Gondwana breakup under the ephemeral look
Abstract
Atalophlebiinae (Ephemeroptera, Leptophlebiidae) is a mayfly subfamily present in temperate and mountainous areas of South America and Australia. We tested the hypothesis that both vicariance and dispersal related to the second phase of Gondwana breakup—which began in the Early Cretaceous and resulted in the separation between Madagascar and India from Antarctica and Australia—contributed to the origin, diversification, and shaped the current distribution of this group. The hypothesis was tested using Bayesian phylogenetic trees, fossil-based molecular dating, and ancestral range estimation to reconstruct the biogeography of the lineages within this group. The results suggested an origin in the late Gondwana supercontinent for Atalophlebiinae (85.76–136.63 mya) after a vicariant event during the Cretaceous period. Subsequently, the lineage diversified into a scenario that refers to a Gondwanic corridor formed by South America, Antarctica, and Australia. At the end of the separation of the continents that made up the Gondwanic corridor, speciation occurred within the current distribution areas. The diversity and current distribution of Atalophlebiinae were shaped by complex processes of vicariance, dispersal, and speciation within the Gondwanic corridor during the second phase of the supercontinent breakup. Mayflies have difficulty in crossing transoceanic barriers, which suggests that most living taxa are the result of more recent local ecological and historical processes.
1 INTRODUCTION
Vicariance and dispersal associated with plate tectonics helped shape diversification and distribution patterns of biodiversity across the planet (Chamberland et al., 2018; Jurado-Rivera et al., 2017; Toussaint, Hendrich, et al., 2017). Gradual breakup of ancient supercontinents contributed to cladogenetic events, dividing lineages by vicariance (see Kim & Farrell, 2015; Sanmartín & Ronquist, 2004; Toussaint et al., 2017) and dispersal through corridors that connected land fragments (see Reguero et al., 2014; Seton et al., 2012). Thus, vicariant events associated with the Gondwana breakup are commonly invoked to explain the disjunct distribution of ancient lineages across continents in the southern hemisphere (McCulloch et al., 2016; Sanmartín & Ronquist, 2004), while transoceanic dispersal would be responsible for this pattern of distribution in more recent lineages (Condamine et al., 2013; Martín-Bravo & Daniel, 2016).
Gondwana breakup occurred gradually and can be divided into two phases. The first began in the Early Jurassic (~180 mya), resulting in the separation of West Gondwana (South America/Africa) from East Gondwana (Madagascar, India, Antarctica, and Australia), ca. 140 mya (Mueller & Jokat, 2019; Seton et al., 2012; Thompson et al., 2019). However, it is important to note that southern South America was connected to the Antarctic Peninsula through the Weddellian Isthmus until the opening of Drake Passage at ca. 35 mya (Elsworth et al., 2017). The second phase began in the Early Cretaceous (~135 mya), and resulted in the separation between Madagascar/India from Antarctica/Australia forming a corridor that connected fragments from South America, Antarctica, and Australia at ca. 100 mya (Gibbons et al., 2013; Seton et al., 2012; Seton et al., 2012; Thompson et al., 2019). Concurrently to these events, rupture between Africa and South America and separation of Madagascar from India took place (Gibbons et al., 2013; Seton et al., 2012; Thompson et al., 2019).
Atalophlebiinae (Ephemeroptera, Leptophlebiidae) are a cool-adapted mayfly subfamily with amphinotic distribution, present in temperate and mountainous areas of South America and Australia (Monjardim et al., 2020; O'Donnell & Jockusch, 2008; Pescador & Peters, 1980; Savage, 1987). Previously, researchers also considered taxa from tropical areas of South America, Africa, and Madagascar as part of this group, suggesting an ancient Gondwana origin of this subfamily (Kluge, 2009; Pescador & Peters, 1980; Savage, 1987). However, recent studies indicate a monophyletic lineage of only amphinotic taxa (Monjardim et al., 2020; O'Donnell & Jockusch, 2008). Ephemeroptera is one of the oldest insect lineages (Misof et al., 2014), and its amphinotic distribution pattern is recurrent in other taxa of the group, such as Ameletopsidae, Coloburiscidae, Nesameletidae, and Oniscigastridae (see Edmunds, 1972; Sartori & Brittain, 2015). Considering the role of plate tectonics in biogeography and our current knowledge of the cool-adapted mayflies, here, we tested the hypothesis that Atalophlebiinae originated and diversified under the influence of both vicariant and dispersal events, during the second phase of Gondwana breakup, which would explain the disjunct distribution between South American and Australian taxa and their absence in tropical Africa, Madagascar, and Indo-Malayan regions.
2 MATERIALS AND METHODS
2.1 Taxon sampling and molecular dataset
We sequenced 76 specimens (Table S1) for two molecular markers (Table S2): the D2–D5 region of the 28S ribosomal RNA gene (Gillespie et al., 2004, 2005) and a partial region of the Cytochrome c oxidase subunit 1 gene (Folmer et al., 1994). We also used 59 sequences available on GenBank to complement our dataset (Table S1). Nine outgroup genera were selected based on a recent phylogeny of the Ephemeroptera (Monjardim et al., 2020; Ogden et al., 2019; O'Donnell & Jockusch, 2008) and the availability of fossils for calibration. Our data matrix comprised 19 genera, representing approximately 55% of the generic composition of Atalophlebiinae. All specimens sequenced in this research are stored in the Museu de Entomologia of the Universidade Federal de Viçosa, under the care of the authors (FFS) or in the Coleção Zoológica Norte Capixaba, Universidade Federal do Espírito Santo, Brazil.
2.2 Phylogenetic analyses
Sequences were aligned in Geneious 9.0 (www.geneious.com), and nucleotide substitution models for each marker were selected using the Corrected Akaike Information Criterion (AICc) in jModelTest2 (Darriba et al., 2012) on CIPRES (Miller et al., 2010). Saturation level of sequences was verified by Xia's test (Xia et al., 2003) in DAMBE 7 (Xia, 2018), and the third codon position of COI was consequently excluded from the analyses. The concatenated molecular data matrix comprised 1622 base pairs (1196 bp from 28S and 426 bp from COI) for 97 operational taxonomic units (Alignment S1). Models selected for each partition were GTR+G to 28S and TrN+G to COI (Table S3).
Phylogenetic tree was inferred using Bayesian inference in MrBayes 3.2.7a (Ronquist et al., 2012) on CIPRES (Miller et al., 2010). Eight Markov chain Monte Carlo (MCMC) iterations were run simultaneously for 1.58 million generations with sampling trees every 1000 generations and 25% of burn-in, until the convergence diagnostic reached the stop value (standard deviation of split frequencies <0.01). Support of nodes was provided by posterior probabilities (PP) as directly estimated from the majority rule consensus topology. Considering recent discussions about statistical significance (Amrhein et al., 2019; Hurlbert et al., 2019; Pike, 2019; Wasserstein et al., 2019), the logic, background knowledge, and experimental design were considered alongside PP to reach a conclusion and decide on its certainty. Therefore, nodes with PP value higher than 0.85 were considered well-supported.
2.3 Divergence times
We used the relaxed uncorrelated lognormal molecular clock with a tree prior using the birth–death incomplete sampling algorithm (Stadler, 2009). Substitution models for each partition (28S and COI) were selected according to AICc (Table S3), and monophyly was forced based on BI results. Clock points calibration was based on seven date priors based on fossils (n = 5), geological event and probabilities of dispersal (n = 1, adapted from Landis, 2017), and secondary data derived from previous analyses (n = 1).
(1) Root was calibrated to represent the minimum and maximum (242–290 mya) ages of fossil species Protereisma permianum Sellards 1907 (Protereismatidae), believed to be one of the stem groups of Ephemeroptera (Godunko et al., 2011; Grimaldi & Engel, 2005; Sroka et al., 2015), and the mayfly Triassonurus doliiformis Sinitshenkova & Papier, 2005 (Siphlonuridae), the lineage with the oldest origin in our dataset (Lognormal distribution, offset = 242.0, Mean = 8.5, Standard deviation = 1.0, mean in real space). (2) Oligoneuriidae initial diversification was calibrated based on Incogemina nubile Storari et al., 2020. (Oligoneuriidae) (Lognormal, offset = 112.6, M = 30.0, S = 1.0, mean in real space). (3) Leptophlebiidae initial diversification was calibrated based on the age of ~175 mya given by Grimaldi and Engel (2005) for origin of the family (Normal distribution, Mean=175.0, Sigma=25.0). (4) Leptophlebiinae initial diversification was calibrated based on the fossil Aureophlebia sinitshenkovae Peters & Peters 2000 (Leptophlebiinae) (Lognormal, offset = 89.3, M = 20.0, S = 1.0, mean in real space). (5) Paraleptophlebia initial diversification was calibrated based on the fossil Paraleptophlebia prisca (Pictet & Hagen, 1856) (Lognormal, offset = 33.9, M = 13.0, S = 1.0, mean in real space). (6) Calibration of the most recent common ancestor of Atalophlebiinae from the lineage that originated Radima Akers, Peters & Peters, 2003 (Lognormal, offset = 85.0, M = 21.0, S = 1.0, mean in real space) considered the final period of separation between Madagascar/India and Antarctica/Australia (~100 mya, Gibbons et al., 2013; Seton et al., 2012; Thompson et al., 2019; White et al., 2013) and low probability of dispersal between these areas after separation (see supplementary data of Landis, 2017; Sanmartín & Ronquist, 2004). 7) Divergence between Atalophlebia Eaton, 1881, and Atalomicria Harker, 1954, was calibrated with the fossil Atalophlebia culleni Etheridge & Olliff 1890 (Lognormal, offset = 2.6, M = 20.0, S = 1.0, mean in real space). Fossil information can be accessed in the Fossilworks Paleobiology Database (http://fossilworks.org).
The input file was constructed in BEAUti v2.5.2 and run in BEAST2 v2.5.2 (Bouckaert et al., 2019) for 100 million generations and trees sampled every 10,000 generations. The convergences of runs and the effective sample size (ESS > 1000) of parameters were examined in Tracer v1.7.1 (Rambaut et al., 2018). A tree with maximum clade credibility topology, using a burn-in of 25%, was constructed with TreeAnnotator v2.5.2, and analyses were run on CIPRES (Miller et al., 2010).
2.4 Ancestral area reconstruction
As suggested by Ree and Sanmartín (2018), the model for reconstruction of ancestral areas was chosen to consider the structure and assumptions of models, and not a statistical method that assumes probabilistic equivalence between different models (e.g., AIC). We selected Dispersal-Extinction-Cladogenesis (DEC; Ree et al., 2005), which is a model that allows the incorporation of fossil and geological information, and to co-estimate phylogeny as a stochastic process in continuous time and incorporates both vicariance and dispersal (Ree & Sanmartín, 2018; Ree & Smith, 2008; Ronquist & Sanmartín, 2011).
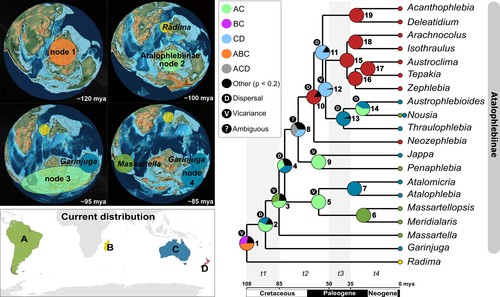
Genera occurrences were defined by presence or absence in South America (A), Madagascar (B), Australia (C), and New Zealand (D). Gondwana breakup is well documented (see Bache et al., 2014; Elsworth et al., 2017; Mueller & Jokat, 2019; Seton et al., 2012; White et al., 2013), and the probability of dispersal was assigned according to the availability of connections between areas across four time slices: (t1) 108 to 85 mya; (t2) 85 to 50 mya; (t3) 50 to 35 mya; and (t4) 35 to 0 mya. As suggested by Landis (2017), the probability of dispersal (constrained to sum to 1) was attributed to short-distances dispersal (s= 0.7); medium-distances dispersal (m = 0.2); and long-distances dispersal (l = 0.1). Thus, probability of medium is implied to exist in short, and long-distance dispersal is implied to exist between all area pairs. Therefore, short distance has value 1 (s+m+l) and medium distance has value 0.3 (m+l) (Table S4; adapted from Landis, 2017).
Time slices and dispersal probabilities considered five geological events: the separation between Madagascar/India and Antarctica associated with low probability of dispersal after separation (~85 mya, supplementary data of Landis, 2017; White et al., 2013; Gibbons et al., 2013; Sanmartín & Ronquist, 2004); Tasman Sea opening (~50 mya, Bache et al., 2014; White et al., 2013); the opening of Drake Passage and Tasman Gateway with consequent Antarctica glaciation (~35 mya, Elsworth et al., 2017; Scher et al., 2015). The analyses were run using the “BioGeoBEARS” package (Matzke, 2014) on R (R Core Team, 2020) under the RASP interface (Yu et al., 2015). The resulting phylogeny from BEAST2 was used as a guide tree (consensus tree).
3 RESULTS
Bayesian inference recovered Atalophlebiinae as monophyletic with high support (Figures 1 and S1) and revealed new phylogenetic relationships among genera in internal clades. Garinjuga Campbell & Suter, 1988, appears as sister to all other Atalophlebiinae genera, including Massartella Lestage, 1930, which, in turn, is sister to the remaining Atalophlebiinae. Two other clades contain genera from South America and Australia (Figure 1).
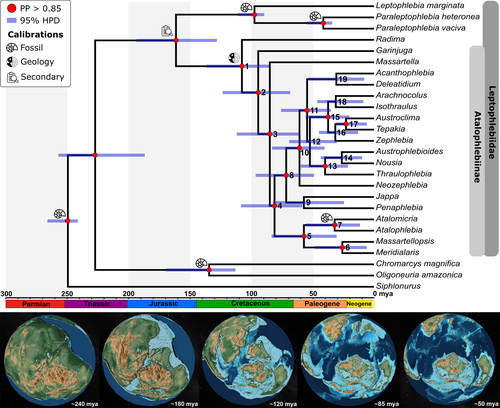
Estimated age for the origin of Atalophlebiinae was 107.83 mya [median age, 95% highest posterior density interval (95% HPD): 85.76–136.63, Figure 1, Table 1, node 1], with initial diversification at 94.52 mya (68.53–123.44, Figure 1, Table 1, node 2). Thus, both the origin and initial lineage divergence occurred in the Cretaceous, during the second phase of Gondwana breakup. The result suggests that most living genera originated within their current distribution area.
| Clade | Divergence time (mya) | DEC | |||
|---|---|---|---|---|---|
| Median | 95% HPD | Ancestral area | Probabilities | Event | |
| Root | 249.95 | 242.09–266.57 | — | — | — |
| Oligoneuriidae + Leptophlebiidae | 227.69 | 187.32–257.53 | — | — | — |
| Oligoneuriidae | 134.62 | 113.24–169.86 | — | — | — |
| (Chromarcys + Oligoneuria) | |||||
| Leptophlebiidae | 161.68 | 128.42–193.84 | — | — | — |
| Leptophlebiinae | 97.58 | 89.77–110.9 | — | — | — |
| (Leptophlebia + Paraleptophlebia) | |||||
| Paraleptophlebia | 41.36 | 34.19–54.5 | — | — | — |
| Node 1 | 107.83 | 85.76–136.63 | ABC | 0.47 | Vicariance |
| Node 2 | 94.52 | 68.53–123.44 | AC | 0.56 | Dispersal |
| Node 3 | 85.05 | 60.11–111.87 | AC | 0.45 | Vicariance |
| Node 4 | 81.21 | 58.67–108.4 | C | 0.39 | Dispersal |
| Node 5 | 57.18 | 30.64–83.5 | AC | 1 | Vicariance |
| Node 6 | 25.83 | 6.09–48.3 | A | 1 | s. w. a |
| Node 7 | 32.1 | 11.53–55.45 | C | 1 | s. w. a |
| Node 8 | 71.66 | 49.07–96.49 | CD | 0.33 | Ambiguous |
| Node 9 | 57.24 | 24.60–82.25 | AC | 1 | Vicariance |
| Node 10 | 60.79 | 40.42–83.02 | D | 0.84 | Dispersal |
| Node 11 | 54.6 | 35.45–75.74 | CD | 0.83 | Dispersal |
| Node 12 | 52.3 | 30.55–69.01 | CD | 0.96 | Vicariance |
| Node 13 | 39.9 | 21.37–59.72 | C | 0.94 | Dispersal |
| Node 14 | 26.26 | 9.83–43.26 | AC | 0.58 | Dispersal |
| Node 15 | 37.44 | 20.24–55.71 | D | 1 | s. w. a |
| Node 16 | 31.55 | 13.05–44.72 | D | 1 | s. w. a |
| Node 17 | 22.8 | 5.95–30.78 | D | 1 | s. w. a |
| Node 18 | 31.17 | 8.74–46.21 | D | 1 | s. w. a |
| Node 19 | 30.7 | 8.05–53.74 | D | 1 | s. w. a |
Inference of ancestral areas (Figure 2, Table 1) suggested that Atalophlebiinae originated in the Gondwana supercontinent after a vicariant event (node 1). Subsequently, this lineage diversified into a scenario that refers to the Gondwanic corridor formed by South America, Antarctica, and Australia (node 2). Then, vicariance again separated the lineage leading to Massartella from the remaining Atalophlebiinae at ca. 85 mya (node 3). Other Australian (Jappa Harker, 1954 and the clade Atalomicria + Atalophlebia) and South American (Penaphlebia Peters & Edmunds, 1972 and the clade Massartellopsis Demoulin, 1955 + Meridialaris Peters & Edmunds, 1972) sister lineages resulted from vicariant events at ca. 57 mya (nodes 5, 9).
4 DISCUSSION
The topology of Atalophlebiinae herein proposed contains four main lineages: Garinjuga from Australia, Massartella from South America, and two others with genera from South America and Australia region (Figures 1, 2, and S1). Garinjuga samples and new sequences addition (Table S1) helped to establish the relationship among main lineages of the group, which in previous research were unclear (see Monjardim et al., 2020). Jappa and Austrophlebioides Campbell & Suter, 1988, belong to the clade supported by node 8 (Figures 1 and 2) diverging from the results of Monjardim et al., (2020). This study did not recover any previously proposed clades within Atalophlebiinae based on morphological data (see Christidis, 2006; Finlay & Bae, 2008; Pescador & Peters, 1980). Considering that they occur in similar environments throughout their distribution, and therefore experience similar ecological filters, many species may have evolved similar characteristics independently (see Bower & Winemiller, 2019). This indicates that some morphological similarities shared between Atalophlebiinae genera may be the result of evolutionary convergence.
The results suggest that the most recent common ancestor between Atalophlebiinae and the lineage that originated the Malagasy group (here represented by Radima) lived in Gondwana in the Cretaceous period (Figures 1 and 2, Table 1, node 1). These lineages diverged allopatrically during the second phase of the supercontinent breakup (Gibbons et al., 2013; Seton et al., 2012; Thompson et al., 2019), and the vicariant event that promoted speciation is probably related to the separation between Madagascar/India from Antarctica/Australia. The breakup process between these areas began at ca. 135 mya, with the migration of the Indian Plate, culminating in the opening of the Indian Ocean at ca. 100 mya (Gibbons et al., 2013; Seton et al., 2012; Thompson et al., 2019).
The initial divergence in Atalophlebiinae occurs in a scenario that refers to the Gondwanian corridor formed by South America, Antarctica, and Australia (Reguero et al., 2014; Seton et al., 2012; Thompson et al., 2019) during the Upper Cretaceous (Figures 1 and 2, Table 1, node 2). The ancestor of the Garinjuga lineage was probably limited to the geographic area where the divergence occurred (area C), which can be explained, for example, by a peripatric speciation, while the remaining lineage (node 3) inherits the entire ancestral range and probably increases its distribution by dispersal within that range (scenario 3 in Ree et al., 2005). Subsequently, a vicariant event isolated an ancestral population (node 3) in South America that gave rise to the Massartella lineage. Changes in sea level, associated with temperature increase on the planet, culminated in several cycles of marine transgressions in Patagonia during the Upper Cretaceous and Paleocene (Haq, 2014; Le Roux, 2012a; Malumian & Nanez, 2011; Parras & Griffin, 2013), which could have prevented dispersal events between South America and the Antarctic Peninsula. In addition, the temperature and precipitation calculated for these periods indicates a climate warm and humid subtropical temperate in this region (Le Roux, 2012b; Varela et al., 2018). This fact may have induced mayfly populations to seek colder habitats in mountains, leaving the lowlands, which could also have promoted allopatric speciation. Events of the same nature could have also been responsible for isolating the lineages that originated Atalophlebia + Atalomicria and Jappa in Australia from Massartellopsis + Meridialaris and Penaphlebia in South America (node 5 and 9). Thus, marine transgressions and/or climate changes were probably strong enough to isolate Atalophlebiinae populations, as this group is adapted to cold streams and rivers and is extremely intolerant of saltwater (Dos Santos et al., 2018).
Massartella descended from the oldest split which produced one lineage presently restricted to South America. Nowadays, it has a wide and disjunct distribution on the tabletops of the Pantepui region and in the mountains of the Atlantic Forest (Domínguez et al., 2006; Pescador & Peters, 1990). Massartellopsis and Meridialaris speciated during the uplift of the Andes (Figures 1 and 2, Table 1, node 6), a region where they are currently found, but where Massartella does not occur (Derka et al., 2009; Domínguez et al., 2006; Hoorn et al., 2010).
New Zealand emerges as an important factor in the diversification of Atalophlebiinae, after events of unclear nature (Figures 1 and 2, Table 1, node 4 and 8), possibly related to extinction events (or with sample gap), during the initial period of its separation from Australia (~85 mya) until the opening of the Tasman Sea (~50 mya, see Bache et al., 2014). An ancestral population isolated in this region (node 10) was the origin of the lineage of Neozephlebia Penniket, 1961, while another lineage increased its range by dispersal to Australia (node 11). Subsequently, peripherical speciation formed the lineage (node 19) in New Zealand region, while others (node 12) inherited the entire ancestral range and increases distribution by dispersal (scenario 3 in Ree et al., 2005). Later, that lineage (node 12) was divided by a vicariant event, probably related to landscape changes during the opening of the Tasman Sea and Tasmanian Gateway at ca. 50–35 mya (see Bache et al., 2014; Scher et al., 2015). After the separation of the Gondwanic corridor (~35 mya, Elsworth et al., 2017; Scher et al., 2015; Seton et al., 2012), speciation occurred within the current distribution areas, indicating that Atalophlebiinae was unable to disperse across transoceanic barriers and suggests that most living taxa are the result of more recent local historical and ecological processes.
The biogeographic history of Atalophlebiinae is congruent with events that occurred during the second phase of the gradual process of Gondwana breakup in the Cretaceous and Paleogene. Our results provided evidence that vicariance and dispersal both played roles in the history of diversification prior to the completion of the second phase of Gondwana breakup. This pattern, together with natural extinction processes and its low dispersal capacity across transoceanic barriers, may explain its absence in other regions that form the circum-Antarctic pattern, such as tropical Africa, Madagascar, and India.
ACKNOWLEDGEMENTS
We thank Juliana Justino for providing crucial support with laboratory procedures, Joyce Prado, Roberta Paresque, Paula Souto, Marina Monjardim, and Sarah Guimarães for helping with data analyses and providing comments to the text. We especially thank Pablo Pesacq, Tomáš Derka, and Rogério Campos for assistance in specimen access. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, productivity grant #309666/2019-8 to FFS), and Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (FAPES).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.




