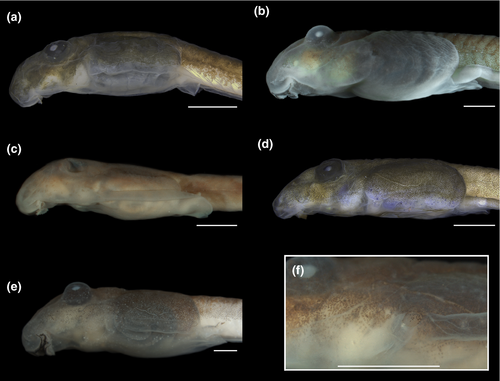
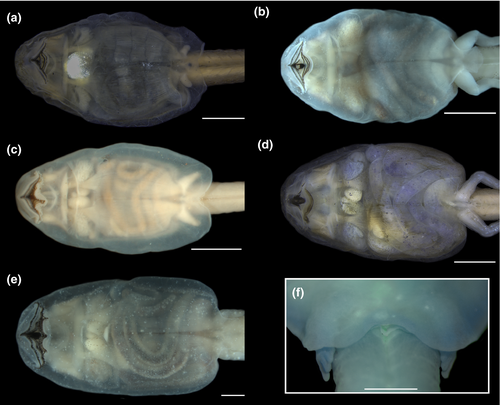
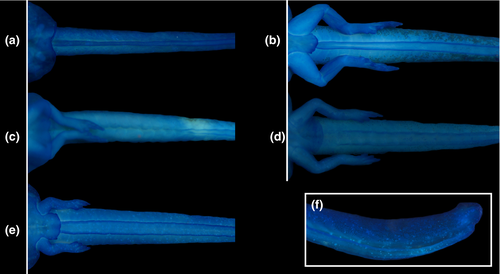
In contrast, some characters are highly variable both among and within genera. A well-developed, expanded abdominal flap is present in all Cycloramphus, T. lutzi, T. petropolitana, T. saxatilis, and T. taophora. In T. megatympanum, as in T. miliaris, it is poorly developed and almost unnoticeable (Figures 4-6); in Zachaenus, it is reduced, forming a small, rounded flap (Almeida-Silva et al., 2019). The posterior margin of the abdominal flap is deeply bilobate in C. lithomimeticus, T. lutzi, and T. petropolitana and unindented in C. boraceiensis, C. brasiliensis, and C. fuliginosus. In T. miliaris, T. petropolitana,T. saxatilis, and T. taophora, the spiracle opens above the midline of the posterolateral region of the flap; in T. lutzi, it opens below it and is directed posteriad; and in C. lithomimeticus, C. boraceiensis, C. brasiliensis, and C. fuliginosus it is aligned with the flap margin, directed posteriad. The ventral fin is low in all tadpoles of Cycloramphus but modified into a groove in all Thoropa. The length of the groove differs interspecifically, being differentiated along the anterior half of the tail in T. lutzi and T. saxatilis, only posteriorly in T. petropolitana, and along the entire fin in T. megatympanum and T. taophora (Figure 7). Unlike T. miliaris, in which the vent tube is free of the ventral fin, the vent tube is attached to the ventral fin in T. saxatilis and T. petropolitana and to the fin and the abdominal flap in T. lutzi and T. taophora. Finally, angular and ventral lateral lines are visible in C. brasiliensis (not observed in T. miliaris).
3.2 Internal morphology
We describe the main buccopharyngeal and musculoskeletal characteristics of larval Thoropa mainly on the basis of T. miliaris, highlighting differences between it and confamiliar tadpoles when pertinent.
3.2.1 Buccopharyngeal cavity (Figures 9 and 10)
Buccal roof triangular, longer than wide (Figure 9a). Prenarial arena long, narrow, devoid of papillae, with arched, posteriorly oriented prominence or ridge. Internal nares elliptical, arranged obliquely; a flap-like papilla projecting mediad from wall lateral to each internal naris, partially occluding narial opening (see detail in T. megatympanum; Figure 10a); narial valve poorly developed or absent. Postnarial arena triangular. Small papillae or ridges oriented obliquely, converging rostrally, weaker than in Cycloramphus tadpoles, forming large flaps almost reaching sagittal axis (Figure 10b). Median ridge trapezoid, low, with smooth or papillate margin in Thoropa; taller and always papillate in Cycloramphus. Single pair of small lateral ridge papilla oriented transversely, bifurcated; larger and flap-like in Cycloramphus. Buccal roof arena rounded, delimited laterally by 5–6 (Thoropa) or 7–10 (Cycloramphus) pairs of conical papillae; pustulation absent. Glandular zone not evident. Dorsal velum with smooth margin, interrupted medially, lacking papillae; some small papillae appear on the velum margin in Cycloramphus.
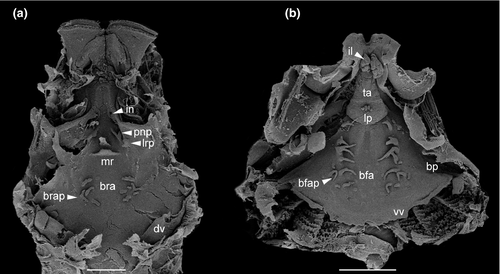

Buccal floor triangular (i.e., narrow anteriorly, wide posteriorly; Figure 9b). Two pairs of infralabial papillae: first pair large and globose (Figure 10c,d); second pair small and conical. Tongue anlage bell-shaped, bearing four conical lingual papillae in Thoropa, two or four in Cycloramphus. Buccal floor arena rectangular, bordered laterally by 10 (Thoropa) or 7–14 (Cycloramphus) pairs of long papillae, the anteriormost deeply bifurcated. Buccal pockets longer than wide, shallow, oriented transversely. Margin of ventral velum with a few secretory pits, laterally smooth, medially undulate with wide median notch. Secretory ridges absent. Branchial basket triangular, shallow, bearing three filter cavities. Branchial plates bearing 6, 5, and 4 filter rows, respectively.
3.2.2 Chondrocranium (Figures 11 and 12)
Chondrocranium longer than wide; greatest width at subocular bar (Figure 11a,b). Suprarostral cartilage tripartite; central corpora fused at midlength by cartilaginous bar; corpus and ala articulated along entire margins; suprarostral alae with well-developed dorsal anterior processes (Figure 11c). In Cycloramphus, the processes are more pronounced and laterally expanded. Ethmoid region very long, with trabecular horns diverging far distally; horns with free portion short, narrowly divergent to almost parallel in Cycloramphus, with expanded distal margin; lateral trabecular process absent. Intertrabecular plate poorly chondrified medially. Basicranial fenestra weakly chondrified, mostly occluded by a thin membrane; carotid and craniopalatine foramina barely visible. Orbital cartilages not contacting otic capsules; dorsal margin of prootic foramen open. Optic and oculomotor foramina present. Frontoparietal fontanelle large, almost rectangular, wider posteriorly; bordered laterally by taenia tecti marginales, anteriorly by planum ethmoidale, posteriorly by tectum synoticum. Otic capsules rhomboid with a thin crista parotica and pointed anterolateral process. Jugulare and inferior perilymphatic foramina visible in ventral view.
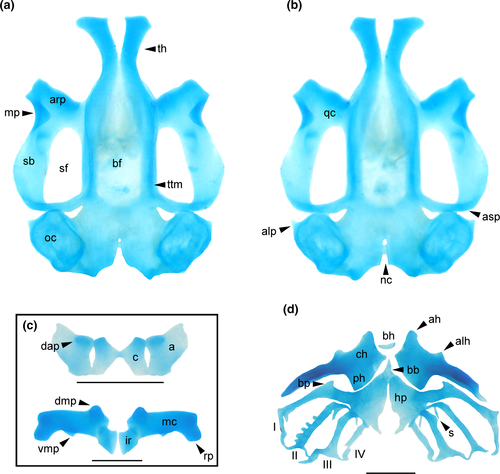
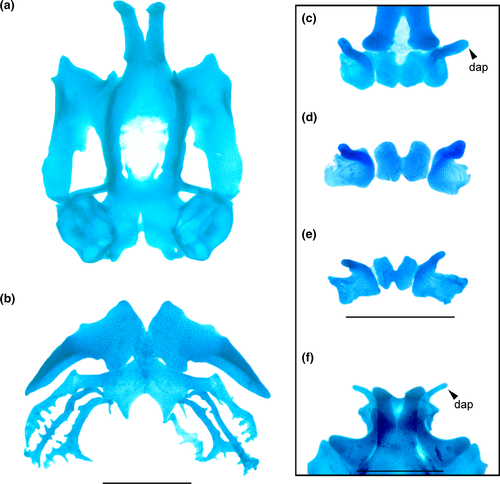
Palatoquadrate C-shaped; articular process very short, wide, with anterior margin slightly sinuous. Quadratocranial commissure thinner than articular process, lacking quadratoethmoid and pseudopterygoid processes. Muscular process triangular, low, thin; hyoquadrate process evident ventrally. Antorbital process weakly differentiated. Ascending process rod-like, thin, slightly curved and arranged perpendicular to sagittal axis, attaching at level of oculomotor foramen (intermediate attachment). Lower jaw (Figure 11c) with L-shaped Meckel's cartilages, orientation deeply oblique relative to main axis of chondrocranium; dorsomedial, ventromedial, and retroarticular processes robust. Infrarostral cartilages rectangular, curved, connected medially by connective tissue.
In the hyobranchial skeleton (Figure 11d), ceratohyals long, flat, thin, subtriangular. Anterior margin bearing tall, rounded anterior process and low, wide anterolateral process. Articular condyle poorly developed. Posterior process triangular, tall, very wide. Pars reuniens poorly chondrified, not joining ceratohyals. Basihyal present, elliptical. Basibranchial weak, narrow, independent of ceratohyals; urobranchial process small. Hypobranchial plates long, triangular, leaving a wide, U-shaped notch between them. Branchial basket with four curved ceratobranchials with numerous lateral projections. Ceratobranchial I continuous with hypobranchial plate, bearing a tall, medially curved branchial process. Ceratobranchials II and IV fused to hypobranchial plate through a thin cartilaginous bridge; branchial process present at ceratobranchials II and III. Ceratobranchials joined distally by terminal commissures. Four long, curved spicules dorsally, the fourth one being wider and finger-like.
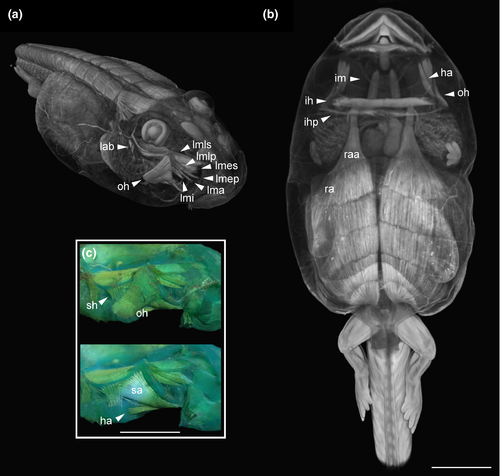
3.2.3 Cranial muscles (Figures 13 and 14)
We identified 33 muscles in tadpoles of Thoropa and Cycloramphus (Table 3). Interhyoideus posterior present as a few loose fibers, continuous medially, originating near lateral edge of ceratohyal (Figures 13b,c and 14b,c). Diaphragmatopraecordalis continuous with interhyoideus posterior. Levator mandibulae externus with two slips (Figure 13a). Origins of levator mandibulae longus superficialis and profundus broadly overlapping. Levator mandibulae lateralis absent. Axial muscles covering dorsal area of otic capsule (Figure 14a).
| Muscle | Insertions |
|---|---|
| Mandibular group, n. trigeminus (c.n. V) | |
| Levator mandibulae longus superficialis | External posterior margin of subocular bar and ascending process—dorsomedial surface of Meckel's cartilage. Via a long tendon |
| Levator mandibulae longus profundus | External margin of subocular bar and ventromedial area of ascending process—external margin of the suprarostral ala. Via a log tendon |
| Levator mandibulae externus superficialis | Inner, dorsal surface of muscular process—anterodorsal surface of Meckel's cartilage |
| Levator mandibulae externus profundus | Inner, middle surface of muscular process—suprarostral ala. It shares a tendon with levator mandibulae longus profundus |
| Levator mandibulae articularis | Inner, ventral surface of muscular process—dorsal surface of Meckel's cartilage |
| Levator mandibulae internus | Ascending process—lateral edge of Meckel's cartilage |
| Intermandibularis | Median aponeurosis—ventromedial surface of Meckel's cartilage |
| Mandibulolabialis | Lower lip—ventromedial surface of Meckel's cartilage |
| Hyoid group, n. facialis (c.n. VII) | |
| Hyoangularis | Anterodorsal, lateral edge of ceratohyal—retroarticular process of Meckel's cartilage |
| Quadratoangularis | Ventral area of palatoquadrate—retroarticular process of Meckel's cartilage |
| Suspensorioangularis | Posterior margin of muscular process—retroarticular process of Meckel's cartilage |
| Orbitohyoideus | Anterior and dorsal margins of muscular process—lateral edge of ceratohyal |
| Suspensoriohyoideus | Posterior margin of muscular process and subocular bar—lateral edge of ceratohyal. Fibers mostly parallel |
| Interhyoideus | Median aponeurosis—ventral surface of lateral edge of ceratohyal |
| Branchial group, n. glossopharyngeus (c.n. IX) and vagus (c.n. X) | |
| Levator arcuum branchialium I | Subocular bar—ceratobranchial I |
| Levator arcuum branchialium II | Subocular bar—ceratobranchial and terminal commissure II |
| Levator arcuum branchialium III | Lateral aspect of otic capsule—ceratobranchial III and peritoneum. Formed of two slips, the anterior with loose fibers, the posterior thick |
| Levator arcuum branchialium IV | Posterolateral aspect of otic capsule—ceratobranchial IV |
| Tympanopharyngeus | Posterolateral aspect of otic capsule—ceratobranchial IV and pericardium anterior to glottis |
| Dilatator laryngis | Posterior aspect of otic capsule—arytenoid cartilage |
| Constrictor branchialis II | Branchial process II—commissura terminalis I. It runs on the inner margin of ceratobranchial I |
| Constrictor branchialis III | Branchial process II—commissura terminalis II. It runs on inner margin of ceratobranchial II |
| Constrictor branchialis IV | Ceratobranchial III—commissura terminalis II. It runs on the inner margin of ceratobranchial III |
| Subarcualis rectus I | Base of posterior process of ceratohyal—ceratobranchial I and aponeurosis common with the rectus abdominis anterior. It includes two slips, the dorsal thin, and the ventral very thick and longer |
| Subarcualis rectus II-IV | Ceratobranchial IV—ceratobranchial I. It crosses the ceratobranchial III ventrally |
| Subarcualis obliquus II | Urobranchial process—branchial process II |
| Diaphragmatobranchialis | Peritoneum—distal point of ceratobranchial III |
| Spinal group, spinal nerve innervation | |
| Geniohyoideus | Hypobranchial plate and ceratobranchial III—infrarostral cartilage. It attaches at the level of ceratobranchial II and III, via tendon |
| Rectus abdominis | Peritoneum (diaphragm)—pelvic girdle. It is formed of six open myotomes of similar lengths |
| Rectus abdominis anterior | Peritoneum, aligned with m. rectus abdominis—aponeurosis shared with the m. subarcualis rectus I |
| Rectus cervicis | Peritoneum—ceratobranchial III. Some loose fibers appear to insert near ceratobranchial III |
| Interhyoideus posterior | Continuous fibers join ventrally the lateral edges of ceratohyals |
| Diaphragmatopraecordialis | Loose fibers surrounding the branchial chamber |
We observed two remarkable conditions in tadpoles of Thoropa and Cycloramphus: (a) the subarcualis rectus I with two slips, one inserting on the dorsal ceratobranchial I and the other on a aponeurosis, in which fibers of the rectus abdominis anterior are also inserted (Figure 14d); and (b) the levator arcuum branchialium III with two slips: one poorly developed and inserting on the distal ceratobranchial III and the second well developed, inserting on the peritoneum (Figure 14e,f). Additionally, the oblique musculature of the body wall is well developed in all species.
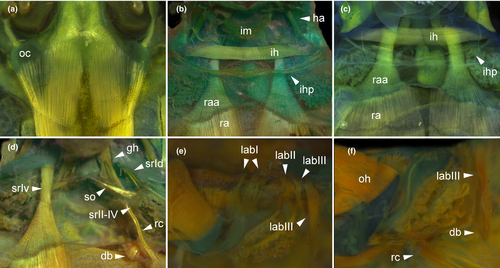
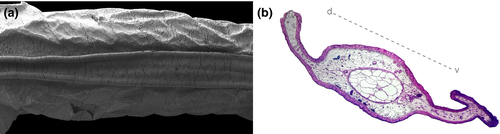
3.2.4 Visceral components (Figure 15)
Gut long, coiled, concealing other organs, switchback point located laterally on the left side (Figure 15a,b). Lungs present, well developed, short, inflated in C. boraceiensis, T. megatympanum, and T. miliaris (Figure 15c), poorly developed, short, deflated in T. petropolitana and T. taophora.
4 DISCUSSION
4.1 Larval morphology and the systematics of Cycloramphidae
The phylogenetic hypotheses presented for Cycloramphidae differ substantially. For instance, Frost et al. (2006) recovered Thoropa as sister to dendrobatoids (i.e., Thoropidae + Dendrobatoidea) and Cycloramphus nested in a clade containing several lineages of Hyloides. Grant et al. (2006) did not recover Thoropa and Cycloramphus as sister taxa either; instead, Thoropa was recovered as sister to a large clade containing Hylorina Bell, 1843, Eupsophus Fitzinger, 1843, Alsodes Bell, 1843, Limnomedusa Fitzinger, 1843, Proceratophrys Miranda-Ribeiro, 1920, and Odontophrynus Reinhardt & Lütken, 1862, whereas Cycloramphus was the sister of Rhinoderma darwinii Duméril & Bibron, 1841. Later, Pyron and Wiens (2011) found Thoropa and Cycloramphus as sister taxa, and both sister to a clade containing Hylodidae + Alsodidae. Grant et al. (2017) found Cycloramphidae to be paraphyletic, with Thoropa as sister to Dendrobatoidea and Cycloramphus + Zachaenus as sister to Alsodidae + Hylodidae. Jetz and Pyron (2018) recovered Thoropa as sister to Cycloramphus + Zachaenus and this clade as sister to a clade containing Hylodidae, Batrachylidae, and Alsodidae. Sabbag et al. (2018) recovered Cycloramphidae as monophyletic—although Zachaenus was nested within Cycloramphus and Thoropa cf. lutzi was sister to Cycloramphus + Zachaenus—and sister to Alsodidae + Hylodidae. de Sá et al. (2019) recovered Cycloramphus and Thoropa as sister taxa with Zachaenus nested within Cycloramphus. Recently, Hime et al. (2020) found Cycloramphidae to be sister of a clade composed by Hylodidae + (Alsodidae + Batrachylidae). Most of these studies employed only DNA evidence and treated insertions and deletions as nucleotides of unknown identity, with few exceptions (e.g., Grant et al., 2017), and all available hypotheses are based on poor taxonomic sampling that does not encompass the diversity of Cycloramphidae. Although a large-scale phylogenetic analysis is beyond the scope of this manuscript, we discuss the putative impact of larval morphology in the systematics of the family.
Our observations of tadpole morphology provide additional support for the monophyly of Cycloramphidae. Specifically, tadpoles of Cycloramphus and Thoropa share the following character-states that we propose as new synapomorphies for the family (Figure 16 and Figure S1): (a) jaw sheaths laterally compressed; (b) posterolateral portion of body integument expanded into an abdominal flap; (c) dorsal fin reduced; (d) ventral fin reduced; (e) dorsal fin originating on the tail; (f) eye meniscus present; (g) second slip of the levator arcuum branchialium III inserting on the peritoneum; (h) rectus abdominis anterior present, (i) inserting on a thin aponeurosis; (j) ventral slip of the subarcualis rectus I attached to a thin aponeurosis common with that of the rectus abdominis anterior (k) axial musculature reaching far anterior on the dorsal aspect of otic capsules; (l) suprarostral corpora fused medially; and (m) secretory ridges of branchial food traps absent. Additionally, all tadpoles of Thoropa have a ventral fin modified into a groove, which we hypothesize to be a synapomorphy for the genus.
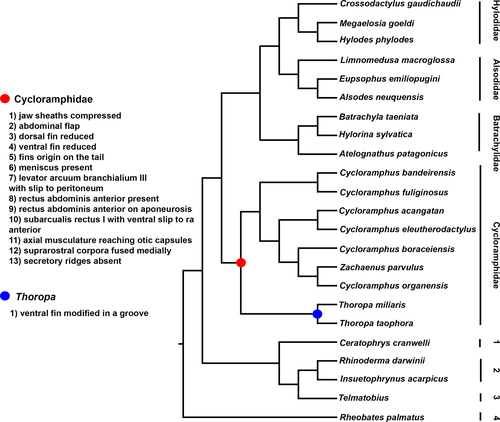
These characters are unique to Cycloramphidae among potential close relatives (some are convergently shared with other semiterrestrial tadpoles; see discussion below). Others are observed in distant related taxa. For instance, reduced tail fins have been reported in several species (e.g., Gephyromantis sculpturatus (Ahl, 1929), Mantellidae; Randrianiaina et al., 2011), and secretory ridges are absent in several macrophagous (e.g., Dendropsophus decipiens (Lutz, 1925), Hylidae; Dias et al., 2019), arboreal (e.g., Triprion spinosus (Steindachner, 1864), Hylidae; Wassersug & Rosenberg, 1979), and endotrophic (e.g., R. darwinii, Rhinodermatidae; Wassersug & Heyer, 1988) larvae. Some characters also deserve future investigation, such as the presence of the interhyoideus posterior; Haas (2003) hypothesized that its absence is synapomorphic for anurans, but he also reported its presence in several clades, (e.g., hylids, odontophrynids, some dendrobatids). We also observed the interhyoideus posterior in hylodids, but the lack of data for other taxa prevents us from further discussion.
Recently, several studies have demonstrated the disproportionate impact of phenotypic characters in phylogenetic analyses of predominantly molecular datasets, changing topologies and increasing or decreasing the relative branch support of multiple clades, (e.g., Cabra-García & Hormiga, 2020; de Sá et al., 2014; Sánchez-Pacheco et al., 2018). We predict that the inclusion of phenotypic characters from larval morphology in a total evidence analysis of Cycloramphidae will impact the systematics of the group and support the monophyly of the family.
4.2 Functional morphology of cycloramphid tadpoles
Thoropa and Cycloramphus larvae exhibit a number of apomorphic character-states that appear to be causally related to semiterrestriality. The modification of the posterolateral surface of the body to form a flattened integumentary expansion appears to play a role in adhering the body to the substrate. Wassersug and Heyer (1983) suggested that surface tension (capillarity) is key to enabling these tadpoles to adhere to rock surfaces. Surface tension increases with the greater area in contact with the rock surface provided by this abdominal flap. In Thoropa, surface tension is further increased by the modification of the ventral fin to form a groove that greatly increases the area of contact between the tadpole and the substrate (Movie S1). Lutz (1929) observed that Cycloramphus tadpoles, which lack this fin groove, adhere to vertical walls without using their tails, which are apparently only involved in propulsion (see also Verdade et al., 2019). Some authors (e.g., Barth, 1956; Bokermann, 1965) have suggested that the oral disc and the well-developed jaw sheaths might be involved in hooking these tadpoles to the rocks. Recently, Verdade et al. (2019) reported that the larvae of Cycloramphus bandeirensis “moved across the wet surfaces of the rocks by affixing their mouth to the rock and then pulling the body forward by abdominal muscular contraction.” Similarly, field observations (G. Colaço, pers. comm.) indicate that the oral apparatus, tail, and abdominal flap, are involved in anchoring the tadpole of T. miliaris to the substrate. Tadpoles of T. saxatilis use their mouths to anchor their bodies and to aid in locomotion. Additionally, they employ alternating movements of the legs and abdominal muscular contraction (Figure 17; Movie S1).
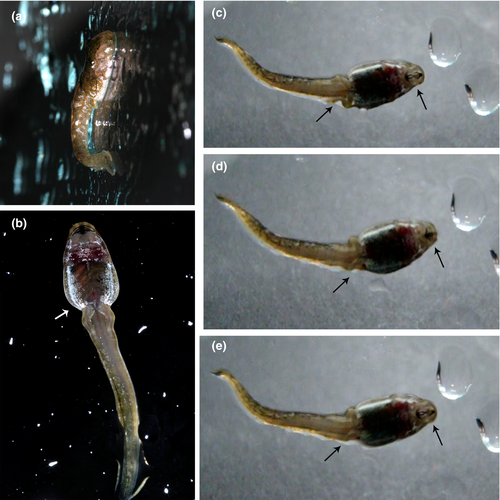
In addition to external morphology, several internal anatomical features are likely related to life and locomotion in rocky environments. The rectus abdominis anterior is present in all cycloramphids examined. This muscle is rare among anuran larvae. Muscles with similar arrangements have been reported in some lineages, including Ascaphus (Gradwell, 1973), Boophis Tschudi, 1838, Hyloscirtus Peters, 1882, Ranoidea Tschudi, 1838 (Haas & Richards, 1998), Leptobrachella Smith, 1925 (Haas et al., 2006), Occidozyga Kuhl & Van Hasselt, 1822 (Haas et al., 2014), Otophryne Boulenger, 1900 (Wassersug & Pyburn, 1987), and Telmatobius Wiegmann, 1834 (Vera Candioti, 2008). We also confirm its presence in Hylodidae and some bufonids and mantellids (P.H.S. Dias, unpubl. data). In the aforementioned species, the muscle is a thin band of fibers that originate near the medial fibers of the rectus abdominis and extends rostrad, usually to insert on the anteroventral palatoquadrate via a very long tendon. In contrast, in cycloramphids, this muscle is a discrete, thick slip of fibers that originate along the whole width of the rectus abdominis and converge to insert on the ventral slip of the subarcualis rectus I, in a condition that is unknown in any other anuran larvae. In tadpoles of Ascaphus, Gradwell (1973) suggested that the rectus abdominis anterior could pull the body toward the substrate after adhesion by the oral disc. It is likely that in cycloramphids the subarcualis rectus I assumes a comparable function by being the effective skeletal insertion of fibers of the rectus abdominis anterior.
The configuration of the levator arcuum branchialium III is another remarkable feature of cycloramphid tadpoles. The thick caudal slip is attached to the peritoneum, instead of the distal edge of ceratobranchials, as it is in most other described larvae. Further, the insertion is well ventral and slightly caudal to the level of the occipital region and close to the attachments of rectus cervicis, rectus abdominis, and diaphragmatobranchialis. A muscle with a comparable disposition was described by Haas et al. (2006) and Haas et al. (2014) in Occidozyga and Leptobrachella but interpreted as the diaphragmatobranchialis. Based on anatomical evidence and direct observations, Haas et al. (2006) showed that fossorial tadpoles of Leptobrachella mjobergi are capable of bending the body at a sagittal plane, and that bending can occur at the atlanto-occipital joint. The resemblance of the arrangement of dorsal axial musculature, and of ventral recti abdominis and cervicis, and diaphragmatobranchialis/levator arcuum branchialium III in Leptobrachella and cycloramphids, leads us to suspect that dorsal extension and ventral flexion of the head are achieved similarly in both groups. Similar to the burrowing movements as in Leptobrachella, head maneuverability might be necessary to achieve the jumping and tail-flipping locomotion described for cycloramphid and other semiterrestrial larvae (Barej et al., 2010; Gopalan et al., 2012; Hoff et al., 1999; Veeranagoudar et al., 2009; G. Colaço, pers. comm.).
Wassersug and Heyer (1983) hypothesized that both Cycloramphus and Thoropa are obligate air breathers. Lutz (1929:20) asserted that Cycloramphus tadpoles “cannot stay in the water unless they emerge at least with the anterior part of their body,” and Bokermann (1965:527; free translation from the Portuguese) reported that Thoropa tadpoles “placed in water, they are clumsy and can only move around by clinging to some support point,” supporting the air-breathing hypothesis. We observed reduced and shrunken lungs in T. megatympanum and small and inflated lungs in T. miliaris, suggesting that lungs do not play an important role in gas exchange for either species. On the other hand, branchial filaments are present in the branchial baskets of cycloramphid tadpoles examined, but the greatly reduced spiracle suggests poor irrigation of gills within the branchial chamber—contra Barth (1956:490), who suggested that Thoropa tadpoles would breath through strong branchial filaments. This morphology is compatible with ecological data showing that most cycloramphid larvae live in saxicolous environments with (sometimes very) limited water volume (e.g., Movie S1). According to Noble (1929), the long tail in tadpoles of Cycloramphus might perform an important role in respiration, and Verdade et al. (2019) suggested that the expanded abdominal flap might also play an important role in gas exchange. Thus, it is likely that most gas exchange in cycloramphid tadpoles occurs through the skin or through combined skin and lung respiration, with branchial respiration being negligible or absent.
Feeding habits of semiterrestrial tadpoles are as interesting as the adhesion mechanism and gas exchange, given that filter-feeding is unlikely in semiterrestrial environments. The semiterrestrial tadpoles of Cycloramphidae have well-developed, fully keratinized, compressed, massive jaw sheaths powered by robust jaw levator muscles, suggesting a role in macrophagous diets. Similarly, the buccopharyngeal cavity presents an overall reduction in papillae and secretory surfaces. Wassersug and Heyer (1983) reported mineral grains, algal filaments, and part of an arthropod exoskeleton in the guts of T. petropolitana and asserted that branchial food trap morphology seems to preclude planktonic suspension feeding. Barth (1956) reported diatoms, pteridophytes, spores, vegetal matter, and rests of small arthropods in the digestive tract of T. miliaris. Interestingly, similar feeding habits have been reported in other semiterrestrial tadpoles. Gut content of the tadpoles of Indirana leithii (Boulenger, 1888) presented filamentous algae and diatoms (Modak et al., 2018), and Wickramasinghe et al. (2007) found microflora, microfauna, detritus, and mineral particles in the tract of tadpoles of N. ceylonensis, as well as conspecific eggs and tadpoles.
4.3 Adaptation for semiterrestrial life? Convergent evolution in semiterrestrial tadpoles
As noted above, semiterrestrial tadpoles are known in Bufonidae, Cycloramphidae, Dicroglossidae, Petropedetidae, Ranixalidae, Ptychadenidae, and Pyxicephalidae. In all seven lineages, they evolved from typical pond or stream dwellers ancestors (Figure 18), and, as early noted by Drewes et al. (1989), converged to very similar phenotypes (Figure 19). Among the material we examined, larvae of A. martiensseni, I. beddomi, P. perreti, and P. vulpiae have oval, elongated bodies; long tails with reduced tail fins; massive, fully keratinized, compressed jaw sheaths; poorly developed spiracle; large, dorsal eyes; long, coiled guts concealing other organs; and long, well-developed, muscular hind limbs. Most of these character-states (with exception of those from internal morphology ones, for which there is a dearth of information) have also been reported in the literature (e.g., Barej et al., 2010; Drewes et al., 1989; Gopalan et al., 2012). Moreover, the tadpoles of P. perreti also possess a short, poorly developed muscular process and strong, well-developed axial muscles covering the otic capsules dorsally; an additional slip of the levator arcuum branchialium III inserting on the peritoneum, and secretory ridges absent (P.H.S. Dias, unpubl. data).
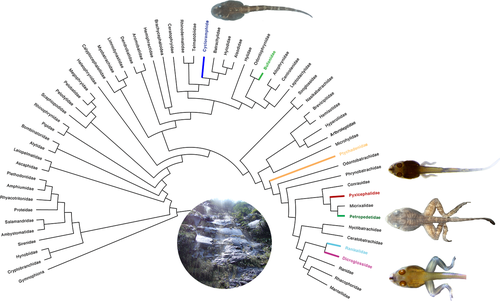

The presence of homoplastic character-states in all semiterrestrial tadpoles of unrelated phylogenetic lineages seems to suggest that these character-states might be adaptations for semiterrestriality. Although adaptation can be difficult to test (Kluge, 2005), the correlation between transformation series (A→A′) and transformation in the ecological aspects (œ→ œ′) provides a strong framework for more rigorous studies in the future (Losos, 2011).
5 CONCLUSIONS
This study provides the hitherto most comprehensive account of semiterrestrial tadpole morphology. We described several new characters, proposed 13 new phenotypic synapomorphies for Cycloramphidae, and discussed the function and the evolution of these remarkable morphologies. Nevertheless, data are restricted to only part of Cycloramphidae, and species representing the C. bolitoglossus and C. eleutherodactylus groups and Zachaenus, which possess endotrophic, nidicolous tadpoles (Heyer, 1983a, 1983b; Heyer & Crombie, 1979), still need to be studied in detail. Additionally, data on the internal morphology of other semiterrestrial tadpoles are almost non-existent, and further research is necessary to understand the extent of the convergence among these lineages.
The importance of larval morphology to understand evolution and diversification of anurans has been recognized for a long time (e.g., Boulenger, 1892; Dias et al., 2013, 2021; Haas, 1995, 2003; Lataste, 1879; Noble, 1929; Orton, 1953), but we are far from fully understanding larval diversity. In the last 20 years, as more species were studied, several new, highly modified larval character-states have been described (e.g., Dias, 2020; Dias et al.,2019, 2021; Gan et al., 2015; Haas et al., 2006, 2014; Peixoto et al., 2003; Rada et al., 2019; Rowley et al., 2012; Vera Candioti et al., 2017; Zachariah et al., 2012). Our study provides another example of how poorly studied groups can present novel and peculiar characters. The configuration in cycloramphid tadpoles of the muscles levator arcuum branchialium, subarcualis rectus I, and the morphology of the rectus abdominis anterior are unique among all anurans tadpoles. Larval morphology is an exciting field, and we predict that further research on poorly known groups will reveal novel phenotypes, synapomorphies, and complex evolutionary histories.
Note: Subsequent to the acceptance of our article, Dubois and colleagues (2021; Megataxa, 1, 1–738) transferred Zachaenus into the synonymy of Cycloramphus.
ACKNOWLEDGEMENTS
S. Richter (University of Rostock) kindly allow us to use the micro-CT in his facilities; S. Scholz assisted us with the micro-CT procedures. D. Baldo (LGE), J. D. Lynch (ICN), J. Pombal Jr. (MNRJ), J. Faivovich (MACN), K. Tighe (USNM), L. F. Toledo (ZUEC), M. O. Rödel (ZMB), P. A. Garcia (UFMG), and R. Glor and Rafe Brown (KU) provided access to the specimens under their care. Financial support and fellowships were provided by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; #2012/10000-5, #2013/20420-4, #2013/50741-7; #2015/11239-0, #2018/15425-0) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Proc. 88887.364687/2019-00). G. C. is supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and T.G., H. R. S. and C.F.B.H. are Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) researcher fellows (306823/2017-9, 306963/2018-3, 306623/2018-8). F. V. C. was supported by CONICET and ANPCyT PICT 2018-3349. G. Fialho helped with the measurements of the tadpoles of T. miliaris. Ê. Mattos and P. Lenktatis assisted us with the SEM procedures. Fieldwork with T. saxatilis was conducted in collaboration with P. Colombo, S. Sánchez-Pacheco, and R. Santos, and T.G. is grateful to P. Colombo for sharing his unparalleled knowledge of the amphibians of Rio Grande do Sul, including T. saxatilis. We are grateful to R. Wassersug, A. Haas, J. Faivovich, and T. Pezzuti, and Gabriela Bittencourt for sharing their known on buccopharyngeal characters, musculoskeletal characters, and semiterrestrial tadpoles, respectively, and J. S. Beneti for comments on the format and structure of an early draft. Fieldwork was authorized by the Instituto Chico Mendes de Conservação da Biodiversidade (SisBio 13173-1 and 13256).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




