Revision of the genus Buccinanops (Mollusca: Neogastropoda: Nassariidae), an endemic group of gastropods from the Southwestern Atlantic, including a new genus and accounts on the Buccinanopsinae classification
Contributing author: Guido Pastorino ([email protected])
Zoobank link: LSID: http://zoobank.org/urn:lsid:zoobank.org:pub:933869D9-25B2-42A0-9B56-115C992ED0FE Online ISSN: 1439-0469
Abstract
The nominal species referred to the endemic southern South American genus Buccinanops are revised, clarifying the definition of Buccinanopsinae. All valid Recent species are redescribed based on type material and other specimens emphasizing anatomy and illustrated using SEM photographs of radulae, embryos, and shell ultrastructure. Only seven of 13 nominal species described are considered valid: Buccinanops cochlidium (Dillwyn, 1817) (type species); B. latus sp. nov. (formerly B. gradatus or B. lamarckii from Brazil only); B. monilifer (Kiener, 1834); B. deforme (King, 1832); B. paytense (Kiener, 1834); B. uruguayense (Pilsbry, 1897) and B. duartei Klappenbach, 1961. A phylogenetic analysis based on morphological characters of these species and 12 other buccinoideans is carried out in order to compare results with recent DNA sequence approaches. The ingroup cladogram is ((B. monilifer (B. latus-B. cochlidium)) (B. duartei (B. uruguayense (B. deforme-B. paytense)))). The former genus Buccinanops, supported by 11 synapomorphies, can be divided, and Buccinastrum gen. nov. (3 synapomorphies of support) is introduced for the 4 latter species in the above cladogram; its type species is Buccinastrum deforme. Buccinanops (8 synapomorphies) is thus restricted to B. cochlidium, B. monilifer, and B. latus, which are large-sized, living in relative deep waters (subtidal), while Buccinastrum includes small-sized species mostly restricted to the intertidal zone. The analysis of morphological characters is suggestive of a traditional subfamily Dorsaninae, with 12 synapomorphies, including four genera—Dorsanum, Bullia, Buccinanops, and the new Buccinastrum, but this result is only informative, as it has been considered paraphyletic in a DNA sequence approach, being subdivided into Dorsaninae, Buccinanopsinae, and Buliinae.
1 INTRODUCTION
The genus Buccinanops d’Orbigny, 1841 presently comprises a group of the common mostly carnivorous gastropods living in sandy beaches along the Southwestern Atlantic coast, from southern Brazil to Tierra del Fuego. Considerable variation of relatively featureless shell morphology and a broad latitudinal distribution combine to obscure the true number and definition of the species belonging in this genus. Nevertheless, new collections, a revision of all existing type materials and museum specimens, and a detailed morphological and anatomical study, allow us to identify more precisely the status of each name recorded for this genus throughout its entire distribution area.
The last comprehensive account of the genus Buccinanops was in the revision of the Argentine and Uruguayan “Dorsaninae” by Carcelles and Parodiz (1939), ~80 years ago. In that pioneering paper, most of the species were included, with good photographs and radula sketches for the first time. However, no anatomical data were included, and the range of each species was taken mostly from old literature, thus veiling the real distribution.
A similar shell morphology probably induced previous authors (i.e., Allmon, 1990; Carcelles & Parodiz, 1939) to include the genus Buccinanops with Dorsanum Gray, 1847 and Bullia Gray, 1833, in various systematic arrangements, always related, despite the fact that the latter genera are currently living in western and southern Africa, and in the Indian Ocean. Pastorino (1993) reported some of the generic and subgeneric combinations of these three genera proposed by different authors. Also, in a comparative scenario among these three genera, Simone and Pastorino (2014) described the anatomy of species of Dorsanum and Bullia from W Africa, concluding that Buccinanops was recognized as a separate genus. The three genera have been considered related, sometimes as members of the same subfamily; sometimes as members of monotypic subfamilies—Buccinanopsinae, Dorsaninae, and Bulliinae (Galindo et al., 2016), but always within the family Nassariidae.
The analysis by Allmon (1990, text Figure 25) recovered a monophyletic group (Dorsanum (Bullia – Buccinanops)), supporting the then new subfamily Bulliinae. However, Haasl (2000, Figure 4) showed the three taxa in a paraphyletic arrangement, with Bullia-Buccinanops in one branch, followed by Dorsanum, as sister taxon of a Photinae/Nassariinae branch. In the molecular genetic study by Galindo et al. (2016), which used mitochondrial (COI, 16S, 12S) and nuclear (28S, H3) genes, the three supposedly closely related genera were retrieved in separated positions in their phylogenetic trees obtained using maximum likelihood and Bayesian algorithms. Buccinanops was placed at the base of the Nassariidae, as its first branch; Bullia appeared in a more internal position, separated from Buccinanops by samples now considered Photinae and Cylleninae (formerly buccinids). Samples of Anentominae and a subfamily-indet. Nassodonta are two branches separating Dorsanum from Bullia, sister group of remaining nassariids allocated in Nassariinae. The three genera—Buccinanops, Bullia, and Dorsanum—were thus included in proper monotypic subfamilies—Buccinanopsinae (described then as new), Bulliinae Allmon, 1990 and Dorsaninae Cossmann, 1901. Curiously, Bullia granulosa did not fall within the Bullia branch, but instead into Nassariine branch of the Naytia group. Only the new subfamily, that is, Buccinanopsinae had characters defined in that paper, the set of features is stressed in discussion section herein.
The Galindo et al. (2016) arrangement has been currently accepted (e.g., WoRMS website, 2020); however, a morphological definition of these taxa, from subfamily to species level, is still mostly wanting. Based on a relatively comprehensive nassariid/buccinid morphological dataset that has been produced by our team, both in Nassariidae in general (e.g., Abbate & Cavallari, 2013; Abbate et al., 2018; Couto & Simone, 2019; Gernet et al., 2019; Simone, 2007, 2011; Abbate & Simone, submitted), and in the taxa Buccinanops/Bullia/Dorsanum in particular (e.g., Abbate & Simone, 2016; Pastorino, 1993a, b; Simone & Pastorino, 2014), it appears plausible that these three genera are more closely related based on morphology, rather than their surprising widespread dispersal among different branches within Nassariidae, proposed by Galindo et al. (2016). It is not our intention to contradict the DNA sequence results (Galindo et al., 2016), as the studied assembly is much smaller and focused on these three genera. Thus, no taxonomical change is proposed here in family/subfamily levels. However, as the search for morphological definitions of the abovementioned taxa has revealed a different scenario, the different result looks interesting to be exposed, representing a counterpoint, and raising interesting and well-based discussion.
The present paper includes type material examination, as well as anatomical features to be added to the conchological and radular characters traditionally assigned to the genus Buccinanops and allies. The main objective was a revision of the Recent species that have been included in Buccinanops s.l.. Furthermore, the relationships between Buccinanops s.l. and other members of the superfamily Buccinoidea were considered, from a phenotypical perspective, and in comparison with results from other approaches (e.g., DNA sequence data). The present database is inserted in an already published phylogenetic approach (Simone, 2011). Additionally, the buccinoidean representation in that scenario is enriched, with the intention of a better-based discussion, saving long explanations on the methodologies.
2 MATERIALS AND METHODS
Most of the material reviewed for this work belongs to the largest collections from South American institutions: Museo Argentino de Ciencias Naturales "Bernardino Rivadavia," Buenos Aires (MACN) and Museo de La Plata (MLP) from Argentina; Museu de Zoologia da Universidade de São Paulo (MZSP) and Museu Nacional, Rio de Janeiro (MNRJ) from Brazil; Museo Nacional de Historia Natural, Montevideo, Uruguay (MNHNM) and United States National Museum, Washington D.C., USA (USNM). The type material is housed in the following European and American museums: The Natural History Museum, London (NHMUK); Muséum d'Histoire naturelle de la Ville de Genève, Switzerland (MHNG); Zoological Museum - Natural History Museum of Denmark, (ZMUC); Museo Nacional de Historia Natural, Montevideo, Uruguay (MNHNM); and Academy of Natural Sciences of Drexel University (formerly Academy of Natural Sciences of Philadelphia) (ANSP). Fresh material was collected from different localities around Brazil, Uruguay, and Argentina throughout the current geographic distribution of the Recent species of Buccinanops.
Dissections were performed on ethanol-preserved specimens for studies of the external morphology and anatomy. Radulae were prepared according to the method described in Pastorino (2005) and observed using a Philips XL 30 scanning electron microscope (SEM) at the MACN. Shell ultrastructure data were procured from freshly fractured collabral sections taken from the central portion of the lip on the last whorl of at least, two individuals per taxon.
Most photographs were taken using a Nikon D100 digital camera with a 60 mm macro lens, except those—usually type specimens—sent by different curators. All images were digitally processed with Photoshop CS6 and Corel PhotoPaint X6 software.
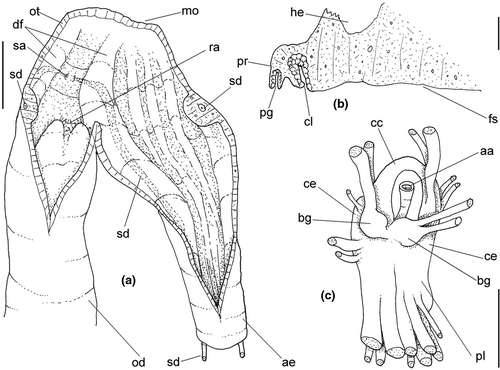
Abbreviations used in anatomical drawings: aa: anterior aorta, ac: siphonal fold anterior to gill, ad: adrectal sinus, ae: anterior esophagus, af: afferent gill vessel, ag: albumen gland, an: anus, au: auricle, bg: buccal ganglion, bc: bursa copulatrix, br: subradular membrane, bv: blood vessel, cc: cerebral commissure, ce: cerebral ganglion or cerebro-pleural ganglion, cg: capsule gland, cl: cement gland, cm: columellar muscle, cs: columellar muscle secondary flap, cv: ctenidial vein, dd: duct to digestive gland, df: dorsal fold of buccal mass, dg: digestive gland, di: diaphragmatic septum, ed: epipodial tentacle, ek: efferent renal vessel, ep: posterior esophagus, es: esophagus, fp: female pore, ft: foot, fs: foot sole/mesopodium, gd: duct of gland of Leiblein, ge: subesophageal/visceral ganglion, gi: gill, gl: gland of Leiblein, gm: gill muscle, go: gonopericardial duct, gp: pleural ganglion, gv: visceral ganglion, he: hemocoel, hg: hypobranchial gland, ig: ingesting gland, in: intestine, ki: kidney, kl: kidney lobe, km: kidney membrane with pallial cavity, m1–m11: odontophore muscles, mb: mantle border, me: mid esophagus, mj: jaw/peribuccal muscle, mo: mouth, mt: mantle, ne: nephrostome, ng: nephridial gland, nr: nerve ring, nv: nerve, oa: opercular pad, oc: odontophoral cartilage, od: odontophore, op: operculum, os: osphradium, ot: oral tube, ov: pallial oviduct, oy: ovary, pa: posterior aorta, pb: proboscis, pc: pericardium, pd: penial duct, pe: penis, pg: anterior furrow of pedal glands, pl: pedal ganglion, pn: penial gland, pp: penial papilla, pr: propodium, pt: prostate, py: pallial cavity, ra: radula, rn: radular nucleus, rm: retractor muscle of proboscis, rs: radular sac, rt: rectum, rv: afferent renal vessel, ry: rhynchostome, sa: salivary gland aperture, sm: stomach muscle, sc: subradular cartilage, sd: salivary duct, se: septum between odontophore and esophageal portions of buccal mass, si: siphon, sg: salivary glands, sp: salivary duct swollen region, ss: subterminal bulged portion of salivary duct, st: stomach, sv: seminal vesicle, te: tentacle, tg: integument, tm: transverse muscle between esophagus and odontophore, ts: testis, vd, vas deferens, ve: ventricle, vg: vaginal atrium, vl: valve of Leiblein, vo: visceral oviduct.
In text spm: specimen(s).
The phylogenetic analysis methodology described by Simone (2011) was applied, which basically used the TNT and Winclada softwares, with no special additional algorithms. A total of 14 new lines were added to the cladogram published by Simone (2011) and filled with characters of each species (Table 1) representing the ingroup Buccinanops and other taxa of Buccinoidea. No character needed to be added to Simone's list of characters, yet, some states were essential to improve the resolution of the buccinoideans currently introduced, as follows:
| Species | Family | Source |
|---|---|---|
| Oligohalinophila dorri (Wattebled, 1886) | Nassariidae | Simone (2007, 2011) (as Nassodonta) |
| Amphissa acuminata (Smith, 1915) | Columbellidae | Simone and Leme (2001); Simone (2011) |
| Amphissa cancellata (Castellanos, 1979) | “ | “ |
| Pustulatirus ogum (Petuch, 1979) | Fasciolariidae | Couto et al. (2015) |
| Hemipolygona beckyae (Snyder, 2000) | “ | “ |
| Pugilina morio (Linnaeus, 1758) | Melongenidae | Abbate and Simone (2015) |
| Pugilina tupiniquim Abbate & Simone, 2015 | “ | “ |
| Buccinum undatum Linnaeus, 1758 | Buccinidae | Wilsmann (1942); person. obs. |
| Engoniophos unicinctus (Say, 1826) | Nassariidae | Abbate et al. (2018) |
| Dorsanum miran (Bruguière, 1789) | Dorsaninae | Simone and Pastorino (2014) |
| Bullia granulosa (Lamarck, 1822) | “ | “ |
| Bullia laevissima (Gmelin, 1791) | “ | Abbate and Simone (2016) |
| Buccinanops monilifer (Kiener, 1834) | “ | Simone (1996, 2011), this study |
| Buccinanops latus sp. nov. | “ | “ |
| Buccinanops cochlidium (Dillwyn, 1817) | “ | This study |
| Buccinastrum duartei (Klappenbach, 1961) | “ | “ |
| Buccinastrum uruguayense (Pilsbry, 1897) | “ | “ |
| Buccinastrum deforme (King, 1832) | “ | “ |
| Buccinastrum paytense (Kiener, 1834) | “ | “ |
2.1 Characters in Simone (2011)
113: added state 5 = pair of posterior epipodial tentacles (Bullia, Dorsanum).
127: added state 3 = located lateral to short transverse flap (Buccinanops, Bullia, Dorsanum).
206: added state 4 = right edge convex (B. deforme, B. paytense, B. uruguayense).
316: added state 3 = pair of lateral, dorso-ventral thin muscles (Buccinanops, Buccinastrum, Bullia, Dorsanum).
332: added state 5 = laterally positioned, like lateral protractors (Buccinanops, Buccinastrum).
340: added state 2 = insertion on radular sac, radular nucleus and posterior end of cartilages (buccinoideans).
341: added state 3 = m2c (along median line – Bullia).
343: added state 3 = as circular thin fibers as opposite of m6 (buccinoideans).
344: added state 12(C) = m3l longitudinal lateral pair (Buccinanops, Buccinastrum) (actually 678).
352: added state 2 = multiple origins in outer edge of cartilages (Buccinanops, Buccinastrum except B. duartei, Bullia, Dorsanum).
359: added state 3 = originated in opposed side of m4 in cartilages (buccinoideans).
377: added states 3 = dorsal, Y-shaped (Buccinanops, Buccinastrum, Bullia, Engoniophos, Dorsanum, Buccinum?); 4 = similar, but multiple (2 or more) (B. deforme, B. uruguayense); 5 = similar, but inserting directly in cartilages instead of m3 (Bullia).
384: added state 4 = originated at cartilages posterior end by side of m5 (buccinoideans).
395: added state 2 = absent but with lateral arrangement connected to anterior end of cartilages only (buccinoideans, volutoideans).
418: added states: 4 = arched, with 2–3 cusps at edge (Pugilina, Pustulatirus, Hemipolygona); 5 = arched, with ~10 cusps at edge (Engoniophos, Buccinanops, Buccinastrum, Buccinum); 6 = arched, with ~20 cusps at edge (Bullia, Dorsanum).
424: added state 4 = wide, oblique, with long cusp in each end, being lateral cusp larger (Pugilina, Bullia, Dorsanum, Engoniophos, Buccinum, Buccinanops, Buccinastrum).
426: added state 3 = presence of intermediary small cusps (Bullia laevissima, Buccinum, Buccinanops).
450: added state 5 = very narrow and long, running attached to esophagus by side of aorta branch (buccinoideans).
452: added states 3 = close to median line, ventral (Pugilina, B. cochlidium, B. latus, B. monilifer); 4 = lateral, close to dorsal folds (Bullia, Dorsanum, Engoniophos, Pustulatirus, Hemipolygona, B. duartei); 5 = lateral, far from dorsal folds (B. deforme, B. paytense); (B. uruguayense = 0).
478: added state 3 = broad, thick walled (Buccinastrum, Bullia).
485: added state 4 = absent, but valve only vestigial (B. latus, B. monilifer, B. cochlidium).
493: added state 2 = similar, but with bulged portion towards posterior (Buccinanops, Buccinastrum, Bullia, Dorsanum) (additive).
554: added state 2 = gonopericardial duct present (Buccinanops, Buccinastrum, Bullia).
575: added state 3 = in subterminal exposed small papilla (Buccinanops, Buccinastrum).
660: added state 2 = with buccal commissure imbibed in nerve ring (Buccinanops, Buccinastrum).
667: added states 2 = concentrated, but with cerebral commissure long, forming small strap-like arc (Buccinanops, Buccinastrum, Bullia, Dorsanum); 3 = same, but with commissure very long (about 2–3 times longer) (B. latus, B. monilifer, B. cochlidium).
The resulted consensus cladogram is presented in the Figure 35, with the synapomorphies of each node presented. A few states presented ambiguity in the optimization in the cladogram, in these cases the acctran algorithm was used in that Figure. The characters with minor ambiguity in some branches are: 9, 180, 331, 377, 418, 424, 426, 478, 498.
3 RESULTS
3.1 Systematics
3.1.1 Subfamily Buccinanopsinae Galindo, Puillandre, Louzet & Bouchet, 2016
Additional diagnosis: Fusiform shell from obese to relatively elongated. Sculptured mainly with undulating growth lines. Suture well-marked. Aperture simple, from 1/3 to 1/2 of shell length. Outer lip simple, non-determinate growth. Siphonal canal simple, wide, non-protruded. Callus normally thin. Head-foot usually unpigmented, lacking eyes; head flap-like, with tentacles on both sides. Siphon long, bearing small fold in its base. Lateral, dorso-ventral muscles uniting anterior regions of esophagus and odontophore. Pair of odontophore retractor muscles (m2) with multiple origins. Rachidian flattened, arched, ~20 cusps in cutting-edge. Stomach with posterior bulge. Penis duct thick-walled, convolute, muscular. Nerve ring with cerebral commissure long, widely arched.
Included taxa: Genera: Buccinanops (type), Buccinastrum gen. nov.
3.1.2 Genus Buccinanops d'Orbigny, 1841
Type species: Buccinum cochlidium Chemnitz, 1795 (=B. cochlidium Dillwyn, 1817) by original designation (d’Orbigny, 1841: 432). Chemnitz's work was rejected by the ICZN Direction 1 (1954). Cernohorsky (1984: 23) mentioned Herrmannsen (1846) as the source of designation. However, while on page 127 of Herrmannsen (1846) it is clearly stated that cochlidium is the type species, it is evident that Herrmannsen was only following d’Orbigny's original designation and not designating the type species himself. Allmon (1990: 20) wrongly stated that the type species was Buccinum globulosum Kiener, 1834. No reference to this type designation can be found in d’Orbigny (1841). Buccianops Adams & Adams, 1858: 113 is a typing error pro Buccinanops. The gender is masculine according to the art. 30.1.4.3 of the ICZN (1999).
Diagnosis: Shell bucciniform, medium or large, smooth, slightly shining; walls thin. Sometimes with shallow subsutural flat cords and/or small sharp nodes or spines along keel in last two or three whorls. Flat cords also covering basal surface of last whorl. First teleoconch whorls with axial riblets. Spire variable, always less than 1/3 of maximum shell height. Weak terminal columellar fold always present. Parietal callus variable, ranging from thin glazed area to thicker protuberant callus. Operculum large with smooth margins and terminal nucleus. Duplicity of pair of extrinsic odontophore muscles m1b; aperture of salivary glands ventral, close to median line; simplified anterior esophagus; papilla-like female genital pore. Radular central tooth multicuspidate, with 5–12 cusps increasing in size towards middle of tooth, rachidian base strongly curved; lateral teeth with inner and outer cusps hook-shaped, always with 1 to 3 intermediate cusps. Egg capsules always attached on the callus area of female shells only. Nurse eggs, non-planktonic larvae and crawling veliger.
Species included: B. cochlidium, B. latus sp. nov., and B. monilifer.
3.1.3 Buccinanops cochlidium (Dillwyn, 1817)


Buccinum cochlidium Chemnitz, 1795: 275, pl. 209, figs. 2053, 2054 (invalid name); Dillwyn, 1817: 627. Kiener, 1834: 10, pl. 6, fig. 17; Anton, 1838: 91; Deshayes, 1844: 187.
Buccinum Lamarkii (sic) Kiener, 1834: 5, pl. 3, fig. 6.
Buccinanops cochlidium: d'Orbigny, [1839] pl. 61, fig. 25; M. E. Gray, 1850: 20, 139, pl. 98, fig. 9; Carcelles & Parodiz, 1939: 751, figs. 2, 6; Carcelles, 1944: 250.
Bullia Cochlidium: Gray, 1839: 126.
Buccinum (Buccinanops) cochlidium: d'Orbigny, [1841]: 434.
Buccinum gradatum Deshayes, 1844: 186.
Bullia gradata: Reeve, 1846: pl. 1, fig. 3; Chenú, 1859: 160; Peile, 1937: 185, fig. 21; Abbott & Dance, 1986: 177, fig. 9.
Bullia cochlidium: Reeve, 1847: pl. 4, fig. 23; Hanley, 1856: 117, pl. 23, fig. 95; Chenú, 1859: 159, fig. 747.
Bullia (Buccianops) (sic) cochlidium: H. & A. Adams, 1858: 113.
Bullia (Buccinanops) cochlidium: H. & A. Adams, 1858: 113; Tryon, 1882: 13, pl. 5, fig. 73, pl. 6, figs. 76, 78.
Buccinanops (Buccinanops) cochlidium: Fischer, 1884: 636.
Bullia (Buccinanops) gradatum: Watson, 1886: 190.
Bullia (Buccinanops) cochlidium: Paetel, 1888: 116.
Bullia cochlidium: Cossmann, 1901: 220.
Bullia gradata pampeana Ihering, 1907: 445; Feruglio, 1933: 236, 239.
Buccinanops (Buccinanops) cochlidium: Thiele, 1929: 32.
Buccinanops gradatum: Carcelles & Parodiz, 1939: 754, pl. 3–5, fig. 3; Barattini & Ureta, 1960: 114; Castellanos, 1970: 92, pl. 7, fig. 6; Rios, 1970: 93, pl. 27, 1985: 103, pl. 35, fig. 453; Penchaszadeh, 1971a, 1971b: 480, figs. 1e, 2f, 2g, 1973: 15, fig. 1; Scarabino, 1977: 188, pl. 5, fig. 4; Cernohorsky, 1984: 28; Farinatti, 1985: 219; Calvo, 1987: 143, fig. 110.
Buccinanops cochlidium: Barattini & Ureta, 1960: 113, 114; Castellanos, 1970: 93, pl. 7, fig. 10; Cernohorsky, 1984: 27, figs. 94–95; Aguirre, 1993: 33, pl. 2, figs. 2 a-b; Pastorino, 1993a: 162, fig. 4–6; 1993b: 153, figs. 2–5.
Buccinanops lamarckii: Richards & Craig, 1963: 140; Rios, 1970: 93; Rios, 1975: 96, pl. 27, fig. 401; Scarabino, 1977: 189, pl. 5, fig. 3; Rios, 1985: 103, pl. 35, fig. 454; Calvo, 1987: 143, fig. 111.
Bullia (Buccinanops) cochlidia: Allmon, 1990: 20, pl. 3, figs. 1, 13, pl. 6, fig. 34.
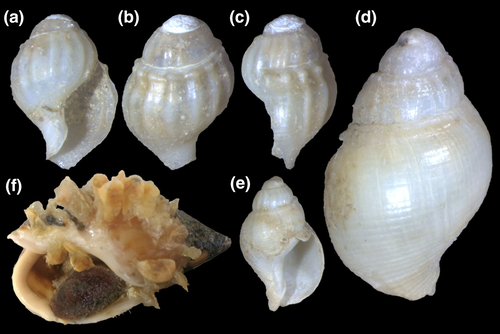
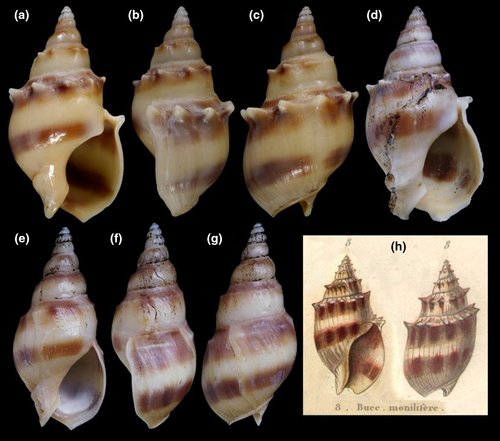
Type material: Buccinum cochlidium: holotype ZMUC, figured by Aguirre (1993a, pl. 2, Figure 2a–b), here in Figure 1a–c; B. gradatum: holotype MHNG 1296/18 (Figure 1d–f); B. lamarckii: holotype MHNG 1296/4 (Figure 1i–k); Bullia gradata pampeana: holotype MACN-Pal 855 (Figure 1g–h).
Type localities: Buccinum cochlidium: Islands of South seas (in error); B. gradatum: New Zealand (in error); B. lamarckii: unknown, no locality mentioned; Bullia gradata pampeana: “dépôts pampiens de Puerto Militar” (Quaternary deposits at Puerto Belgrano, Buenos Aires province).
3.1.4 Description
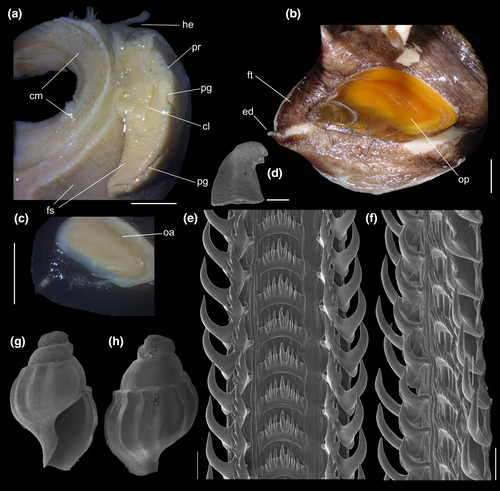
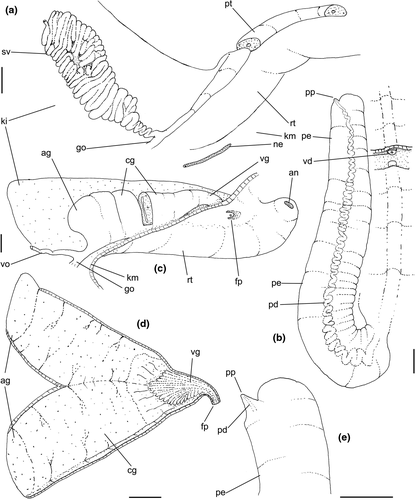
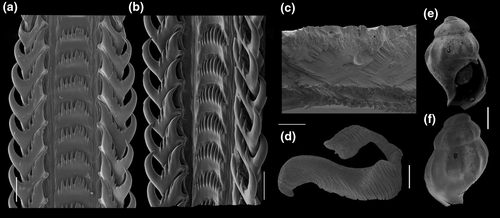
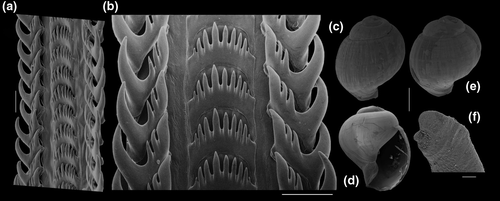
Shell: (Figures 1, 2) Large (maximum height 95 mm), somewhat variable, usually fusiform. Protoconch paucispiral of 1.5 to 2 whorls, 1.5–1.7 mm wide in second whorl; first whorl smooth, followed by 10–12 weak axial riblets after second whorl (Figure 3a–b). Teleoconch of 6–7 shouldered or globose whorls. Spire rounded, ~1/3 of total shell length. Suture well defined, impressed; blunt subsutural carina in ~50% of specimens; aperture large, usually half of total shell length. Parietal callus usually weak, stronger in large specimens. Sculpture absent except for growth lines and shallow undulations. Left edge of siphonal canal with pointed anterior beak of ~30°. Ultrastructure of shell usually with three layers, outermost layer very thin or absent, of amorphous calcite, intermediate layer thicker, of crossed-lamellar aragonite, with crystal faces oriented collabrally, and innermost layer thinner, of crossed-lamellar aragonite oriented perpendicular to outer lip.
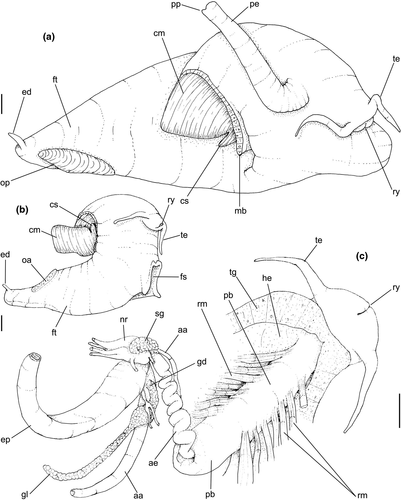
Head-foot: (Figures 3a–c, 4a–c) Head weakly protruded, somewhat socket-like, narrow (~50% of head-foot width); color dark-brown, almost black (Figure 3c) in exposed areas (except for foot sole and opercular pad), particularly darker in head and in median-dorsal area of foot. Tentacles well-separated from each other; elongated and narrow, eyes absent (Figure 4a–c). Rhynchostome as longitudinal slit located in middle region of lightly protruded head ventral surface (Figure 4a–c: ry). Foot broad, of about 3/4 whorl when retracted. Sole rather triangular, edges thick and rounded (Figure 4a–b: fs). Anterior furrow of pedal glands almost imperceptible, marked by narrow furrow in anterior edge (Figures 3a: pg, 4b); pedal gland thick in medial region, immersed in pedal musculature (Figure 3a: superior pg). Single, small epipodial tentacle (ed) located in dorso-posterior end of foot, originating in dorsal region of edge posterior to operculum. Opercular pad elliptical, ~50% as wide as dorsal surface of foot; attachment with operculum occupying ~80% of its area (Figures 3c, 4b: oa). Columellar muscle thick, spanning, ~1.5 whorl; distal end bifid, right element wide (cm), left element narrow (cs), encased in furrow kept by columellar fold (Figure 4a-b). Female with small orifice of cement gland located on median line of anterior sole region (Figure 3a: cl); inner space wider than narrow duct.
Operculum: (Figures 2m-n, 4a: op) Elliptical, corneous, pale brown, translucent. Nucleus terminal in inferior corner. Outer edge straighter than inner edge. Outer surface with concentric growth lines, forming undulations. Scar oval, occupying about 80% of inner area, somewhat dislocated closer to inner edge.
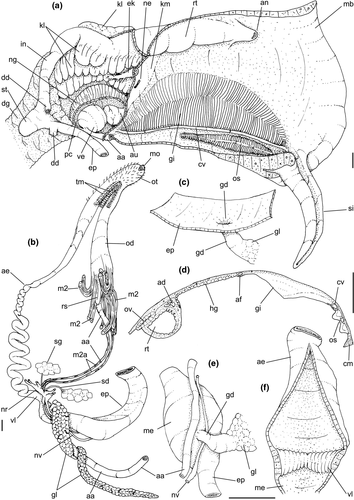
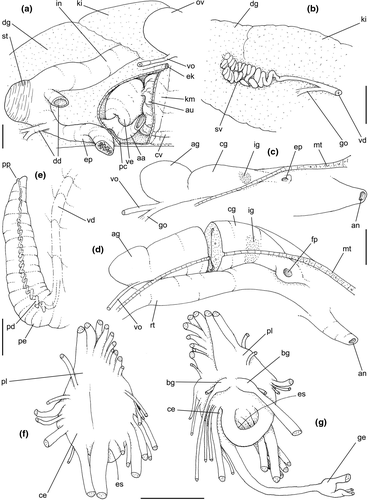
Pallial cavity: (Figure 5a, d) Mantle edge simple, thick. Siphon long, slender, ~10% of mantle edge length, almost as long as 2/3 of pallial cavity when retracted (Figure 5a: si); edges simple. Osphradium of ~1/2 length, and 1/10 width of pallial cavity; anterior end pointed, but more rounded than narrow, tail-like posterior third (Figure 5a: os); osphradium filaments symmetrical, relatively low, isosceles-triangular (Figure 5d: os). Very narrow area between osphradium and gill. Ctenidial vein (cv) narrow, with uniform width along its length. Gill (gi) ~80% of pallial cavity length, ~1/2 its width at widest middle region; anterior end broadly pointed, located at some distance from mantle edge. Posterior gill end slightly more pointed than anterior end, touching pericardial area. Afferent gill vessel very narrow, lying at short distance from right edge of gill (Figure 5d: af). Gill separated from right edge of pallial cavity by area equivalent to its width. Hypobranchial gland thin, light-brown, covering most of area between gill and rectum, slightly thicker in anterior quarter, vaguely pigmented light-brown. Rectum broad, with thick walls, running along right edge of pallial cavity (Figure 5d: rt). Anus simple, siphoned (terminally detached), located near mid length of pallial cavity (Figures 5d, 7c: an). Pallial gonoducts located between rectum and pallial right edge as described below.
Visceral mass: (Figure 5a) Anterior quarter of whorl mostly occupied by kidney (km) and pericardium (pc). Digestive gland light-brown, located along inferior (siphonal) region of each visceral whorl, covering middle digestive tubes and two whorls posterior to stomach. Gonad pale light-brown, lying along superior and columellar surfaces of visceral whorls posterior to stomach. Stomach small, located half whorl posterior to pallial cavity (Figure 5a: st).
Circulatory and excretory systems: (Figure 5a) Pericardium located just posterior to gill, along left anterior region of visceral mass (pc), slightly dislocated anteriorly along right side of posterior 1/10 of gill. Auricle (au) small, attached to anterior surface of pericardium, with ctenidial vein entering from left and connected to kidney at its right end. Auricle connected to anterior surface of ventricle. Ventricle (ve) large, rounded. Aortas located along postero-left region of ventricle. Kidney volume equivalent to ~1/4 of pallial cavity volume, 4-times larger than pericardium; located along middle and right regions of anterior end of visceral mass. Nephridial gland (ng) triangular in section; lying along dorsal-right region of reno-pericardial wall. Renal lobe (kl) in three longitudinal, thick flaps, turned to left, transversely folded, occupying most of kidney's interior volume (Figure 5a: kl); intestine running along kidney right side. Efferent renal vessel (ek), well-developed, inserted in dorsal kidney lobe flap.
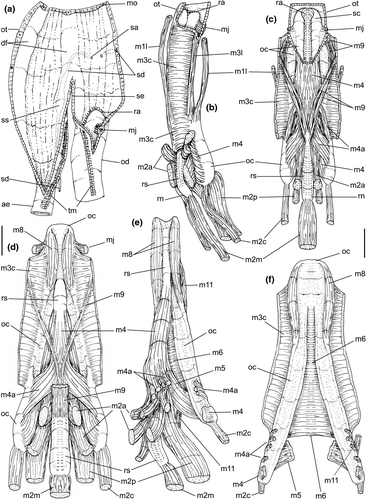
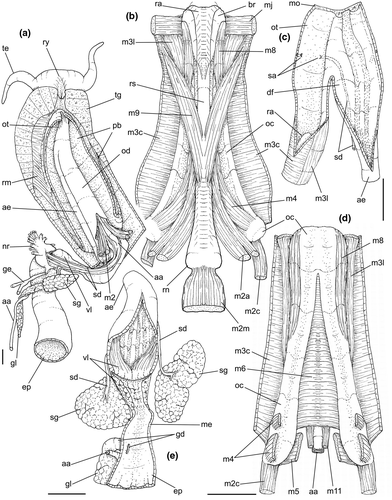
Digestive system: (Figures 4c, 5b, c, e–f, 6) Proboscis as long as foot, 1/4 of head-foot width (Figure 4c: pb). Circular mouth at proboscis tip (Figure 5b: mo). Buccal cavity with pair of broad and low dorsal folds (Figure 6a: df), occupying ~1/3 of dorsal wall, with interval between them slightly broader than each fold. Odontophore oval, as long as retracted proboscis (Figure 5b: od). Odontophore tube connecting it with buccal cavity, length equivalent to ~1/5 of odontophore length, possessing mostly longitudinal muscles (Figure 6a, c: ot). Odontophore muscles (Figure 6a–f): m1, several small jugal muscles connecting buccal mass to adjacent inner surface of proboscis; one pair more developed originating in peribuccal lateral region of proboscis, running posteriorly, inserting in lateral side between middle and posterior thirds of odontophore (Figure 6b–c: m1l); tm, pair of transverse muscles connecting lateral walls of odontophore with adjacent wall of esophagus (Figures 5b, 6a: tm), along anterior third of odontophore; set of m2, several retractor muscles of odontophore, part of them working also as dorsal tensor muscles of radula, all originating in ventral surface of hemocoel, in region just posterior to proboscis, running anteriorly, some median fibers running through nerve ring, insertions as following: m2m, median m2, single, inserting on radular nucleus together with small branch of aorta (Figure 6b–e); m2a, broad and thick pair auxiliary of m2 and dorsal tensor muscles of radula, with 4–5 separated pairs of bundles, inserting along radular sac in its middle-lateral regions (Figure 6b–e); m2c, lateral odontophore retractors, inserting at posterior end of each odontophore cartilage (Figure 6b–e); m3c, single circular, constrictor muscle covering? dorsal surface of odontophore, inserting along outer side of dorsal edge of cartilages at along ~70% their length (Figure 6b–f); m3l, pair of narrow, lateral longitudinal muscles, originating in lateral region of odontophore tube, running posteriorly attached to odontophore lateral surface, inserting in fan-like manner lateral side of m3c posterior surface (Figure 6b); m4, strong pair of dorsal tensor radular muscles, originating in odontophore cartilages on posterior-ventral surface, running toward dorsal surrounding lateral surface of cartilages, inserting laterally along radular sac (Figure 6b–f); m4a, 4–5 small pairs auxiliary of m4, of similar attributes, but originating in bundles along outer-dorsal edge of cartilages, in their posterior quarter (Figure 6c–f); m5, pair of secondary dorsal tensor muscles of radula, originating in posterior-dorsal surface of posterior end of cartilages, running dorsally and medially mixing with m4, inserting into radular sac alongside and medial to m4 insertion (Figure 6e–f); m6, thin horizontal muscle, uniting both odontophore cartilages just posterior to anterior fusion, along ~70% of cartilage length, inserting along ventral and internal edge of cartilages, becoming gradually wider posteriorly (Figure 6e–f); m8, pair of cartilages shortener muscles, located along dorsal edge of anterior region of cartilages, along almost 1/3 their length, thicker anteriorly, gradually narrowing posteriorly (Figure 6d–f); m9, two pairs of narrow protractor muscles of radula, one located just anterior from another (posterior slightly broader), originating on posterior half of dorsal edge of cartilages, running medially towards anterior, inserting along m3c anterior fibers (Figure 6c–d); m11, pair of ventral tensor muscles of radula, thin, narrow, originating in posterior-ventral end of cartilages, running anteriorly, and medially covering m6, inserting into ventral edge of radula and subradular cartilage, and some inner portion preceding this (Figure 6e). Subradular cartilage expanding in exposed region of radula into buccal cavity, covering neighboring surface of radula (Figure 6c: sc); oc, odontophore cartilages, elongate, furrow-like, flattened, ~20 times longer than wide; fusion between both cartilages in anterior-medial end, along ~20% their length (Figure 6e–f); subradular membrane, covering inner surface of subradular cartilage and radula, having insertions of m4, m5 and m11 (Figure 6d–e). Radula (Figure 3e–f): slightly longer than odontophore, ~50 rows of teeth; rachidian tooth wide, comb-like, occupying ~half of radular width; base curved, width ~3 times greater than its length; ~9 long, sharp pointed cusps of similar size, except for some diminishment toward lateral edges, lateral cusps very small, sometimes vestigial; distance between rachidian and lateral tooth equivalent to 1/3 of rachidian width; lateral tooth with two major and 2–4 minor long cusps; base equal in width to rachidian tooth; base deflected posteriorly by ~50 degrees; outer cusp about as long as tooth base, scythe-shaped; inner cusp shorter, stout, with 2–4 shorter cusps of decreasing size emerging from its surface outer cusp and set of inner cusps separated from each other by smooth area equivalent to 1/4 tooth's width. Salivary glands enveloping esophagus along region of valve of Leiblein and nerve ring (Figures 4c, 5b: sg), size slightly larger than nerve ring; their ducts very narrow, running along anterior esophagus wall and, more anteriorly, inside dorsal folds of buccal cavity (Figure 6a: sd); opening with very small pore (Figure 6a: sa), into anterior-middle region of dorsal folds of buccal cavity; salivary ducts with enlarged bulges along middle area of dorsal folds (Figure 6a: ss). Anterior esophagus with somewhat thick walls, twice length of odontophore; inner surface with 12–15 narrow, uniform longitudinal folds; posterior half zigzagging intensely (Figures 4c, 5b: ae). Valve of Leiblein almost imperceptible externally; inner structure only marked by transverse wide glandular fold (Figure 5f: vl), lacking any valve or long cilia; smooth area anterior and posterior to transverse fold (Figure 5f); oblique furrow (bypass) absent. Middle esophagus ~5% of anterior esophagus length (Figure 5e: me), walls thin; inner surface like that of anterior esophagus; aperture of gland of Leiblein as a minute pore. Gland of Leiblein narrow, elongated (Figures 4c, 5b: gl), ~70% posterior esophagus length; anterior region ~double of middle esophagus width, gradually tapering toward posterior; middle portion surrounding local portion of anterior aorta (Figures 4c, 5b: aa). Duct of gland of Leiblein short, narrow, simple (Figure 5b, e: gd). Posterior esophagus (Figures 4c, 5b: ep) ~70% anterior esophagus length, very wide (4–5 times wider than wider portion of anterior esophagus), slightly flattened, narrowing gradually posteriorly (Figure 5a: ep) in same width as anterior esophagus; inner surface with 12–15 narrow, tall, irregular, sometimes coalescent longitudinal folds. Stomach small blind sac, ~1/4 width of adjacent visceral whorl, ~1/4 whorl long; located ~half whorl posterior to pallial cavity, approximately in middle position of that whorl (Figure 5a: st). Esophageal insertion and intestinal origin in anterior side of stomach relatively well-separated from each other; 2 narrow ducts to digestive gland, being one in esophageal insertion, turned posteriorly, another in intestinal origin, turned left (Figure 5a: dd). Gastric inner surface mostly smooth. Intestine about as wide as adjacent posterior esophagus; weakly sigmoid visceral loop (Figure 5a: in) immersed initially in digestive gland, afterwards passing though ventro-left side of kidney, immersed in renal lobe. Rectum and anus described above (pallial cavity).
Genital system: Male: (Figures 4a, 7a, b, e) Gonad along columellar region of each visceral whorl. Intensely coiled seminal vesicle (Figure 7a: sv) along ~1/2 whorl in region preceding pallial cavity; placed slightly obliquely; more posterior region narrower, gradually widening anteriorly; narrowing in region preceding pallial cavity in short zigzag in central-left region of visceral anterior border, just posterior to nephrostome (Figure 7a: ne). gonopericardial duct relatively wide, inserting in vas deferens at end of its coiled portion (Figure 7a: go). Prostate spanning about 1/3 pallial cavity length, narrow, closed (tubular), lying ventrally and to right of rectum (Figure 7a: pt). In region posterior to anus, vas deferens gradually crossing to pallial cavity floor, narrow; walls relatively thick, running relatively straight up to penis base (Figure 7b: vd) immersed in pallial floor integument. Penis slender, curved backwards in base, about ~80% of pallial cavity length, ~15 times longer than its width, dorso-ventrally flattened (Figures 4a, 7b: pe); base narrow, located at some distance from right tentacle (Figure 4a); middle level twisting, narrowing gradually up to blunt tip, with small right papilla (Figures 3d, 7b, e: pp). Penial duct running approximately along penis center, narrow, intensely coiled along its entire length, slightly less coiled and narrow in distal 1/5 (Figure 7b: pd). Penial aperture in apex of papilla (Figure 7e: pd), very small.
Female: (Figure 7c–d) Visceral oviduct narrow, running along middle region of columellar surface of last whorl of visceral mass, closely preceding pallial cavity (Figure 7c: vo). Posterior region of pallial oviduct protruding into kidney. Albumen, whitish, (Figure 7c: ag) and capsule, pale beige, (cg) glands adjacent, albumen gland spanning posterior 1/5 of pallial oviduct. Seminal receptacle absent. Capsule gland with flat lumen, vaginal furrow running along its left edge, with surface smooth (Figure 7d: cg). Vaginal duct as tapering region preceding female pore (Figure 7c–d: vg), wall thin, inner surface narrow longitudinal folds. Female pore narrow, weakly protruded, papilla-like, sheltered in shallow cavity (Figure 7c: fp). Bursa copulatrix absent. Cement gland in pedal sole described above (head-foot) (Figure 3a: cl).
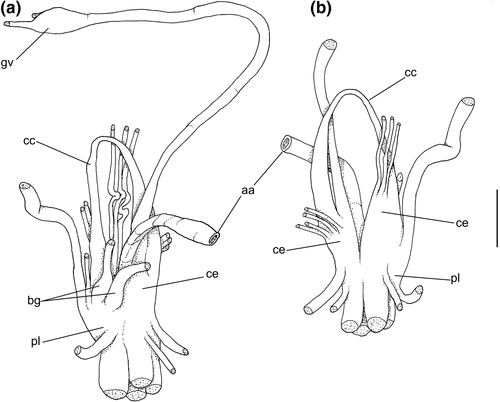
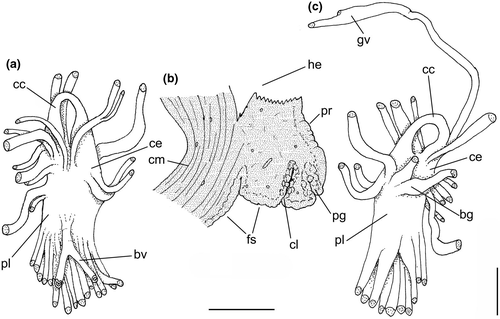
Central nervous system: (Figure 8a–b) Well-concentrated, most connectives and commissures indistinct; total nerve ring volume ~1/30 that of hemocoel. No distinction between pleural and cerebral ganglia (ce). Each cerebral/pleural ganglia ~1/4 of nerve ring volume. Cerebral commissure very long, strap-like, extending dorsally ~30% more than nerve ring size; narrow in middle, thickening on both sides, without clear distinction with cerebral ganglia (Figure 8a–b: cc). Pedal ganglia (pl) slightly smaller than cerebro-pleural ganglia; located just ventral to cerebro-pleural ganglia as almost single mass; deep furrow separating both pedal ganglia (Figure 8b: pl). Buccal ganglia (Figure 8a: bg) small, located side by side in region close to left cerebral ganglion. Visceral ganglion (Figure 8a: vg) ~1/4 of pedal ganglion size, located 3-times nerve ring length posteriorly to it. Subesophageal ganglion not detected, possibly embedded within nerve ring.
Habitat: Inhabits sandy bottoms, from 6 to ~50 m depth. It feeds primarily on dead fishes and crustaceans. It is frequently found associated with the sea anemone Antholoba achates (Drayton in Dana, 1846) (as Phlyctenanthus australis in Pastorino, 1993) in the Peninsula Valdes area (~42°S), living on the dorsum of the shell. Occasionally it has been observed feeding on live bivalves.
Distribution: From Rio Grande do Sul, Brazil (~32°S) to Puerto Madryn, Golfo Nuevo, Chubut, Argentina (~42°S).
Material examined: BRAZIL. Rio Grande do Sul. Rio Grande, Tramandaí, MZSP 77556, 1 spm, MNRJ 6092, 2 spm; Praia do Imbe, MNRJ 2923, 2 spm, MNRJ 6062, 2 spm; Cassino, MZSP 61463, 7 spm, MZSP 19197, 3 spm, MZSP 44023, 2 spm, MACN-In 8221, 2 spm, MNRJ 690, 2 spm, MNRJ 3467, 3 spm, MNRJ 4172, 1 spm; Albardão, MZSP 16203, 2 spm; MZSP 51731, 1 spm in 13 m, 32°58'S, 52°30'W; MZSP36633, 1 spm; MZSP 53201, 3 spm; 531, 6 spm; MZSP 19178, 3 spm; MZSP 19179, 5 spm; MZSP 19180, 3 spm; MZSP 19181, 1 spm; MZSP 19182, 3 spm; MZSP 19184, 1 spm; MZSP 19186, ~10 spm; 29°43′S 49°55′W, 24 m, MZSP 19187, 1 spm (GEDIP sta. 1715, RV W. Besnard col, 8.iv.1972); MZSP 19188, 5 spm; MZSP 19189, 1 spm, Albardão-Sarita, 18–28 m, MNRJ 2872, 3 spm; Pta. Cidreira, MNRJ 2922, 3 spm; São Jose do Norte, Praia Grande, MNRJ 691, 2 spm; Ponta da Cidreira, MNRJ 2177, 2 spm; No precise locality, MZSP 19190, 1 spm; MZSP 19192, 2 spm; MZSP 19197, 1 spm; MZSP 32892, 3 spm; MZSP 32902, 3 spm; MZSP 51313; 33°24′S, 52°47′W, MACN-In 15347, in 13 m, 3 spm. URUGUAY. Rocha; MZSP 51679, 2 spm, MZSP 52424, 1 spm; Cabo Santa Maria, MZSP 13394, 1 spm; MACN-In 23569, 6 spm; USNM 331316, 1 spm; La Coronilla, USNM 359259, 1 spm; La Paloma, MZSP 19183, 2 spm, MZSP 19196, 3 spm; MZSP 33348, 1 spm; MZSP 33400, 2 spm, MNRJ 1236, 3 spm, MNRJ 6546, 2 spm, MACN-In 15151, 14 spm; MACN-In 30484, 3 m, 3 spm, MACN-In 15314, 6 spm. Maldonado; MZSP 10050, 10 spm, MZSP 51654, 2 spm; Punta del Este, MZSP33353, 1 spm; Piriapolis, MACN-In 18158, ~30 spm, MZSP 57510, 34°5′S 53°30′W, in 20 m, MACN-In 15922, 34°38′S, 52°15′W, 128 m, 1 spm. ARGENTINA. Buenos Aires; MACN-In 15851, 35°24′S, 53°10′W, 28 m, 1 spm, MACN-In 25200, 35°40′S 56°03′W, 9 m, 22 spm, MACN-In 15980, 35°08′S 52°35′W, 6 spm, MACN-In 24147 36°24′S 55°53′W; MACN-In 24300, 36°44′S 56°34′W, 9 m, 9 spm; off Ostende, MACN-In 21779, 1 spm; Mar Chiquita, MACN-In 3922, 1 spm; Mar del Plata, MACN-In 10287, 2 spm, MACN-In 19140, 3 spm, MACN-In 19273, 8 spm, MACN-In 10768, 5 spm, MACN-In 12069, 1 spm, MACN-In 11999, 3 spm, MACN-In 9363-12, MACN-In 19139-1, 1 spm; Villa Gesell, MACN-In 28827, 2 spm; 38°08′S 56°58′W, MACN-In 23380, 60 m, 4 spm; Puerto Quequén, MACN-In 23206, 26 spm, MACN-In 18672, 5 spm; Necochea, MACN-In 9368-28, 2 spm; Ostende, MACN-In 20099, 7 spm; Monte Hermoso, MACN-In 6619–31, 8 spm, MACN-In 11230, 18 spm, MACN-In 9209-17, 3 spm, MZSP 51650, 10 spm, MZSP 389, 7 spm, 10056, 1 spm; USNM 219862, 6 spm; USNM 465581, 1 spm; Cabo San Antonio, MACN-In 16306, ~36 spm; Carmen de Patagones, MZSP10058, 10 spm; Arroyo Parejas, Bahía Blanca, MACN-In 11232, 12 spm; Puerto Belgrano, MACN-In 11123–1, many spm; Bahía San Blas MACN-In 22943, 3 spm, MACN-In 20273–1, 2 spm; Mouth of Rio Negro river, MACN-In 20526, 1 spm; 40°56′S 62°28′W, 17 m, MACN-In 20671, 2 spm. Rio Negro; Puerto San Antonio, USNM 568254, 2 spm; San Antonio, MZSP52101, 1 spm; Faro Punta Villarino, San Antonio Oeste, MACN-In 13146-2, 2 spm, MACN-In 9376; MACN-In 9379–30, 4 spm, MACN-In 9152, MACN-In 9152-14, 18 spm, MACN-In 9379–31, ~26 spm, MACN-In 23705, 1 spm. Chubut; Golfo San Matias, MZSP 51328, 1 spm, MZSP 10057, 2 spm, MACN-In21203, 4 spm, MNRJ 36, 1 spm; Faro Punta Villarino, MACN-In 13146-1, 1 spm; Puerto San José, MACN-In 9174, 2 spm; Playa Larralde, MACN-In 37814, 12 spm; Puerto San José, MACN-In 26435, 128 m, 14 spm; Golfo San José, MACN-In 9174–15, 2 spm, MACN-In 9441-6, 9 spm; Playa Villarino, 42°24′S 64°17′W, MACN-In 38162, 10 m, 25 spm; Peninsula Valdes, MNRJ 2927, 2 spm, MNRJ 9399, 2 spm; Puerto Pirámides, MACN-In 26434, 14 spm. MACN-In 11484, 7 spm; Punta Norte, MACN-In 9158-7, 2 spm; Puerto Madryn, MZSP10054, 2 spm.
Remarks: Buccinum cochlidium Chemnitz, 1795 was rejected by ICZN Opinion 184 (1944) and Direction 1 (1954), which rejected and made invalid all new specific names proposed in that work. The next available name in line was also Buccinum cochlidium, changing the author to Dillwyn (1817). When Deshayes (1844) reviewed the 2nd edition of Lamarck's Histoire Naturelle des animaux sans vértebrés, he described Buccinum gradatum based on the specimen housed in Lamarck's collection and figured by Kiener (1834: 10, pl. 6, Figure 17) (Figure 1d) as Buccinum cochlidium. The material is now at MHNG (Figure 1e–f). Tryon (1882) raised some doubts about the validity of B. gradatum; however, the name was sustained by most later authors. The probable holotype of Buccinum lamarckii Kiener, 1834, housed in MNHNG (Figure 1i–k) clearly represents the southern morph of Buccinanops cochlidium.
A. d'Orbigny (1839, pl. 61, Figure 25) (reillustrated by M. E. Gray, 1850) depicts an apparently juvenile specimen of Buccinanops cochlidium. Later, Pilsbry (1897) considered it as Bullia uruguayensis. The figure is not clear and based on a juvenile specimen and could be any one of these species. The Buccinanops material from d’Orbigny's collection, housed in NHMUK was examined, and we conclude that it contains several lots with the following species: B. deforme: 1854.12.4.469, 4 sp., 1854.12.4.470, 11 sp.; 1854.4.466, 6 sp.; B. uruguayense 1854.12.4.467, 12 sp.; B. monilifer: 1854.21.4.465, 9 sp.; B. paytense: 1854.21.4.463, 1 sp.; B. cochlidium: 1854.21.4.461, 4 sp.; 460, 2 sp.; 462, 1 sp.; 464, 3 sp.; 459, 1 sp.; 458, 1 sp.
Ihering (1907) described Bullia gradata pampeana, a fossil subspecies from Quaternary deposits of Pleistocene age according to Parodiz (1996). Ihering (1907) also mentioned doubts about the differences between his Bullia gradata and B. cochlidium, showing that the typical form without subsutural cord (B. cochlidium) gradually change to that of thicker shell, with subquadrate profile and rectangular whorls (B. gradatum). Cernohorsky (1984) also mentioned the possibility that Buccinanops gradatum could be only a variation of B. cochlidium. However, several modern authors (e.g., Calvo, 1987; Rios, 1985) keep both names in use. Later, Allmon (1990) included B. gradatum in the list of synonyms of B. cochlidium. Allmon (1990) also subordinated Buccinanops as a subgenus of Bullia which was discussed by Pastorino (1993a) who reinstated the full generic status.
Buccinum turritum Perry, 1811, was mentioned by Deshayes (1844) as a dubious synonym of B. cochlidium Chemnitz. Petit (2010) stated that Perry's name could be nomen dubium until “specialist with all the available types rule out about the validity of this name.” We have seen all the existent type material related with these names, and there is none with real similarity to the picture of B. turritum. The regular axial ribs, particularly on the last whorl, appear as a characteristic feature of B. turritum, which are absolutely absent in any species of Buccinanops. We agree with Petit's designation of B. turritum as a nomen dubium and according to our observations, without any relationship with the genus Buccinanops.
Tryon (1882) and Allmon (1990) synonymized Buccinum labyrinthum Gmelin, 1791 from the Netherlands coast with Buccinanops cochlidium. Pastorino and Kantor (1998) discovered that Gmelin's species is actually an anomalous specimen of Buccinum undatum L. with no connection to the South American species.
Penchaszadeh (1971, 1973) described the egg capsules of several species of Buccinanops including material of the form "gradatum" from Mar del Plata, Buenos Aires, Argentina. The morphology and ornamentation of the capsules are exactly the same of those from specimens from Puerto Pirámide, off Chubut Province where the only available form is "cochlidium."
3.2 Buccinanops latus sp. nov.

Buccinanops lamarckii: Rios, 1970: 93; 1975: 96, pl. 27, fig. 401; 1985: 103, pl. 35, fig. 454; 2009: 246 (fig.) (non Kiener, 1834).
Buccinanops gradatum: Rios, 1970: 93, pl. 27; 1985: 103, pl. 35, fig. 453; 2009: 246 (in syn); Rios & Calvo, 1987: 143, fig. 110 (non Deshayes, 1844).
Buccinanops gradatus: Simone, 1996: 88–96, figs. 1–3, 6, 12–30; Galindo et al., 2016: 342, fig. 1 (non Deshayes, 1844).
urn:lsid:zoobank.org:act:52D86791-0AA4-4E0D-A258-F82317E0257C.
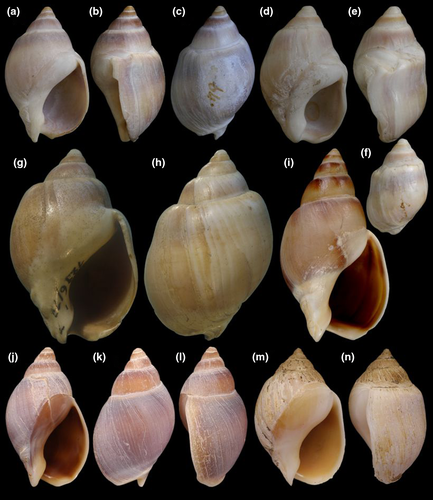
Type material: Holotype MZSP 28190 (Figure 9a–c). Paratypes: Brazil. Rio de Janeiro; São Tomé, off Cabo de São Tomé, MZSP 72606, 2 shells (Figure 9i–j) (Femorale; trawled, 60 m, fishermen, viii/2002). São Paulo; off Ubatuba, off Flamengo Beach, MZSP 51656, 3 spm (W Narchi leg, 14/vii/1957) (Figure 9f–h); Guarujá, Praia do Perequê, MACN-In, 3 spm (Figure 9d–e); Santos, Barra de Santos, 46°19′S 24°04′W, MZSP 28078, 1 shell, MZSP 28079, 1 shell (21.ix.1970); Cananéia, off Bom Abrigo Island, 25°08′S 47°50′W, 8 m, MZSP 35775, 1 spm (otter trawl, Magenta leg., viii/2002) (Figure 9k–l), MZSP 47125, 13 spm (BIOTA sta. 83i, 16.x.2001).
Type locality: BRAZIL. São Paulo; Praia Grande, off Boqueirão Beach, 24°04′S 46°28″W, ~8 m depth (otter trawl, local fishermen col., i/1994).
Etymology: The new species is named in reference to the broad profile of the shell, from Latin latus, meaning broad, wide.
Diagnosis: Shell up to 80 mm; usually simple, globose. Spire angle 65–70°. Left edge of canal with blunt beak of ~90°. Head-foot mostly colorless. Kidney tissue with two longitudinal folds. Single odontophore pair m9; pair m2a reduced. Radular rachidian with 7–8 uneven cusps. Stomach larger. Anal gland present. Nerve ring with cerebral commissure slightly shorter than ring itself.
Description: This supplements the conchological and anatomical descriptions provided by Simone (1996) (at that time as B. “gradatus”).
Shell: (Figures 9, 10) Size large (to 80 mm), spire short (half shell length), and wide. Spire angle 65–70°, obtuse. Protoconch of ~2.8 mm, first two whorls smooth, with axial riblets appearing subsequently, from suture to suture, each riblet with clear subsutural node (Figure 10a–c); riblets gradually disappearing after two whorls, leaving only growth lines and shallow spiral cords (Figure 10d–e), clearer along adapical half of each whorl, ~10 per whorl. Shouldered shells (Figure 9f–h) more uncommon than in preceding species (~10%). Left (adaxial) edge of canal with blunt anterior beak of ~90° (Figure 9a, d, f, i, k).
Head-foot: Color of exposed areas lighter, practically light tan, except for some sparse pale-brown spots, varying in density among specimens. Females with well-developed cement gland, similar to that of preceding species, but shorter and wider (Figure 11b: cl).
Operculum: Similar to B. cochlidium.
Pallial cavity: Features similar to preceding species, except for more bluntly pointed anterior end of gill (in Simone, 1996: figure 12, gi).
Circulatory and excretory systems: Heart similar to that of B. cochlidium. Kidney also similar, except for renal lobe divided into two longitudinal folds (instead of three), each fold with different glandular constitution (Simone, 1996: figure 28: gk, vp) (uniform in B. cochlidium).
Digestive system: Most characters similar to those of B. cochlidium. Differences include the following: Odontophore muscles described in Simone (1996). Table 2 shows a correlation of muscles terminology. Odontophore muscle m9 as single pair, bifurcating from each other more anteriorly (Simone, 1996: figure 21: m5); pairs m2a more poorly developed, being narrower fibers (Simone, 1996: figure 21: m4). Radula: rachidian with 7–8 cusps, each cusp broader, flatter, and more uneven than those of preceding species; lateral teeth with inner set of cusps with only three cusps, being medial cusp ~3 times larger than remaining cusps (Simone, 1996: figure 6). Transverse muscles between esophagus and odontophore (Figure 5b: tm) poorly developed. Anterior esophagus much simpler, practically straight (Simone, 1996: figure 15: ao). Pair of salivary glands ~twice size of those in preceding species. Valve of Leiblein much less well developed, only marked by sudden change of inner esophageal folds (Simone, 1996: figure 16: vl). Stomach ~50% larger and internally more complex (Simone, 1996: Figure 18). Inner anal gland subterminal, close to anus (Simone, 1996: figure 19: ga).
| In Simone (1996) | Current | Name/function/topology |
|---|---|---|
| m1 | m1 | Jugal muscles |
| m2 | m3c | Dorsal circular tensor muscle |
| m3 | m2c | Retractor muscles of odontophore, cartilage component |
| m4 | m4+m2a | Main dorsal tensor muscles of radula |
| m5 | m9 | Radular dorsal protractor muscles |
| m6 | m8 | Antero-dorsal tensor muscles of cartilages |
| m7 | m9 | Radular dorsal protractor muscles (a secondary branch) |
| m8 | m9 | Radular dorsal protractor muscles (a secondary branch) |
| m9 | m4 | Main dorsal tensor muscles of radula (a secondary branch) |
| m10 | m5 | Auxiliary dorsal tensor muscles of radula |
| m11 | m6 | Horizontal muscle (a ventral circular tensor muscle) |
| m13 | m11 | Ventral tensor muscles of radula |
Genital system: Most features similar to preceding species. Distinctions include the following: Seminal vesicle (sv) slightly smaller; despite in also having gonopericardial duct (go), additionally bearing aperture to pallial cavity in its posterior pallial portion (Simone, 1996: figure 29: va). Penis with subterminal papilla slightly more developed, and penis duct slightly less coiled. Pallial oviduct similar, including vaginal atrium length and papilla-like female aperture. Cement gland slightly shorter (Figure 11b), well-separated from hemocoel.
Central nervous system: (Figure 11a, c) Nerve ring similar to that of preceding species, including concentration of ganglia, indistinction of some ganglia by fusion, and position of buccal ganglia. Cerebral commissure (cc) about half length and thicker than that of preceding species, relatively uniform in width along its length. Visceral ganglion (gv) relatively smaller, located closer to nerve ring, distance equivalent to length of nerve ring.
Development: Egg capsules attached to callus parietal region of female's shell (Figure 10f). Each egg capsule of ~10 mm, oval, flattened, with thin walls, translucent, containing ~15 embryos.
Habitat: Sandy mud and muddy bottoms, 5–40 m, normally collected by otter trawl.
Distribution: Espírito Santo (~20°S) to north Rio Grande do Sul (~31°S), Brazil.
Material examined: BRAZIL. Espírito Santo; Guarapari, MZSP 39453, 1 spm, MZSP 77303, 2 spm. Rio de Janeiro; Cabo de São Tomé, MZSP 28184, 1 spm, MZSP 34820, 1 spm, MZSP 34870, 1 spm, MZSP 72606, 2 shells, 100–120 m (Femorale, viii.2002); off Ilha de Santana, Macaé, MNRJ 8838, 2 spm, MNRJ 1194, 2 spm, MZSP 73814, 60 m, 1 shell (otter trawl, Femorale, viii/2000); off Ponta de Guaratiba, 35–40 m, MNRJ 8836, 3 spm; Cabo Frio, MZSP 51658, 1 spm; Niterói, Itaipu, off Ilha do Pai, 30–40 m, MNRJ 8837, 2 spm; off Ilha de Guanabara, MNRJ 8835, 2 spm; Rio de Janeiro, MZSP 33399, 1 spm, Copacabana, MZSP 51651, 1 spm; Ilha Grande, MZSP 51378, 1 spm, MZSP 51629, 1 spm; Angra dos Reis, MZSP 51320, 2 spm, MZSP 51341, 1 spm, MZSP 51641, 2 spm, MZSP 51647, 1 spm, MZSP 51390, 1 spm; Paraty, MZSP 48307, 1 spm. São Paulo; Ubatuba MZSP 28080, 6 spm, 28081, 1 spm, 33443, 1 spm, 28186, 2 spm, 37921, 2 spm, 37925, 3 spm,; 38000, 6 spm, 38001, 1 spm, 38330, 19 spm, 38002, 2 spm, 38498, 13 spm, 38499, 8 spm, 38336, 1 spm, 40010, 3 spm, 40020, 5 spm, 45467, 1 spm, 45505, 3 spm,; 47092, 5 spm, 47126, 17 spm, 51314, 7 spm, 51316, 3 spm, Barra Seca, MZSP 51676, 1 spm, Praia de Maranduba, MZSP 51802, 1 spm, Praia do Flamengo, MZSP 51661, 2 spm, 51656, 1 spm (W. Narchi col, 14.vii.1957), Praia de Itaguá, MZSP 51645, 3 spm, 51633, 1 spm, 64201, 3 spm; Caraguatatuba, MZSP 40229, 1 spm, 40278, 1 spm, 38332, 8 spm, 37923, 2 spm, 38338, 1 spm, 38340, 2 spm, 45245, 1 spm, 45252, 1 spm, 45253, 1 spm, 45480, 9 spm, 45526, 3 spm, 45647, 4 spm, 45648, 3 spm, 45649, 3 spm, 45664, 67 spm, 45665, 23 spm, 45666, 20 spm, 47086, 2 spm, 47135, 1 spm, 47099, 2 spm, 47125, 13 spm, 54189, 1 sp; São Sebastião, MZSP 37922, 1 spm,; 38003, 1 spm, 38004, 4 spm, 38331, 12 spm (23°52.330′S 45°34.227′W, Biota-Fapesp, sta. 163i, 27/vi.2002), 38333, 2 spm, 38334, 2 spm, 38337, 2 spm, 38339, 1 spm, 38341, 21 spm, 38343, 1 spm, 38402, 1 spm, 45239, 2 spm, 45539, 1 spm, 45543, 3 sp.; 47131, 3 spm, 10053, 3 spm, Praia do Gato, MZSP 14259, 6 spm; Ilha dos Buzios, MZSP 15339, 1 spm; Bertioga, MZSP 61464, 40 spm, 61448, 17 spm, 61451, 2 spm, off Boracéia Beach, MZSP 112055, 1 shell (Ex Colella col., i/1971); Guarujá, MZSP 57506, 2 spm, Praia do Perequê, MZSP 28188, 1 spm, 28192, 20 spm, 43579, 6 spm, 43736, 1 spm, Praia do Góis, 2 m, MZSP 44007, 18 spm, 61160, 1 spm MZSP 33429, 1 spm; Santos, MZSP 33475, 1 spm, 33344, 1 sp, 35397, 2 spm, 51631, 1 spm, 51642, 1 spm, 61443, 4 spm, 36627, 2 spm, 51352, 1 spm, 51639, 1 spm, 32 m, 51804, 1 spm, off Santos, MZSP 36466, 2 spm (Coltro leg, iv/2003), 24°05.704′S 46°20.270′W, 22 m, MZSP 108269, 2 spm (otter trawl, Instituto de Pesca, 4/vii/2012); between Ilha da Queimada Grande and Lage de Santos, MZSP 78246, 12 spm, 30–35 m (otter trawl, xii/1998); São Vicente, MZSP 61151, 1 spm; Praia Grande, off Praia do Boqueirão (type locality) MZSP 27319, 2 spm, 28190, 2 spm, 60047, 10 spm, 61081, 100 spm, 61147, 1 spm, 61148, 3 spm, 61149, 3 spm, 61152, 5 spm, 61153, 7 spm, 61442, 15 spm, 61444, 9 spm, 61445, 9 spm, 61446, 1 spm, 61447, 2 spm, 61449, 2 spm, 1450, 3 spm, 61453, 10 spm, 61466, 35 spm, 61467, 20 spm, 61469, 1 spm, 28079, 1 spm, 51681, 2 spm, 47141, 3 spm, 28082, 18 spm, 28183, 17 spm, 28193, 7 spm, 35392 ~30 spm; Mongaguá, MZSP 51385, 1 spm; Itanhaém, MZSP 33398, 6 spm; Peruíbe, MZSP 61150, 1 spm, Praia das Enchovas, MNRJ 2925, 3 spm, Icaparra, MZSP 51649, 4 spm; Iguape, MZSP 51333, 1 spm, 51807, 1 spm. Paraná; Camburiú, MZSP 14768, 1 spm; Paranaguá, MZSP 34494, 1 spm. Santa Catarina; Penha, 40–50 m, MZSP 53552, 34 spm; Itajai, MZSP 61465, 40 spm; Porto Belo, MZSP 51329, 1 spm, 51330, 3 spm, 51331, 3 spm, 51655, 3 spm, 32867, 1 spm, 44019, 2 spm, 17908, 1 spm; Florianópolis, MZSP 33442, 1 spm, Praia de Piçarras, MZSP 51675, 11 spm, Itaporocóia, MZSP 15792, 1 spm; Barra Velha, MNRJ 2929, 2 spm; Itajubá, MNRJ 3498, 2 spm; Pântano do Sul da Ilha de Santa Catarina, MNRJ 2932, 2 spm, MZSP 16157, 2 spm.
Remarks: Buccinanops latus sp. nov. is morphologically close to the type species. It was apparently identified under three names, that is, B. cochlidium, B. lamarckii, or B. gradatus by different authors. All these nominal species were described without accurate type locality (i.e., southern seas for B. cochlidium), wrong (i.e., New Zealand for B. gradatum), or unknown. Buccinanops lamarckii is a clear synonym of B. cochlidium and B. gradatum was split from B. cochlidium, probably based only on very few shells. When several hundred lots are compared, from a large latitudinal range, a single variable species could be recognized. Our anatomical studies, and a DNA sequence treatment produced elsewhere (B. gradatus in Galindo et al., 2016, based on sample MZSP 108269, also included herein), suggested the presence of a new species with a more restricted range but a comparable morphology. Once we established the existence of a new species, the use of one of these old names was attempted. However, we decided that with the available knowledge (i.e., only shells with no locality to backup those names) no clear proof were enough to use any of them, cochlidium or gradatus, for the new entity. On the contrary, we have abundant evidence, anatomical and DNA sequence, with a considerable amount of lots, sufficient to describe a new species. Additionally, there are conchological differences explored in the distinctive description above and in the discussion below.
The shell in B. latus is smaller (up to 80 mm, against ~120 mm of B. cochlidium) and has a shorter spire (up to half of total shell length, while B. cochlidium usually has a longer, slender, spire), and the left (adaxial) edge of the canal usually has an anterior blunt beak of ~90° versus ~30° of B. cochlidium. However, some immature specimens could be dubious. The head-foot color is also diagnostic as B. cochlidium is almost black in exposed areas (Figure 3c) while B. latus mostly lacks pigmentation or shows only some sparse brown spots. The remaining anatomical differences are explored above, drawing attention to the distinctions in the nerve ring, as B. cochlidium has a much longer and narrower cerebral commissure (Figure 8a–b: cc) than that of B. latus (Figure 11a, c). In addition, the valve of Leiblein is much more reduced in B. latus than in B. cochlidium (Figure 5f). Most specimens of the new species have shells of uniform light-brown color (Figure 9a–c, f–l); however, about 30% of the specimens also have axial irregular bands (Figure 9d–e). The periostracum is thin, velvet-like in freshly collected specimens (Figure 9a–d, f–h). B. cochlidium and B. latus do not overlap in geographic distribution according to our data. B. latus has as southernmost limit 31°S, while the northernmost limit of B. cochlidium is 32°S.
Compared the egg capsules of B. cochlidium illustrated by Penchaszadeh (1971, 1973) and Averbuj and Penchaszadeh (2010a), B. latus (Figure 10f) has more rounded and flattened capsules without ornamentation.
3.3 Buccinanops monilifer (Kiener, 1834)
Buccinum moniliferum Valenciennes in Kiener, 1834: pl. 3, fig. 8; Anton, 1839: 91; Deshayes, 1844:191; d’Orbigny, 1845: 199.
Bullia armata Gray, 1839: 126; Reeve, 1846, pl. 1, fig. 2 a, b; Kobelt, 1877: 290.
Buccinum (Buccinanops) maniliferum [sic]: d’Orbigny, 1846: 434.
Bullia (Buccianops) armata: H. & A. Adams, 1853: 113.
Buccinanops cochlidium (var 3): Gray, 1854: 40 non Chemnitz, 1795 nec Dillwyn, 1817.
Bullia (Buccinanops) moniliferum: Chenu, 1859: 160, fig. 750.
Bullia (Buccinanops) armata: Tryon, 1882: 14, pl. 6, figs. 82, 83; Paetel, 1888: 116.
Dorsanum armatum: Cossmann, 1901: 218.
Dorsanum moniliferum: Carcelles & Parodiz, 1939: 747, figs. 1, 2; Carcelles, 1944: 249; Barattini & Ureta, 1960: 113; Rios, 1970: 92, pl. 28; Rios, 1975: 95, pl. 27, fig. 398.
Bullia (Dorsanum) monilifera: Cernohorsky, 1982: 17, fig. 17, 871.
Bullia monilifera: Abbott & Dance, 1983: 177.
Buccinanops moniliferum: Cernohorsky, 1984: 29; Pastorino, 1993a, figs. 1–3.
Buccinanops (Dorsanum) moniliferum: Rios, 1985: 103, pl. 35, fig. 456.
Buccinanops moniliferum: Calvo, 1987: 143, fig. 112.
Bullia (Buccinanops) monilifera: Allmon, 1990: 25, pl. 2, fig. 12; pl. 6, figs. 1, 2.
Buccinanops moniliferus: Simone, 1996: 96–100, figs 4–5, 7–8, 10–11, 31–42.
Type material: Buccinum moniliferum: unknown, never found (not in MHNP, neither Geneva); Bullia armata: unknown (not in NHMUK).
Type locality: B. monilifer: “Terra Nova”; B. armata: unknown.
Distinctive description: This supplements the conchological and anatomical description provided by Simone (1996).
Shell: (Figure 12) Large (up to ~55 mm), fusiform, with 6–7 slightly convex whorls. Color variable, usually dirty whitish, creamy with two thick brownish bands, one subsutural and one along middle of last whorl (Figure 12c, g). Protoconch large, 2.5 whorls, 2.2 mm wide in 2nd whorl, with 8–10 riblets per whorl (Figure 13e–f). Transition to teleoconch almost indistinct. Suture well defined, impressed. Spire high, 1/2 to 1/3 of total shell height; first two whorls with incomplete axial riblets, 3rd and subsequent whorls with 10–14 subsutural peripheral spines or knobs, somewhat blunt, and of variable size or development. Sculpture absent, except for growth lines; base of last whorl with few spiral threads. Parietal callus weak but visible (Figure 12d). Aperture smaller than half of total length, 60–70% of shell width; subcircular, posterior notch usually present, with thicker area on parietal side of anal angle (Figure 12a, d, e). Outer lip profile slightly sinuous (Figure 12b, f). Inner lip with strong inflexure at base of siphon (Figure 12a, d, e).
Ultrastructure of shell of three layers, outermost layer of amorphous calcite, intermediate thicker of crossed-lamellar aragonite; innermost thinner also of crossed-lamellar aragonite (Figure 13c). Remaining characters similar to preceding species.
Head-foot: Characters similar to those in B. cochlidium. Female cement gland (Figure 14b: cl) more anteriorly located, shorter and broader. Dark pigment absent.
Pallial cavity: Characters like those of B. cochlidium, except for more elongated gill and osphradium, both with more uniform width throughout their length (Simone, 1996: figure 33: gi, os).
Digestive system: Buccal mass organization similar to preceding species, differences explored in Simone (1996: figure 34). Salivary ducts anterior region running along dorsal folds (Figure 14a; sd), lacking subterminal bulged region, strong curve preceding its aperture towards medial; salivary aperture ventral, at short distance from median line, approximately in middle level between mouth and odontophore (Figure 14a: sa). Radula (Figure 13a–b), central tooth with 5–13 cusps of variable size, some specimens uniform, others with central cusp larger. Rachidian base curved. Lateral teeth with outer cusp hook-shaped, inner cusp thicker than 2–5 intermediate cusps, sometimes with different number on each side; lateral tooth base with blunt inner cusp. Valve and gland of Leiblein, and stomach similar to those of B. latus, rather than B. cochlidium.
Genital system: Male genital structures mostly similar to those of preceding species (see Simone, 1996: figs. 39–41). Penis with blunt tip, almost bilobed, with papilla in its mid region (Figure 13d). Females with pore preceded by rather bulged, muscular region.
Central nervous system: (Figure 14c) Closer to that described for B. latus, except for relatively shorter cerebral commissure (cc), and clearer pair of buccal ganglia (bg).
Habitat: Sandy mud and muddy bottoms, 5–25 m, normally collected by otter trawl.
Distribution: From Rio de Janeiro, Brazil (~23°S) to mouth of Rio Negro area (~41°S), Argentina.
Material examined: BRAZIL. Rio de Janeiro; MZSP 34771, 1 spm; Recreio dos Bandeirantes, MNRJ 6059, 3 spm; Cabo Frio, MNRJ 6462, 2 spm; Cabo de São Tomé, MZSP19591, 1 spm. São Paulo; MZSP 34443, 10 spm, MZSP 35404, 2 spm, MZSP 51673, 8 spm; Ubatuba, MZSP 33401, 1 spm, MZSP 33406, 1 spm, MZSP 38349, 1 spm, MZSP 48192, 1 spm, MZSP 51287, 1 spm, MZSP 38005, 2 spm, MZSP 51662, 2 spm; São Sebastião, MZSP 398, 16 spm, MZSP 51664, 3 spm, MZSP 53330, 1 spm; Caraguatatuba, MZSP 37924, 1 spm; Bertioga, MZSP 61404, 1 spm, MZSP 61426, 14 spm; Guarujá, Praia de Pernambuco, 91.5 m, MZSP51399, 2 spm; Santos, MZSP 51388,1 spm, MZSP 51805, 4 spm, MZSP 51799, 2 spm, MZSP 35403, 1 spm, MZSP 15233, 1 spm, MZSP 33402, 2 spm, MZSP 78817, 2 spm; São Vicente, Ponte Pensil, MZSP 61423, 4 spm; Praia Grande, MNRJ 1523, 2 spm, off Boqueirão, MZSP 26865, 2 spm, MZSP 27320, 3 spm, MZSP 28151, 56 spm, MZSP 28152, 18 spm, MZSP 28153, 18 spm, MZSP 28175, 15 spm, MZSP 28176, 28 spm, MZSP 28177, 9 spm, MZSP 34444, 14 spm, MZSP 34445, 19 spm, MZSP 61398, 3 spm, MZSP 61399, 2 spm, MZSP 61400, 14 spm, MZSP 61401, 1 spm, MZSP 61402, 1 spm, MZSP 61403, 1 spm, MZSP 51332, 1 spm, MZSP 61406, 7 spm, MZSP 61407, 14 spm, MZSP 61409, 3 spm, MZSP 61410, 6 spm, MZSP 61411, 2 spm MZSP 61413, 5 spm, MZSP 61414, 4 spm, MZSP 61415, 6 spm, MZSP 61416, 8 spm MZSP 61417, 6 spm MZSP 61418, 6 spm, MZSP 61420, 15 spm, MZSP 61421, 12 spm, MZSP 61422, 2 spm, MZSP 61424, 2 spm, MZSP 61425, 35 spm, MZSP 61427, 26 spm, MZSP 61428, 20 spm, MZSP 61430, 20 spm, MZSP 61432, 20 spm, MZSP 61435, 36 spm, MZSP 61436, 60 spm, MZSP 61437, 80 spm, MZSP 61438, ~100 spm, MZSP 61439, 70 spm, MZSP 35841, 1 spm, MZSP 60070, 2 spm, MZSP 61450, 2 spm; Iguape, MNRJ 11427, 2 spm; Suarão, MZSP 15208, 1 spm; Itanhaém, MZSP 28180, 1 spm, MZSP 33357, 68 spm, MZSP 51338, 2 spm, MZSP 53589, 5 spm, MZSP 51793, 1 spm; Peruíbe, off Praia Central, MZSP 15127, 2 spm, MZSP 61412, 10 spm, MZSP 61408, 8 spm, MZSP 61431, 12 spm; Iguape, MZSP 10059, 6 spm, MZSP 14145, 2 spm, MZSP 51657, 3 spm, MZSP 51663, 9 spm, MZSP 51803, 1 spm, MZSP 47227, 2 spm, MACN-In 1855, 19 spm. Paraná; Ilha do Mel, MZSP 17855, 2 spm, MZSP 17854, 1 spm; Paranaguá, MZSP 51348, 1 spm; Guaratuba, MZSP 51653, 2 spm. Santa Catarina; Piçarras, Praia Alegre, MZSP 61419, 6 spm, MNRJ 2931, 2 spm. Rio Grande do Sul; MZSP 32901, 4 spm, MZSP 19191, 9 spm, MZSP 19195, 12 spm, MZSP 43088, 1 spm, MNRJ 3868, 1 spm; off Torres, MZSP 32724, 4 spm; Vitoria do Palmar, 15–25 m, MZSP 51666, 5 spm; Cassino, MZSP 61405, 2 spm, MNRJ 3470, 2 spm, MNRJ 7806, 1 spm, MACN-In 8219, 3 spm; Rio Grande, MZSP 40341, 8 spm; Mostardasto São José do Monte, 8–20 m, MZSP 62976, 3 spm, MZSP 62976, 2 spm; off Chui, MACN-In 15346, 33°24′S 52°47′W, 13 m, 12 spm. URUGUAY. Montevideo; MZSP 10039, 3 spm. Rocha; La Paloma, MZSP 33397, 9 spm, MZSP 60048, 2 spm, MACN-In 35383, 5 spm, MACN-In 15149, 4 spm, MACN-In 30486, 2 spm, MNRJ 1243, 2 spm, MNRJ 6527, 2 spm; Cabo Santa Maria, MACN-In 15374, 34°53′S 55°19′W, 20 m, 2 spm, MACN-In 11593 1 spm. Maldonado; Maldonado, MACN-In 9356–6, 1 spm. ARGENTINA. MZSP 51345, 15 spm, MZSP 51342, 12 spm. Buenos Aires; littoral, MNRJ 34, 35 spm, MZSP 51344, 2 spm, MZSP 51345, 3 spm; Punta Médanos, MACN-In 25197, 35°40′S 56°03′W, 10 m, ~80 spm, MACN-In 23517, 35°55′S 55°19′W, 21 spm, MACN-In 11843, 2 spm; Mar del Plata, MZSP 10037, 3 spm, MACN-In 16544, ~50 spm, MZSP 22389, ~40 spm, MACN-In 4660–1, 32 spm, 19274, 38 spm MACN-In 22389–1, 2 spm; MZSP 19139, 8 spm, MACN-In 9217–4, 6 spm, MZSP 11372, 4 spm, MZSP 11998, 7 spm, MZSP 10109, 2 spm, MZSP 29459 ~ 200 sp., MACN-In 8587–6, 2 spm, MZSP 9490, 1 spm, MZSP 8734, 1 spm, MZSP 12221, 1 spm, MZSP 9361, 2 spm; Miramar, MACN-In 9247–6, 6 spm, MACN-In 9252–2, 2 spm; Necochea, MZSP 51660, 9 spm, MACN-In 29661, Punta Negra, 1 spm; Monte Hermoso, MZSP 10035, 9 spm, MACN-In 9209–16, 7 spm, MACN-In 6619–28, 4 spm, off Faro Recalada, 1 spm; Punta Carballido, Quequén, MACN-In 26281, 8 spm; Puerto Quequén, MACN-In 18671, 30 spm; Bahía Blanca, MACN-In 14867, 30 spm; Arroyo Parejas, MACN-In 29663, 2 spm, MACN-In 11232–2, 1 spm; Puerto Belgrano, MACN-In 11233, 2 spm, 6620–18, 1 sp.; Bahia Blanca, MACN-In Caleta/Brightman, 6 spm; Isla Trinidad, MACN-In 19669, 7 spm; Bahía San Blas, MACN 20273, 1 spm; Carmen de Patagones, MZSP 10034, 16 spm; Buenos Aires, MACN-In 30025, 4 spm, MACN-In 11696, 27 spm, MACN-In 20670, 40°47′S 62°15′W, 15 m, 9 spm. Río Negro; MACN-In 20527, 9 spm, MZSP 51678, 3 spm, E of mouth of Río Negro, MZSP 51363, ~60 spm.
Remarks: This is probably the most common species of Buccinanops in Buenos Aires province, Uruguay and southern Brazil. Usually found subtidally. Buccinanops monilifer is easy to recognize, because it is the only species of Buccinanops with subsutural spines, despite some rare specimens in which these are very weak of even obsolete (Figure 12e–g; Simone, 1996: figure 11). The shell is slender, and the coloration is diagnostic in those cases with short or weak spines. It was cited as belonging to the genus Dorsanum by Cossmann (1901), followed by local authors (e.g., Carcelles & Parodiz, 1939; Castellanos, 1970; Rios, 1994). The attribution is probably because of some shell similarity with Dorsanum miran (Bruguière, 1789), the West African genotype. The distinction between Buccinanops and Dorsanum is explored elsewhere (Pastorino, 1993a; Simone & Pastorino, 2014), restricting Dorsanum to the African coast. Penchaszadeh (1971) and Averbuj and Penchaszadeh (2010b) reviewed the reproductive biology and the development of imposex in the females of this species (as Dorsanum moniliferum). Martorelli (1991) pointed out the presence of cercarian parasites in adult specimens of B. monilifer (as Dorsanum moniliferum).
3.4 Genus Buccinastrum gen. nov.
Diagnosis: Shell bucciniform to nassariform, medium to small; outer surface normally mat; walls thick. Sculpture of axial growth lines only; whorl profile rounded, rarely shouldered; low irregular subsutural undulations, sometimes with few faint cords. Last whorl smooth, usually lacking cords. First teleoconch whorls smooth. Left, adaxial edge of canal preceded by usually strong transverse fold (Figure 15i: blue arrow). Parietal callus normally well-developed. Radular rachidian with more uniform cusps. Extrinsic odontophore muscles m1b single. Aperture of salivary glands lateral. Anterior esophagus with longitudinal folds. Valve of Leiblein well-developed. Stomach with single duct to digestive gland. Female genital pore as wide, simple pore.
urn:lsid:zoobank.org:act:3D715FEE-89DD-4FB7-A46F-1F66D827C6AA.
List of included taxa: Buccinastrum deforme (type species), B. duartei, B. paytense, B. uruguayense (all new combinations).
Etymology: the suffix astrum is a diminutive, jointed to Buccinum a genus of similar shell shape; Buccinastrum = little Buccinum. Gender neuter.
3.5 Buccinastrum deforme (King, 1832) new combination

Buccinum deforme King, 1832: 349.
Buccinum globulosum Kiener, 1834: 12, pl. 10, Figure 33.
Buccinanops globosum var.: d'Orbigny, [1839] pl. 61, Figure 24.
Buccinum (Buccinanops) globulosum: d'Orbigny, [1841]: 435.
Buccinanops globulosum: d'Orbigny, [1842]: 157; Carcelles & Parodiz, 1939: 764, Figures 7-9; Carcelles, 1950: 63; Carcelles & Williamson, 1951: 299; Barattini & Ureta, 1960: 117; Castellanos, 1970: 90, pl. 7, Figure 5; 1992: 13, pl. 2, Figure 10; Penchaszadeh, 1971a, 1971b: 477, Figure 2 a–b; Scarabino, 1977: 189, pl. 5, Figure 5; Cernohorsky, 1984: 28; Farinatti, 1985: 219, pl. 1.
Buccinum ampullaceum Deshayes, 1844: 203, nomen novum pro Buccinum globosum Kiener (in error pro globulosum) non Gmelin, 1791, nec Quoy & Gaimard, 1833.
Bullia deformis: Reeve, 1845, pl. 3, Figure 21; Adams & Adams, 1858:113; Kobelt, 1877: 290; Tryon, 1882: pl. 5, fig. 51; Ihering, 1895: 227; 1907: 425; Feruglio, 1933: 36.
Bullia (Buccinanops) deforme: Paetel, 1888: 116; Allmon, 1990: 16, fig. pl. 2, 13.
Bullia globulosa: Reeve, 1846 pl. 1, Figure 5; H. & A. Adams, 1858: 113; Tryon, 1882: 11, pl. 5, fig. 60; Pilsbry, 1897: 6; 1899: 127; Ameghino, 1906: 279; Ihering, 1907: 405; Feruglio, 1933 pl. 9, Figure 30 a, b; Abbott & Dance, 1986: 178, Figure 1.
Bullia (Buccinanops) globulosum: Paetel, 1888: 116.
Buccinanops globulosum var. elata Strebel, 1906: 155, pl. 11, figs. 75 a–b.
Buccinanops globulosa: Peile, 1937: 185, Figure 20.

Buccinanops squalidus: Richards & Craig, 1963: 140 (non King, 1832).
Bullia (Buccinanops) globulosa: Allmon, 1990: 20, pl. 2, Figure 14.
Type material: Buccinum deforme: NHMUK 1854.12.4.470, 2 syntypes (Figures 34–36). B. globulosum: holotype MHNG 1296/17/1 (Figs. 40–41).
Type locality: Buccinum deforme; ad flumen Plata (Rio de La Plata), Gorriti [Island], Uruguay; B. globulosum: unknown; Buccinanops globulosum var. elata: Puerto Madryn, Argentina; B. ampullaceum: unknown.
3.5.1 Distinctive description
Shell: medium size (to 70 mm, with usually 45 mm), globose. Protoconch (Figure 16c–e) of 2–2.5 whorls, of ~1.3 to 1.6 mm wide in second whorl (n=3), smooth or with weak sparse spiral lines (Figure 16e); whorls convex, globose. Spire short, ~40% of length; last whorl very globose, of ~60% of total length. Suture well defined. Sculpture wanting, except for growth lines (Figure 16c, h, k). Aperture large, oval, ~60% of shell length and width. Siphonal fasciole simple, rising below anterior half of callus. Columellar callus defined, weak (Figure 16a, j) or strongly pronounced (Figure 16d, g, m). Columella curved, ending in characteristic terminal plait. Outer lip barely sigmoid (Figure 16b, e, l, n).
Head-foot and operculum: Similar to those of preceding species (Figure 17a). Clearly bilobed posterior end of columellar muscle (cm), left-slender component tall (cs). Females with cement gland in middle region of anterior third, ending away from hemocoel (Figure 17d: cl). Operculum (Figure 17o, p) large, suboval, with subterminal nucleus.
Pallial cavity: (Figure 17c, e) Features similar to preceding species. Gill (gi) proportionally broader, occupying ~half of pallial roof; gill filaments relatively lower, with right edge usually convex. Osphradium slightly asymmetrical, with left filaments ~20% smaller than right filaments. Small fold separating anterior end of gill from osphradium, perpendicular to mantle border (ac).
Circulatory and excretory systems: (Figures 17b–c, 19a) Most characters alike those of preceding species. Kidney with two flaps of renal lobes (kl), both connected to efferent renal vessel (ek) and to intestine (in). Hollow inner space wider, mainly in posterior region.
Digestive system: (Figures 18, 19) Most features similar to those of preceding species. Exclusivities and notable characters are lateral transverse muscles (tm) slightly less developed (Figure 18a), odontophore cartilages slightly broader and shorter, with pair m8 longer (~45% of cartilage's length); fusion of cartilages along ~25% of their length (Figure 18d: oc); pair of muscles m9 as single, broad bulk in each side (Figure 18b: m9); pair m4 with broader origins (Figure 18d: m4); posterior end of m6 and m3c closer to posterior end of cartilages (Figure 18d), at ~1/7 of cartilages length from their posterior end. Radula: rachidian tooth with 6–12 cusps increasing towards center; rachidian base strongly curved; lateral teeth with outer cusp hook-shaped, inner cusp larger than 2–3 intermediate cusps. Lateral teeth base with blunt inner cusp. Salivary gland ducts lacking subterminal bulged region (Figure 18c: sd); salivary aperture close to median line on ventral wall, at middle level of oral tube (Figure 18c: sa); inner surface with 5–6 longitudinal, low folds, separated from each other (Figure 18e: ae). Anterior esophagus simpler, almost straight (Figure 18a: ae). Valve of Leiblein well-developed (Figure 18a, e: vl), with an inner transverse glandular fold and long iridescent cilia turned proximally; no bypass furrow. Middle and posterior esophagus internally smooth (Figure 18e: me, ep); middle esophagus very short; posterior esophagus gradually acquiring 5–6 inner, longitudinal folds (Figure 19a: ep). Gland of Leiblein narrow, shorter (Figure 18a: gl), its duct short, simple, hollow; opening as simple pore (Figure 18e: gd). Pair of ducts to digestive gland (Figure 19a: dd), being esophageal duct ~half of intestinal duct. Stomach bulged backwards, with strong circular muscle (Figure 19a: st); internally with two transverse folds, one in esophageal insertion, another in intestinal origin.
Genital system: (Figure 19b–e) Most attributes similar to those of preceding species, distinctions following. Male seminal vesicle slightly less-developed (Figure 19b: sv), with gonopericardial duct present (go). Prostate simple, narrow, simply as duct swelling. Penis broad in base (Figure 17a: pe), tapering gradually and uniformly up to bluntly pointed tip (Figures 19e, 21a: pe); penis duct smoothly zigzagging along penis center (pd); papilla very small, subterminal (pp). Female pallial oviduct with shallow constriction between albumen and capsule glands (Figure 19c–d: ag, cg), albumen ~half of capsule gland length; inner lumen very flat. Vaginal atrium ~1/5 of pallial oviduct length, with posterior, dark-colored ingesting gland (ig). Female pore simple, with elevated edges (Figure 19c: fp).
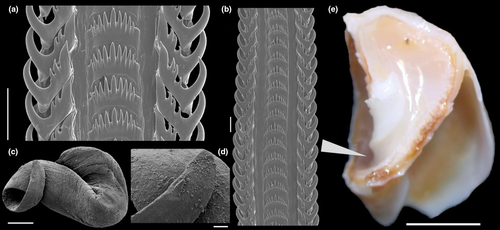
Central nervous system: (Figure 19f–g) Characters similar to those of preceding species, being slightly broader. Cerebral commissure (cc) relatively short, broad, rounded. Pair of buccal ganglia (bg) located slightly separated from each other.
Habitat: Sandy and sandy-mud beaches, intertidal to shallow subtidal levels.
Distribution: Cabo Santa Maria, Uruguay to southern Santa Cruz, Argentina.
Material examined: URUGUAY. Rocha; Cabo Santa Maria, USNM 340683, 3 spm. Maldonado; USNM 334528, 2 spm, USNM 270864, 1 spm; Punta del Este, MZSP 32081, 5 spm, MZSP 43098, 1 spm; MZSP 51347, 2 spm, MZSP 51628, 6 spm, MNRJ 6163, 2 spm; Ilha Dos Lobos, MNRJ 1215, 1 spm, USNM 359242, 2 spm; Solis, MACN-In 9536–2, 2 spm; Piriapolis, MACN-In 15433, 4 spm; Punta Negra, MNRJ 1772, 2 spm, USNM 359231, 4 spm. Canelones; Montevideo, MZSP 52109, 6 spm, USNM 364197, 7 spm; Isla Flores, MACN-In 28970, 2 spm; MNRJ 856, 3 spm. ARGENTINA. MZSP 54048, 2 spm. Buenos Aires; Cabo San Antonio, MACN-In 16310, 10 spm; Mar del Plata, MACN-In 8653–7, 2 spm, MACN-In 9155–4, 3 spm; Punta Carballido, Puerto Quequén, MACN-In 26280, 1 spm; Monte Hermoso, MZSP 51592, 7 spm, MZSP52112, 2 spm, MACN-In 11235, 23 spm, USNM 219861, 1 spm, USNM 465578, 2 spm; Bahía Blanca, MZSP 10045, 3 spm, MACN-In 14868, 40 spm, MACN-In 11236, 21 spm; Puerto Belgrano MACN-In 11236–1, 2 spm, MACN-In 6620–17, 2 spm; Arroyo Parejas, MACN-In 22471, 2 spm, MACN-In 11234, 3 spm; Bahia San Blas, MACN-In 20252, 61 spm, MACN-In 20273–2, 3 spm; Isla Trinidad, MACN-In 19678, 1 spm; Carmen de Patagones, MZSP 52104, 15 spm; MZSP 10048, 4 spm, MZSP 52102, ~100 spm; mouth of the Río Negro, MACN-In 20528, 17 spm; Río Negro; Puerto San Antonio, MACN-In 9379, 3 spm, MACN-In 9379–28, 14 spm, MACN-In 9152–13, 12 spm, MACN-In 13359, 15 spm, MACN-In 9379–24, 2 spm, MACN-In 9379-26/27, 113 spm, MACN-In 9379, 25 spm, USNM 346842, 3 spm; Puerto San Antonio Este, MACN-In 23703, 12 spm, MACN-In 13358, 6 spm, MACN-In 29138, 2 spm, MZSP 52103, 3 spm, MZSP 10032, 2 spm, MZSP 78270, 3 spm, MACN-In 29662, 2 spm, USNM 381691, 3 spm; Punta Villarino, MACN-In 13145, 16 spm, MACN-In 13146–3, 5 spm; San Antonio Oeste, USNM 807981, 2 spm Chubut; Golfo San Matias, MACN-In 33041, 3 spm; Puerto San José, MACN-In 9174–13, 5 spm; Puerto Madryn, MACN-In 38163, 42°24′S 64°17′W, 3 spm, MACN-In 9676–1, 5 spm, MACN-In 9281–2, 2 spm, MACN-In 22477, 35 spm, MACN-In 10953, 41 spm, MACN-In 9171–20, 25 spm, MACN-In 9040, 12 spm, MACN-In 9171–31, 3 spm, MACN-In 9675, 32 spm, MZSP 52111, 8 spm; Golfo San José, MACN-In 9032–14, 12 spm; Punta Norte, MACN-In 11485, 51 spm, MACN-In 9158–6, 5 spm; Puerto Pirámides, MACN-In 26400, ~100 spm, MACN-In 26402, 3 spm, MACN-In 9177–4, 12 spm; Bahía Craker, MACN-In 26437, 20 spm; Bahía Bustamante, MACN-In 26436, 2 spm, MACN-In 14447, 1 spm, MACN-In 26401, 2 spm, MACN-In 9032–34, 3 spm; Camarones, MACN-In 26438, 11 spm; Rada Tilly, MACN-In 30881, 28 spm; Comodoro Rivadavia, MACN-In 6826, 12 spm; Golfo de San Jorge, MNRJ 2069, 2 spm. Santa Cruz; Caleta Olivia, MACN-In 35384, 3 spm.
Remarks: Buccinastrum deforme (King, 1832) was described in one of the first molluscan reports from southern South America. However, the type was never illustrated. Kiener (1834) described Buccinum globulosum without any reference to King (1832). Later authors (e.gBarattini & Ureta, 1960; Carcelles & Parodiz, 1939; Castellanos, 1970; Cernohorsky, 1984) used both names as distinct species. The two syntypes housed at the NHMUK (Figure 16a–f) clearly show extremes of variation of only one species (Figure 16g–h). Usually, individuals living in Buenos Aires province and Uruguay have shorter spire, heavier columellar callus, and reduced aperture. This is probably related to the selective predation of crabs of the genus Danielethus Thoma, Ng & Felder, 2012 which fracture the shell using a special denticle on the master claw. Along the coast of Chubut province and southwards, these crabs are absent. Thus, the shells develop a higher spire, thinner whorls, and larger aperture (Figure 16j–l). Gomez Espinosa et al. (2018) showed the relationship between the crab D. crenulatus and B. deforme. Narvarte (2006) studied the population ecology of the so-called B. globulosum. It is sympatric with B. cochlidium particularly around Peninsula Valdes area; however, both species live at different depths. While B. deforme is found in intertidal settings—usually never deeper than 6 m—B. cochlidium never is exposed during low tide, living in considerably deeper environments. The interaction between the American Oystercatcher (Haematopus palliatus) and B. deforme (as B. globulosum), which feeds on the leftovers of the crabs (Cyrtograpsus angulatus), was studied by Daleo et al. (2005).
In the original description of B. deforme, King (1832: 349) mentioned the presence of “eggs” attributed to it. According to his description, those capsules—“transparent nidus, the size of a turtle's egg”—actually belong to the volutid Adelomelon brasiliana (Lamarck, 1811), a species fairly common in the area.
The radula of B. deforme is uniform in interpopulational studies. There is no sexual dimorphism, neither differences between distant populations nor variation correlated with size in specimens (Avaca et al., 2010).
3.6 Buccinastrum duartei (Klappenbach, 1961) new combination
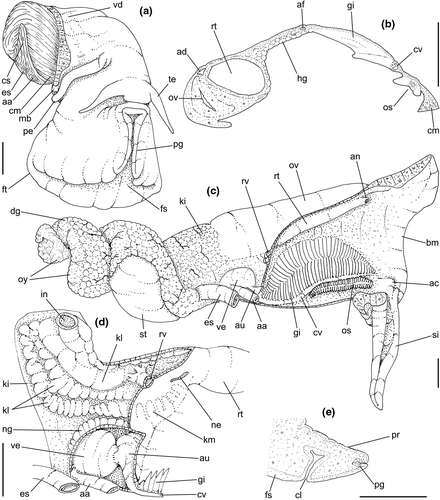
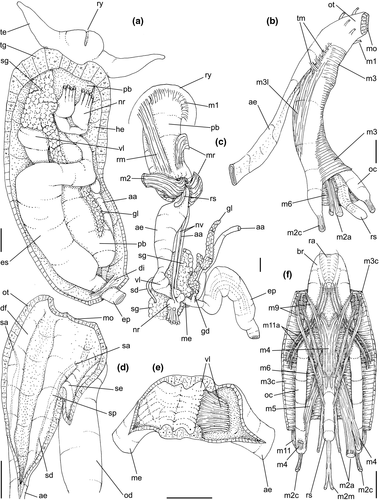
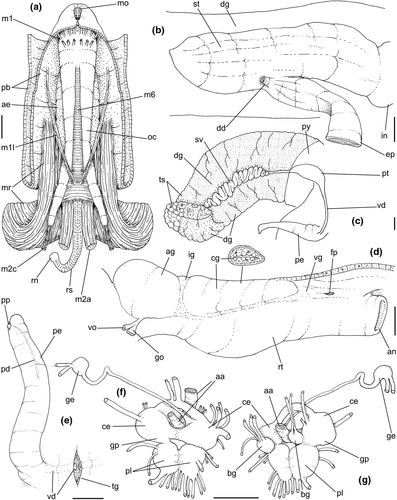
Buccinanops duartei Klappenbach, 1961: 87, Figure 1a, b; Rios, 1970: 92, pl. 27; 1975: 96, pl. 27, fig. 400; 1985: 103, fig. 452; 1994: 130, pl. 41, fig. 559; 2009: 246, fig.; Castellanos, 1970: 94, pl. 8, Figure 13; Cernohorsky, 1984: 28; Allmon, 1990: 27, pl. 2, Figure 11.
Type material: holotype MNHNM 769 (Figure 20a–c), Paratypes, MNHNM 770, 2 spm (Figure 20d), MNHNM 523, 6 spm, MNHNM 893, 1 spm, MNHNM 992, 5 spm.
Type locality: La Coronilla, Rocha Department, Uruguay.
3.6.1 Distinctive description
Shell: (Figures 20e–i, l–n, 21e) Medium size (up to 35 mm), fusiform. Protoconch paucispiral of 2 globose, smooth whorls. Teleoconch up to 6 flat whorls. Growth lines all over shell, otherwise smooth. Most grown specimens usually with small scars—probably boring sponges (Clionaidae) (Figure 20h–i). Color pinkish, purplish, or clear brownish; irregular subsutural white line usually present. Spire less than half of total shell length. Suture impressed, sometimes irregular, overgrown, showing enameling. Aperture large, usually half of total shell length (Figure 20e, h, l). Parietal callus usually well-developed, reducing aperture. Inner lip concave, siphonal inflexure strong. Outer lip barely convex, almost straight (Figure 20f, m). Inferior inner groove internally in each whorl (Figure 21e: arrow).
Shell ultrastructure similar to other Buccinastrum spp.
Head-foot and operculum: (Figures 22a, 23a) Characters similar to preceding species. Operculum (Figure 20j–k) large, suboval, margin smooth, subterminal nucleus. Female cement gland close to anterior edge, depth almost touching propodium (Figure 22e: cl).
Pallial cavity: (Figure 22b–c) Most features similar to those of preceding species. Those unique to this species are as follows: Siphon (si) proportionally longer, small pallial fold between anterior ends of gill and osphradium (ac), perpendicular to mantle border; cavity slightly shallower, ~3/4 whorl; gill (gi) and osphradium (os) proportionally broader; wider portion of gill close to rectum; gill anterior and posterior ends bluntly pointed; osphradium with uniform width along its length; osphradium less sessile, with base smaller than filaments (Figure 22b: os); Gill filaments relatively tall, tip protruded, right edge straight (Figure 22b: gi).
Circulatory and excretory systems: (Figure 22c–d) Similar to that of preceding species, with following differences: relatively small kidney ventral lobe attached to intestine, forming glandular cover of intestine (Figure 22d: superior kl); presence of dorsal lobe, formed by pair of zigzagging glandular folds surrounding posteriorly and left lobe attached to intestine (Figure 22d: left kl); efferent renal vessel large (rv), connected only to ventral lobe.
Digestive system: (Figures 23a–f, 24a) General organization similar to those of preceding species, with following interesting features: Proboscis retractor muscles thick posteriorly (Figure 24a: mr), mainly in ventral branches; sparse lateral transverse muscles connecting esophageal origin and odontophore (Figure 23b: tm). Odontophore muscles: pair m1l inserted more posteriorly and medially (Figure 24a); retractor set of muscles m2 (m2m, m2a, m2c) relatively thinner and slender (Figure 23b, f); m3l as continuous dorsal cover with longitudinal fibers (Figure 23b); pair m4 as single bundle originated at posterior end of cartilages (Figure 23f); m11a, presence of two pairs of auxiliary ventral tensor muscles of radula (beyond m11), originated on inner surface of m3c, in its posterior region, running anteriorly covered by m9, inserting at end of radular ribbon, ventrally to insertions of pair m11. Odontophore cartilages slenderer, anterior fusion at ~1/5 of their length (Figures 23f, 24a: oc). Radula (Figure 21a–b): central tooth with 7–10 cusps usually of equal size, except lateral cusps slightly smaller, central cusps larger or thicker; rachidian base slightly curved. Lateral teeth outer cusp hook-shaped, inner cusp larger, thicker than cusps 2 or 3; sometimes asymmetric intermediate cusps; base with faint blunt inner cusp. Salivary ducts with small subterminal bulged region (Figure 23d: sp); salivary apertures in lateral region of dorsal folds, in posterior level of oral tube (Figure 23d: sa). Anterior esophagus wide and flattened, smooth inner surface (Figure 23b: ae). Valve of Leiblein well-developed, including transverse glandular fold and long cilia turned anteriorly (Figure 23c, e: vl), no bypass groove. Middle esophagus with clear glandular surface, small longitudinal folds, length ~15% of anterior esophagus (Figure 23c: me). Gland of Leiblein slender and sinuous, ~half of anterior esophagus length and width (Figure 23a, c: gl); duct small, simple, hollow. Posterior esophagus very wide, flaccid walls; internally sparse, narrow longitudinal folds (Figure 23a, c: ep). Stomach as blind sac, ~1/3 whorl long, ~half local visceral width; posterior end bluntly pointed; walls thin; inner surface with sparse longitudinal, narrow folds directed toward intestine (Figure 24b: st); single small duct to digestive gland in esophageal insertion (Figure 24b: dd). Anus wide, shortly projected (Figures 22c, 24d: an).
Genital system: (Figure 24c–e) Most characters like those of preceding species. Distinctions following. Male with seminal vesicle narrow, as simple zigzagging wide tube along ~1 whorl (Figure 24c: sv). Prostate simple, short (~1/3 length of seminal vesicle), elliptical (Figure 24c: pt). Penis (Figures 21c–d, 22d, 24c, e) relatively narrow, weakly narrowing along its length, distal half flattened; tip rounded; penis duct narrow, weakly zigzagging along penis middle region (Figure 24e: pd); papilla subterminal, very small (Figure 24e: pp). Pallial oviduct (Figure 24d) preceded by gonopericardial duct (go) inserting close to visceral oviduct (vo); capsule gland (cg) as continuation from albumen gland (ag), separated by loose ingesting gland (ig); albumen with ~60% of capsule gland length, but broader than it. Vaginal atrium (Figure 24d: vg) ~1/6 of pallial oviduct length, walls flaccid, hollow, tapering toward pore. Female pore as simple, longitudinal, small aperture (Figure 24d: fp), located posterior and to right of anus.
Central nervous system: (Figure 24f–g) Most characters similar to those of preceding species. Cerebral commissure (cc) narrow, short (slightly shorter than each cerebral ganglion width). Shallow constrictions between dorsal and ventral set of ganglia. Small orifice between both cerebral/pleural set and both pedal ganglia; anteriorly edged by apparent extra cerebro-pleural commissure (Figure 24f). Buccal ganglia (bg) lacking clear commissure between both. Subesophageal/visceral ganglion (ge) small, spherical, connected by narrow nerve with ~double nerve ring length.
Habitat: Intertidal, in sandy and sandy-mud beaches.
Distribution: Rio de Janeiro (~23°S), Brazil to Miramar, Buenos Aires province (~38°S), Argentina.
Material examined: BRAZIL. Rio de Janeiro; Recreio Dos Bandeirantes, MNRJ 9104. Santa Catarina; Florianópolis, MZSP 51771, 2 spm, MZSP 51659, 1 spm, Praia da Armação, MZSP 51322, 2 spm. Rio Grande do Sul; MACN-In 25624, 1 spm; off Rio Grande, USNM 654363, in 25 m, 2 spm; Tramandaí, MZSP 33413, 4 spm, MZSP 52160, 8 spm, MNRJ 6051, 2 spm, USNM 710317, 4 spm; Torres, MZSP 16131, 3 spm, MZSP 77556, 2 spm; Pinhal, MZSP 27314, 2 spm; São Jose do Norte, MNRJ 1156, 2 spm; Tamandaré, MNRJ 2928, 2 spm; Ponta da Cidreira, MNRJ 9332, 3 spm. URUGUAY. Rocha; USNM 381150, 11 spm; Chuy, barra del arroyo, USNM 875579; La Coronilla, MZSP 33408, 23 spm, MACN-In 28772, 6 spm, MZSP 51616, 2 spm; Cabo Santa Maria, MNRJ 1771, 2 spm, MNRJ 6541, 2 spm; La Paloma, MACN-In 30480, 5 spm, MACN-In 30171, 2 spm. Maldonado; Punta del Este, MACN-In 15375, 34°55′S 55°19′W, 20 m, 1 spm. ARGENTINA. Buenos Aires; Villa Gesell, MACN-In 29373, 6 spm; Mar del Plata, MACN-In 9363–10, 5 spm, MACN-In 10240, 2 spm; Miramar, MACN-In 8451–25, 1 spm, MACN-In 8421–25, 2 spm.
3.7 Buccinastrum paytense (Kiener, 1834) new combination

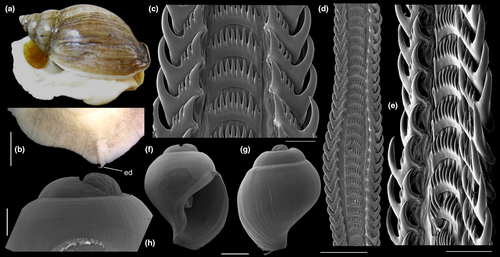
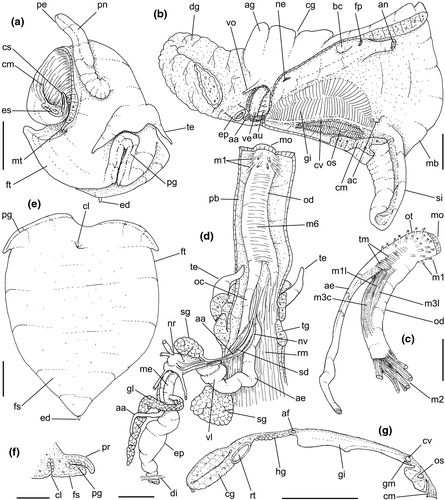
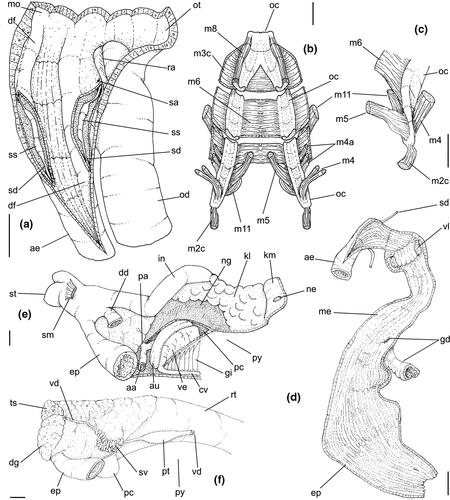
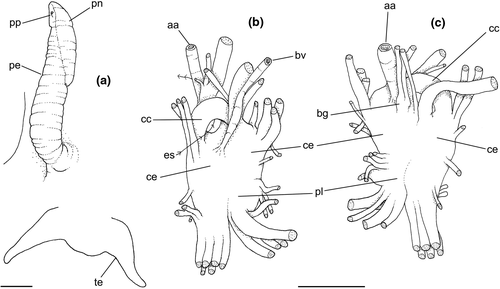
Buccinum squalidum King, 1832: 349 (non Gmelin, 1791).
Buccinum Paytense Val. Kiener, 1834: 17, pl. 6, Figure 16.
Buccinum citrinum Reeve, 1846 pl. 9, fig. 70 (non Wood, 1828); Strebel, 1906: 153, pl. 11, fig. 74 a, b.
Bullia squalida: Reeve, 1847, pl. 4, Figure 26; H. & A. Adams, 1858: 113; Cunningham, 1870: 476; Kobelt, 1877: 289; Ihering, 1907: 537.
Bullia (Buccinanops) squalida: Paetel, 1888: 117.
Buccinanops squalidum: Carcelles & Parodiz, 1939: 758, Figure 10; Castellanos, 1970: 95, pl. 7, Figure 7; Castellanos, 1992: 12, pl. 2, Figure 11.
Buccinanops squalidus: Carcelles, 1950: 62, pl. 2, fig. 40; Carcelles & Williamson, 1951: 299.
Buccinanops paytense: Cernohorsky, 1984: 30.
Bullia (Buccinanops) paytensis: Allmon, 1990: 25, pl. 2, Figure 9.
Type material: B. squalidum: NHMUK 20010205 (Figure 25a–c); B. paytense: not found, probably lost; B. citrinum: NHMUK 1874.12.11.149, illustrated (Figure 25g–i).
Type localities: Buccinum squalidum: unknown; B. paytense: Paita, Perú (in error); B. citrinum: unknown.
3.7.1 Distinctive description
Shell: (Figures 25a–d, j–l, 26a) Medium (up to 57 mm), fusiform. Color reddish brown. Protoconch paucispiral of 1 1/2 to 2 whorls, 1.6 mm wide in second whorl (Figure 26f–h). Sculpture wanting, except for growth lines. Teleoconch of 6–7 slightly convex whorls, spire shorter than 1/2 of shell length. Suture well defined, impressed; 2–3 subsutural spiral cords, sometimes vanishing, very weak. Aperture large, sub elliptic, more than half of total shell length. Parietal callus very weak, but visible (Figure 25a, d, g, j). Inner lip shallowly concave; siphonal inflexure weak. Outer lip simply convex (Figure 25b, h).
Head-foot and operculum: (Figures 26a–b, 27a, e) General features similar to preceding species. Epipodial tentacle small, preceded by medial white region (Figure 26b). Females with cement gland close to anterior end of sole (Figure 27e: cl); relatively short, ending far from hemocoel (Figure 27f: cl). Operculum more rounded, mainly close to nucleus (Figure 25e–f).
Pallial cavity: (Figure 27b, g) Most attributes like those of preceding species, with a few distinctions: Siphon (si) relatively shorter, stubby. Gill (gi) and osphradium (os) relatively shorter and broader; right edge of gill close to tectum. Gill filaments low, with right edge highly convex (Figure 27g: gi). Osphradium with base narrower than filaments (Figure 27g: os).
Circulatory and excretory systems: (Figures 27b, 28e) Characters similar to those of preceding congeneric species, except for single kidney lobe, not organized in folds (Figure 28e: kl).
Digestive system: (Figures 27c–d, 28a–e) Most attributes alike those of preceding congeneric species, distinctive features following. Buccal mass with lateral transverse muscles very developed (Figure 27c: tm). Odontophore with narrow and thin pair m1l (Figure 27c), originating in septum between esophagus and odontophore, running posteriorly at short distance from median line, covering m3c, multiple insertions distributed in postero-dorsal region of odontophore surface; narrow and thin pair m3l, similar to m1l, but originated from lateral surface of odontophore in level of esophageal origin (Figure 27c: m3l); m3c and m6 relatively shorter, keeping free ~1/3 of cartilage length (Figure 28b–c); pair m4 separated in 5–6 separated, narrow bundles (Figure 28b), main posterior branch (m4), another smaller set antero-lateral (m4a); pair m11 relatively thick and broad. Radula (Figure 26d–c): central tooth with 9 cusps of similar size, last two cusps of each margin smaller; rachidian base curved. Lateral teeth with outer cusp large, hook-shaped; inner cusp larger than 3, similar sized, intermediate cusps. Lateral teeth base with blunt inner cusp weakly developed. Salivary ducts possessing subterminal bulged portion (Figure 28a: ss); salivary aperture in lateral side of oral tube, in its posterior level (Figure 28a: sa). Anterior esophagus relatively simple, wide, flattened (Figure 27c, d: ae). Inner surface of entire esophagus with longitudinal, low, narrow folds, slightly more numerous in posterior esophagus (Figure 28d). Valve of Leiblein well-developed, complete, with transverse glandular fold and long cilia (Figures 27d, 28d: vl). Middle esophagus with ~1/3 of anterior esophagus length. Hollow gland of Leiblein relatively long, transversely folded on one side; aperture simple (Figure 28d: gd). Posterior esophagus wide, walls thick, ~as long as anterior esophagus (Figure 28e–f: ep). Stomach (st) with slightly bilobed posteriorly bulged region, walls thick but weakly muscular, but possessing strong gastric muscle (Figure 28e: sm) originated in columella close to end of columellar muscle, running for a short distance up to ventral wall of stomach. Duct to digestive gland single, wide (Figure 28e: dd), located close to intestinal origin.
Genital system: Most features similar to preceding species, except for following: Male with seminal vesicle of medium size (Figure 28f: sv), very narrow caliber; no detected gonopericardial duct. Prostate as simple bulged region in middle portion of pallial vas deferens, preceding its crossing to pallial floor (Figure 28f: pt). Penis (Figures 27a, 29a: pe) possessing elliptical penial gland in dorsal side, from its middle level up to its tip (Figure 29a: pn); penis papilla very small, subterminal (Figure 29a: pp). Female with shallow constriction between albumen and capsule glands (Figure 27b: ag, cg), with albumen gland ~half of capsule gland. Genital atrium with small muscular bursa copulatrix (Figure 27b: bc) in its ventral side. Female aperture as simple transverse pore (fp).
Central nervous system: (Figure 29b–c) Closely similar to preceding species, but with cerebral commissure short, broad, and rounded (cc). Pair of buccal ganglia very small (bg), located close to each other.
Habitat: This is a locally common species that hides buried in sandy beaches during low tide. It can be found because of a low bulge where the animal waits for the rising tide. Averbuj and Penchaszadeh (2016) described the reproductive biology of this species.
Distribution: South of Santa Cruz province, to Tierra del Fuego Is., in Argentina to Punta Arenas, Straits of Magellan in Chile.
Material examined: ARGENTINA. Santa Cruz; San Julián, MACN-In 9200–5, 1 spm, MACN-In 9040–22, 1 spm. Tierra del Fuego; USNM 126896, 1 spm; San Sebastián, MACN-In 12576, 6 spm; Punta Maria, Rio Grande, MACN-In 35385, 20 spm, MZSP 37899, 24 spm; Rio del Fuego, MACN-In 12574, ~80 spm; Bahía San Martín, MACN-In 12578, 1 spm; Punta Sinaia, MACN-In 12577, 3 spm; Ushuaia, MZSP 12574, 1 spm, MZSP 52158, 1 spm; Cabo Peñas, MACN-In 12575–1, 7 spm. CHILE. Magallanes; Bahia Gregorio, USNM 102533, 15 spm, USNM 96528, 1 spm; Punta Arenas, MACN-In 12135–1, 3 spm; Isla Dawson, MACN-In 12135, 2 spm, MACN-In 14135, 3 spm; Straits of Magellan, San Gregorio, MNRJ 1563, 2 spm.
Remarks: King (1832) described this species without illustrating it. He did not notice the previous usage of the name by Gmelin (1791), making his name a homonym. It was later figured and described as Buccinum paytense by Kiener (1834) and as Buccinum citrinum by Reeve (1847). Kiener (1834: 17) cited Valenciennes as author of the species; however, the latter author never published any mention of B. paytense. It was erroneously recorded from Payta, Perú, but it was never recorded further north than Punta Arenas, in the Chilean Pacific.
3.8 Buccinastrum uruguayense (Pilsbry, 1897) new combination

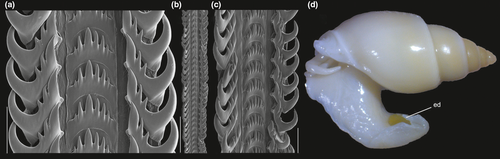
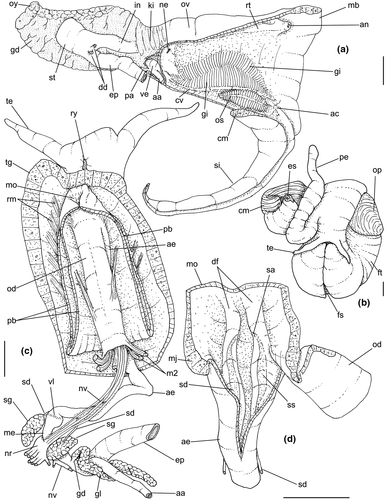
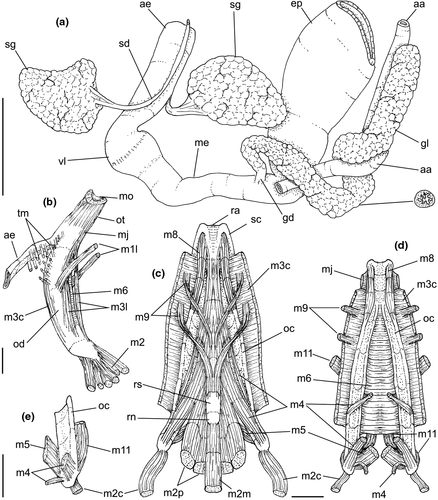
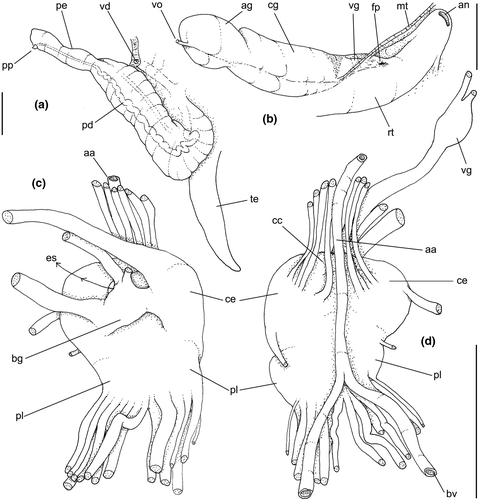
Bullia Uruguayensis Pilsbry, 1897: 6.
?Buccinanops cochlidium: d'Orbigny, 1839, pl. 61, fig. 25; Pilsbry, 1897: 7 (non Dillwyn, 1817).
Buccinanops uruguayensis: Carcelles & Parodiz, 1939: 760, figs. 5 (radula), 12; Castellanos, 1970: 91, pl. 7, fig. 14; Rios, 1985: 103, fig. 456; 1994: 130, fig. 562.
Buccinanops uruguayense: Cernohorsky, 1984: 30.
Bullia (Buccinanops) uruguayensis: Allmon, 1990: 26, pl. 2, fig. 8.
Type material: ANSP 70507, 8 syntypes (Figure 30a–c).
Type locality: Maldonado Bay, Uruguay.
3.8.1 Distinctive description
Shell: (Figure 30a–p) small to medium size (to 31 mm), fusiform. Protoconch paucispiral, of 2 globose, smooth whorls. Teleoconch up to 7 weakly convex whorls, spire around 1/2 of total shell length. Suture well defined, impressed. Sculpture 3–4 subsutural spiral lirae present in most of specimens, vanishing in middle of whorl, reappearing at base of last whorl (Figure 30c, f, h–i, n–o). Aperture large, shorter than half of total shell length. Parietal callus weak (Figure 30a, d, g, j, l, p); inner lip weakly concave; siphonal inflexure relatively strong. Outer lip profile almost straight (Figure 30b, e, k, m).
Head-foot and operculum: Most features similar to those of preceding species (Figure 32b). Colorless, with epipodial tentacle well-developed (Figure 31d: ed). Operculum elongated, pointed close to nucleus (Figure 30q–r).
Pallial cavity: (Figure 32a) Attributes alike those of preceding species, with following special features: Siphon very larger (si). Gill (gi) and osphradium (os) with anterior and posterior ends more sharply pointed. Wide space between gill and rectum. Gill and osphradial filaments similar to B. deforme.
Circulatory and excretory systems: (Figure 32a) Similar to preceding species. Heart proportionally smaller (ve). Kidney lobe with two flaps attached to intestine (ki), transversely folded.
Digestive system: (Figures 32c–d, 33) General characters similar to those of preceding species. Interesting features following. Transverse lateral muscles connecting esophageal origin and odontophore composed of more sparse fibers (Figure 33b: tm). Odontophore muscles: pair m1l inserted in middle level of odontophore length (Figure 33b: m1l); ventral pair m3l broad (Figure 33b); pair m4 divided into three portions, larger bundle originated at posterior end of cartilage, smaller bundle originating preceding posterior 1/4 of cartilages (Figure 33c–d), another very small bundle originating in median side of main bundle (Figure 33e: m4); pair m9 divided into two separated bundles, anterior one inserted at middle length of radular sac (Figure 33c). Pair of odontophore cartilages slenderer, fused portion shorter than 1/4 of length (Figure 33c–d: oc). Radula (Figure 31a–c) central tooth with 7–10 cusps, usually of same size, except for usually smaller lateral cusps. Rachidian base curved. Lateral teeth with outer cusp hook-shaped; inner cusp larger, thicker than 2 intermediate cusps; lateral teeth base with very weak blunt inner cusp. Pair of salivary ducts possessing bulged subterminal portion (Figure 32d: ss); salivary aperture on medial edge of dorsal folds, at middle length of oral tube (Figure 32d: sa). Portions of esophagus similar to those of B. paytense (Figures 32c, 33a: ae, me, ep); inner surface with longitudinal, irregular, narrow folds. Valve of Leiblein complete, lacking bypass (Figures 32c, 33a: vl). Gland of Leiblein small, anterior half hollow (Figure 33a: transverse section), with 5–6 longitudinal, glandular folds; duct very short (gd) simple. Stomach with non-muscular walls (Figure 32a: st), as deep blind-sac, ~1/3 whorl long; pair of small ducts to digestive gland located in esophagus-intestine intersection (Figure 32a: dd).
Genital system: Most structures similar to those of preceding species, remarkable features following. Male with seminal vesicle of medium size. Prostate like that of B. paytense. Penis marked by sudden bottleneck portion, producing slender, elongated distal region of ~1/3 length of penis (Figures 32b, 34a). Penis duct (pd) convolute, running closer to right side, except for slender distal portion, containing duct narrow, straight, running centrally; penis apex blunt, flattened; papilla small, subterminal (Figure 34a: pp). Female with clear constriction separating albumen and capsule glands (Figure 34b: ag, cg), capsule gland ~twice length of albumen gland. Vaginal atrium (vg) relatively long, ~1/4 of total pallial oviduct length, tapering toward female pore; brown triangular portion of ingesting gland in its posterior region, small muscular concavity in its right-side corresponding to a small bursa copulatrix. Female pore (fp) simple.
Central nervous system: (Figure 34c–d) As concentrated and with similar attributes as in preceding species. Cerebral commissure broad and short (cc). Pair of buccal ganglia apparently fused with each other (bg). Visceral ganglion located posteriorly at a distance equivalent to nerve ring length (Figure 34d: vg).
Habitat: rare, usually subtidal up to 30 m depth.
Distribution: Angra dos Reis, Rio de Janeiro, Brazil to Bahía San Blas, Buenos Aires province, Argentina.
Material examined: BRAZIL. Rio Grande do Sul; Pinhal, MZSP 27314, 2 spm; GEDIP expedition MZSP 33750, 1 spm, MZSP 33898, 2 spm, MZSP 57522, 5 spm; off Mostardas, 31°09′S, 50°43′W, 21 m, MZSP 51405, 4 spm (sta. 1725). URUGUAY. Rocha; La Paloma, MZSP 51406, 1 spm, MACN-In 28784, 2 spm, MACN-In 30481, 1 spm, MACN-In 30487, 1 spm, MACN-In 30171–2, 2 spm, MNRJ 6543, 2 spm, MACN-In 15151, 1 spm, MNRJ 1248, 2 spm, MZSP 78248, 33°54.7′S, 53°17.6′W, 15 m; Cabo Santa Maria, MACN-In 23582, 1 spm. Maldonado; Punta del Este, MZSP 51617, 2 spm. ARGENTINA. Buenos Aires; Necochea, MACN-In 25199, 35°40S 56°03 W, 9.1 m, 14 spm, MACN-In 24202, 35°27′S 54°11′W, 23 m, MACN-In 24146, 36°24S 55°53 W, 16 m, 6 spm, MACN-In 24295, 37°18′S 56°49′W, 8 m, 2 spm, MZSP 10046, 1 spm; Mar del Plata, MACN-In 25364, 17 spm, MACN-In 4660–4, 2 spm; Miramar, MACN-In 9247–14, 12 spm; Monte Hermoso, MACN-In 6619–53, 5 spm, MACN-In 11230–1, 1 spm, MZSP 10052, 16 spm; Arroyo Parejas, MACN-In 11232–1, 3 spm; Puerto Belgrano, MACN-In 11341, 3 spm; Bahía San Blas, MACN-In 20254, 23 spm, MACN-In 20273–3, 1 spm.
Remarks: Buccinastrum uruguayense has been scarcely mentioned in the local or international literature since its original description. Probably it was not easily recognized because of the similarity with young specimens of B. cochlidium and others, one of the locally most common species of Buccinanops. In addition, Ihering (1907: 445) stated that it can be only a local variety of B. cochlidium, an assumption that can justify its absence in subsequent surveys. Despite the size, which is clearly smaller, and the slender spire, the presence of several subsutural lirae in the first whorls (Figure 30h–i, k, n, o) as well as on the base of the last whorl, are distinguishing characters. In addition, the shape and ornamentation of the egg capsules are different from those of B. cochlidium which has a characteristic and unique sculpture on one side. Averbuj and Penchaszadeh (2016) provided some data on the reproductive biology of this species.
4 PHYLOGENETIC ANALYSIS
As explained above, a sample of 19 buccinoideans (Table 1) were analyzed using the Maximum Parsimony methodology outlined in Simone (2011, 2020), including the 7 species studied here. Nine additional species of buccinoideans, particularly of columbellids, fasciolariids, melongenids, nassariids, and buccinid, were also included, based on descriptions published elsewhere (Table 1), although five species were already treated by Simone (2011): two of Buccinanops, Oligohalinophila dorri (Wattebled, 1886) (as Nassodonta) and the two species of Amphissa H. & A. Adams, 1853. The main objective is to check the monophyly of the Buccinanops/Buccinastrum clade and to provide inputs for a wide discussion and analysis of its relationships. Although the Buccinanops/Buccinastrum clade includes all Recent species, it is acknowledged that the representatives of the other buccinoideans taxa are far from being well-represented, as the families are diverse. However, some superficial and preliminary inferences are possible and are explored below.
The matrix resulting from the insertion of the 14 buccinoideans in the matrix published by Simone (2011) is enormous; thus, only the lines concerned with the present paper are reproduced in Table 3. The remaining portions of the matrix were identical to the matrix by Simone (2011). Anyhow, it is important to emphasize that some states were inserted into that matrix (see material and methods above) in order to improve the resolution of the present ingroup.
- Abbreviations: Bops. = Buccinanops; Btrum. = Buccinastrum; Eng. = Engoniophos; Hemipol. = Hemipoligona
The resulting cladogram is mostly the same as the one shown by Simone (2011: figure 20), with an expansion and increased resolution of the nodes 214 to 217 shown in this paper (Figure 35), using the same node labels as in Simone (2011: figure 20), adding letters to newly formed nodes (A–O) that are used in the discussion below. Figure 35 also shows the synapomorphies of each node displayed by symbols (i.e., black square = non homoplastic synapomorphy; white square = reversions; black circle = convergencies – of course these optimizations take into account the entire cladogram by Simone, 2011).
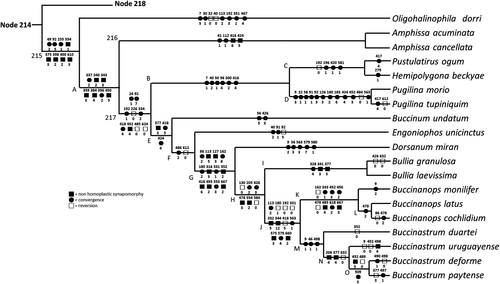
A total of 12 cladograms were obtained in the analysis, the cladogram portion of the ingroup did not possess any polytomy in the strict consensus, and it is reproduced here (Figure 35), with 3131 steps, CI = 51, RI = 94.
5 DISCUSSION
According to the analysis of the material studied, Buccinanops and Buccinastrum are typical representatives of both malacological provinces: Argentine and Magellanic. There are no species of these genera ranging beyond these biogeographical areas.
All species have anatomical features and shell morphology, including reproductive biology traits, that help us in the decision that Buccinanops and Buccinastrum are valid genera. This has been robustly suggested phylogenetically, with representatives present at least since the Neogene in deposits from Southern Patagonia (Griffin & Pastorino, in prep.). The DNA sequence phylogenetic calibration has shown an age—for Buccinanops—of at least lower Miocene (Galindo et al., 2016: 12); and the DNA sequence cladistic arrangement is compatible with the present result, with the single studied Buccinastrum, B. globulosus, as sister of a monophyletic branch that included the three Buccinanops here considered (Galindo et al., 2016, Figure 2).
Interestingly, Galindo et al. (2016) recognized some common characters uniting the three genera (Buccinanops, Bullia, and Dorsanum), a branch accepted by Allmon (1990), Brown (1982), Cernohorsky (1984), Haasl (2000), and Simone and Pastorino (2014), as “the result of convergence during nassariid evolution.” For example, the loss of the planktotrophic mode of larval development and the presence of multicuspid radular lateral teeth. Allmon (1990) concluded that the Nassariidae were monophyletic, with Dorsaninae-Bulliinae being the sister group to both Cylleninae and Nassariinae; he also found a (Dorsanum (Bullia – Buccinanops)) clade, that Haasl (2000) recovered as paraphyletic (CI = 0.381, RI = 0.639).
5.1 Taxonomical comparisons
In this discussion, the character numbers are the same as in Simone (2011). The most important features that can be used for distinguishing the species formerly considered Buccinanops are properly explored above, along the distinctive descriptions. Here, only the more relevant aspects are discussed.
The morpho-anatomical investigation of the seven valid Recent species formerly considered Buccinanops (Figure 35, node J) easily shows two reasonably distinct groups. One of them clusters relatively large species with specimens easily reaching 80–100 mm that live in subtidal waters and are mostly sampled by otter trawl or divers from 10 to 50 m depths. Anatomically, there are eight synapomorphies supporting this clade (Figure 35: node K), some of them are the duplicity of the pair of extrinsic odontophore muscle m1b (character 333, state 1); the ventral aperture of the salivary glands, close to median line (ch. 452, st. 3); the simplification of the anterior esophagus (ch. 478, reversion to 0); the papilla-like female genital pore (ch. 618, st. 2). Nevertheless, the most important synapomorphy is the reduction in the valve of Leiblein, which is only vestigial (ch. 485, st. 4), marked only by a transverse fold in B. monilifer and in B. cochlidium (Figure 5e–f), and only by a change of the internal pleating in B. latus (Simone, 1996: figure 16). The length of the cerebral commissure is another interesting feature of this branch, as it tends to be very long (Figures 8a–b, 11a–c, 14c), particularly in B. cochlidium. The node K, which includes the type species B. cochlidium, represents the revised concept of the genus Buccinanops, grouping only three living species. Its single internal division clusters B. cochlidium with B. latus (Figure 35, node L), in which the synapomorphy is the elongated rather sinuous anterior esophagus (ch. 478, st. 2), particularly in B. cochlidium (Figure 5b: ae).
The other group formerly classified as Buccinanops is the node M (Figure 35), which includes the other four ingroup species. Three synapomorphies support this node, including the presence of irregular subsutural nodes (character 9, state 1); a well-developed, folded callus (ch. 46, st. 1); and the presence of a single duct to the digestive gland in the stomach (ch. 498, st. 1) (e.g., Figure 28e). However, other additional attributes can be added to this branch: the relatively smaller size of the shell, usually smaller than 50 mm; the intertidal habitat, rarely occurring subtidally; the invariably smooth first whorl vs. the presence of axial riblets, and the more robust, thick-walled shell, possibly to resist the waves. Based on this set of characters, the new genus, Buccinastrum gen. nov., is introduced to designate this node. Its more basal branch is B. duartei, with the other three species united at node N. Three synapomorphies support this taxon, including the low gill filaments with convex right edges (character 206, state 4); the multiple pairs of odontophore muscles m9 (ch. 377, st. 4), and the separation of the albumen gland from the capsule gland (ch. 632, st. 0). The following branch separates B. uruguayense from node O, which groups B. deforme and B. paytense. These synapomorphies support node O, including the lateral positioned salivary glands aperture (ch. 452, st. 5); the lack of folds in middle esophagus (ch. 489, st. 0); and a broad duct to digestive gland (st. 509, st. 3).
A set of 11 synapomorphies support node J (Figure 35), that is, Buccinanops + Buccinastrum, previously considered Buccinanops. The most important characters are the single posterior epipodial tentacle (character 113, state 2); the osphradium filaments widely attached to mantle (ch. 192, st. 0); the lack of the odontophore muscle “ma” (ch. 331, st. 0); the presence of lateral buccal mass protractor muscles (m1a – ch. 332, st. 5); the longitudinal, lateral pair of odontophore muscle m3 (ch. 344, st. 12/C); the arched rachidian, with ~10 cusps at the edge (ch. 418, st. 5); the small, simple papilla at the tip of penis (ch. 563, st. 1), located subterminal (ch. 575, st. 3); and the commissure between buccal ganglia incorporated to the nerve ring (ch. 660, st. 2).
The node I (Figure 35), which represents the west and south African genus Bullia, is supported by three synapomorphies, including the oral tube muscles forming a circular, sphincter-like band (character 328, state 4); the presence of the odontophore muscle pair m2c running along median line (ch. 341, st. 3); and the odontophore pair m9 multiple, inserting directly in cartilages instead of in m3 (ch. 377, ct. 5). Bullia granulosa, one of the species included here, resulted in a peculiar allocation using a DNA sequence data: it fells in the nassariine Naytia branch together with four other species (Galindo et al., 2016) formerly considered Nassarius. All these four species have small, sculptured shells with a large callus, very typical of what formerly was considered Nassarius. The inclusion of B. granulosa in this branch renders difficult a definition based on non-genetic-molecular characters. Notwithstanding, all species are now considered Naytia (WoRMS website), a very heterogeneous taxon so far lacking definition. As no Naytia species is included in the present study, no additional comment is possible to be taken from a morphological viewpoint.
Bullia (Figure 35, node I) is the sister-group of the Buccinanops-Buccinastrum branch (node J) in node H, supported by six synapomorphies, including the loss of the eyes (character 130, reversion to state 0); the flat form of the gill filaments (ch. 205, st. 2); the presence of intermediate small cusps in radular lateral teeth (ch. 426, st. 3); the thick-walled anterior esophagus (ch, 478, st. 3), and the presence of gonopericardial duct (ch. 554, st. 2).
The node G (Figure 35) has a branch representing the subfamily Dorsaninae as defined and Galindo et al. (2016) (only having Dorsanum), and the node H. The node G is Dorsaninae as defined by Allmon (1990), with the three genera. It is supported by 12 synapomorphies, reuniting Dorsanum with node H. The main synapomorphies are the tendency for an unpigmented head-foot (character 86, state 2); the slightly protruded head, flap-like, with a tentacle at each lateral end (ch. 127, st. 3); a small fold in the base of the siphon (ch. 162, st. 8); the presence of a pair of thin lateral, dorso-ventral muscles uniting esophagus and odontophore (ch. 316, st. 3); the multiple origins in outer edge of cartilages of the odontophore muscle m2 (ch. 352, st. 2); the flattened, arched radular rachidian, with ~20 cusps in cutting edge (ch. 418, st. 6); the gastric form bulging posteriorly (ch. 493, st 2); the thick-walled, convolute, and muscular penis duct (ch. 553, st. 3); the relatively long cerebral commissure forming small strap-like arc (ch. 667, st. 2). Another synapomorphy (ch. 113, st. 5) refers to the presence of a pair of epipodial tentacles, which actually is shared with most nassariids. Its presence in this node is because of the lack of epipodial tentacles in the nassariid representative Engoniophos Woodring, 1928 (see discussion below), but possibly the state would support a different branch if more nassariids were included. In any case, the typical nassariid pair of epipodial tentacles is large, while those of Dorsanum and Bullia are very small. This difference can be indicative of different origins. Additional discussion on the epipodial tentacles in nassariids is found elsewhere (Allmon, 1990; Galindo et al., 2016; Haasl, 2000).
The remaining portion of the shown cladogram (Figure 35) refers to outgroups, considered only for improving the present discussion. The character survey is thus not as exhaustive. Contrarily, it was only sufficient to arrange the closer taxa, to test the ingroup monophyly, and for permitting a better-based argumentation. Considering this scenario, the ensuing information can be given.
The node F gathers Engoniophos with the dorsanines-bulliines-buccinanopsines (sensu Galindo et al., 2016) (node G). It is supported by two synapomorphies, including an elongated, relatively reduced gland of Leiblein (character 486, state 2); and the loss of the anterior bursa copulatrix in the pallial oviduct of females (ch. 612, st. 0). Engoniophos unicinctus (Say, 1826) is one of the representatives of the family Nassariidae, a position that it has acquired recently (Abbate et al., 2018; Galindo et al., 2016), as it was previously assumed to be Buccinidae. The inclusion of this nassariid in the dorsanids is not a surprise, as the latter has been considered a subfamily of the former. However, as stated above, it is interpreted that there are enough differences to support a distinction of both groups at subfamily rank.
The node E reunites Buccinum undatum with the nassariid branch (node F). Buccinum undatum is the type species of the genus that is the type of Buccinoidea; it was thus included as a good representative of Buccinidae, as well as its superficial shell similarity with Buccinanops (literally a Buccinum lacking eyes). Three synapomorphies support this node, including a dorsal, Y-shaped pair m9 in odontophore (character 377, state 3); and the wide, oblique radular lateral tooth, with long cusp at each end, the lateral cusp being larger (ch. 424, st. 4).
The two representatives of the Fasciolariidae, Pustulatirus ogum (Petuch, 1979) and Hemipolygona beckyae (Snyder, 2000), are united by the node C (Figure 35). Four synapomorphies support this node, including osphradium as wide as gill (character 196, state 1); arched lateral edges of radular rachidian (ch, 420, st. 1); and penis sharply pointed (ch. 581, st. 1). Both representatives of the Melongenidae, Pugilina morio (Linnaeus, 1758) and P. tupiniquim Abbate & Simone, 2015, are united by node D, which is supported by 12 synapomorphies. The more interesting ones are the shouldered shell (ch. 9, st. 3); the hairy periostracum (ch. 22, st. 1); the head preceded by a long neck (ch. 92, st. 3), with very reduced tentacles (ch. 126, st. 3); the osphradium with pointed filaments (ch. 183, st. 6); the salivary glands aperture in median-ventral region of the oral cavity (ch. 452, st. 3); the loss of the valve of Leiblein (ch. 484, st. 0) and a simple papilla on the penis (ch. 536, st. 1).
Both, fasciolariids and melongenids, are joined at node B, supported by six synapomorphies. The more interesting are elongation of the siphon (character 50, state 1); multiple pairs of proboscis retractor muscles (ch. 98, st. 4), with muscles surrounding the base of the proboscis (ch. 300, st. 2); and radular rachidian with 3 cusps at tip (ch. 416, st. 4).
Node 217 (Figure 35) gathers all buccinoideans excluding the columbellids (node 216). It is supported by nine synapomorphies, being the more interesting the shell internal furrow in each whorl (character 24, state 1); the not (or weakly) protruded head (ch. 92, st. 7); the osphradium filaments with tips wider than their bases (ch. 192, st. 1); the loss of the anal gland (ch. 226, st. 0); the pair of retractor muscles of buccal mass, m2, narrow, long, inserting posteriorly in odontophore cartilages (ch. 334, st. 2); the loss of the bypass of the valve of Leiblein (ch. 485, st. 0); and the loss of the bursa copulatrix (ch. 624, st. 0).
Node 216 brings together both species of Amphissa, which are the Columbellidae representatives. The node has four synapomorphies supporting it, including the presence of an anal canal in the aperture (character 41, state 1); a rounded edge of the foot sole (ch. 112, st. 1); radular rachidian reduced or absent (ch. 416, st. 6); and radular lateral tooth arched inwards, with bifid tip (ch. 424, st. 3).
Node A includes the columbellids (node 216) and node 217 (Figure 35), with seven synapomorphies supporting it. The more important ones are a small portion of the pair m2 passing through the nerve ring (character 337, state 2); the odontophore pair m2 inserting on radular sac, radular nucleus and posterior end of cartilages (ch. 340, st. 2); the odontophore muscle m3 as circular thin fibers opposite to m6 (ch. 343, st. 3); the pair m5 originated in opposed side of m4 in cartilages (ch. 359, st., 3); the odontophore pair of ventral radular tensors, m11, originated at posterior end of cartilage alongside m5 (ch. 384, st. 4); transverse muscles (mt) as two bands (ch. 396, st. 2); and pair of salivary ducts very narrow and long, running attached to esophagus alongside aorta branch (ch. 450, st. 5). The branch with Oligohalinophila dorri interposes between nodes 215 and A (Figure 35), preceded by eight more synapomorphies. From those, the more interesting are the anal notch at shell aperture close to suture (ch. 49, st. 2); a socket-like head (ch. 92, st. 5); a branch of the odontophore pair of muscles m4 connected at posterior vertex of cartilages (ch. 354, st. 1); the pair m8 lying on outer-dorsal edge of odontophore cartilages (ch. 375, st. 3); odontophore cartilages several times longer than wide (ch. 398, st. 2); and the albumen and capsule gland at pallial oviduct difficult to separate from each other (ch. 610, st. 3). O. dorri has been considered a nassariid and supposedly should be allocated after node F (Figure 35). Its strange allocation may be thus considered an artifact caused by missing data, as the available sample of this species does not permit a full anatomical investigation. Possibly, if more details are available, a better resolution of O. dorri, and its reallocation closer to nassariids could be obtained. Under this setting, the synapomorphies of both nodes 215 and A can be considered synapomorphies of the Buccinoidea. Interestingly, the allocation of O. dorri was also problematic following a DNA sequence approach, with low support (Galindo et al., 2016).
An uncontroversial phylogeny of the Neogastropoda is far from being well-established, both via morphological and DNA sequence approaches (e.g., Colgan et al., 2007; Fedosov et al., 2017, 2019; Hayashi, 2005; Modica et al., 2011; Simone, 2004, 2017; Yang et al., 2019). Despite that, based on most results in the literature and in the present one, Buccinoidea appear to have the Columbellidae at the base (Figure 35, node 216). This node has the Fasciolariidae-Melongenidae in a branch (Figure 35: node B), and node E (Figure 35). The node E apparently groups the Buccinidae together with a branch with Nassariidae including Dorsaninae (sensu Allmon, 1990) (Figure 35: node F). It is important to emphasize that the present phylogeny has the major concern to provide a better-based discussion, with no intention of challenging the current taxonomy of the Neogastropoda, mainly concerned to the nassariid buccinoideans and allies (Dorsanidae, Buccinanopsidae, etc.). It is, anyway, a step forwards, basing the discussion of future additional phylogenies.
6 CONCLUSIONS
- The previous concept of the genus Buccinanops has alluded monophyly, supported by 11 synapomorphies, and was restricted to SE South American coast, with only one species reaching the southern-east Pacific (B. paytense).
- The phylogenetic arrangement of the species that was previously considered Buccinanops allows its subdivision in two genera, Buccinanops (8 synapomorphies) restricted to three large species from relatively deep waters, and the new Buccinastrum gen. nov. (3 synapomorphies) including four small species—with B. deforme as type—mostly occurring intertidally.
- Based on the differences and exclusive morphological characteristics, the nassariid subfamily Dorsaninae appeared to be valid. It is supported by 12 synapomorphies and includes four genera—Dorsanum, Bullia, Buccinanops, and Buccinastrum gen. nov. However, it is recognized that it has emerged as paraphyletic in DNA sequence studies and the present study does not include all taxa included in those.
- The putative subfamily Dorsaninae is part of the Nassariidae, which is sister of Buccinidae, part of the Buccinoidea, sharing the 15 synapomorphies of the superfamily.
ACKNOWLEDGMENTS
We are grateful to Jose C. Tarasconi (Porto Alegre), who provided information and specimens from Brazil and the following researchers and curators helped in many ways to find type material and send pictures: A. Pimenta (MNRJ), Yves Finet (MHNG), G. Darrigran (MLP), A. Tablado (MACN), F. Scarabino, W. Serra, S. Wlodek (MNHNM), P. Callomon (ANSP); E. Strong and J. Harasewych (USNM), V. Heros, L. A. Galindo and P. Bouchet (MNHNP). Four anonymous referees and editor made important comments on the manuscript. Special thanks are due to M. Griffin (MLP) for his thoughtful suggestions. We acknowledge funding by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina, to which G.P. belongs as member of the “Carrera del Investigador Científico y Técnico.” This work was supported in part by the Project PICT No. 942 from the National Agency for Scientific and Technological Promotion, Argentina. A grant from the FAPESP allowed G.P. to visit the collections of MZUSP in 2006.
Open Research
DATA AVAILABILITY STATEMENT
The raw data and the data basing this paper are entirely reported in it and also in giving open-accessed references, mainly Simone (2011).




