Transalpine dispersal: Italian barred grass snakes in southernmost Bavaria—This far but no further!
Contributing authors: Marika Asztalos ([email protected]), Frank Glaw ([email protected]), Michael Franzen ([email protected]), Carolin Kindler ([email protected])
Abstract
Based on 1031 samples of grass snakes from Central Europe, we examine the recently reported occurrence of the southern subspecies of the barred grass snake (Natrix helvetica sicula) in southern Bavaria, Germany. Using 13 microsatellite loci and mtDNA coding for the cytb gene and the partial ND4 gene plus adjacent tRNAs, we show that N. h. sicula is restricted to a few river valleys (Inn, Isar, Loisach) in southernmost Bavaria and adjacent Tyrol, Austria. At the widening of the river valleys into the pre-Alpine plains, N. h. sicula hybridizes locally with the common grass snake (Natrix natrix) in a bimodal hybrid zone. Our study provides evidence that Central Europe was colonized by Natrix helvetica over two distinct immigration routes. In addition to the previously known western route of the nominotypical subspecies, leading to the colonization of the Rhine region, N. h. sicula crossed the Alps, most likely using the Brenner Pass and/or the Reschen Pass. Our study underlines that the Alps are not an impermeable biogeographic barrier, as often assumed. North of the Alps, the combination of geographic setting (occurrence of N. h. sicula in sheltered Alpine valleys) and population-density-dependent blocking of immigrants by the resident species (N. natrix), acting in concert with intrinsic genetic factors, prevented the formation of a geographically more extended hybrid zone. Unlike N. helvetica, the two subspecies of N. natrix hybridize north of the Alps broadly, in accordance with their better genetic compatibility. Many populations of the resident Central European subspecies (Natrix natrix natrix) have been “genetically swamped” by Natrix natrix vulgaris immigrating from the Balkans. This led to the complete replacement of N. n. natrix by N. n. vulgaris in some regions, where today only the mtDNA of the nominotypical subspecies persists.
1 INTRODUCTION
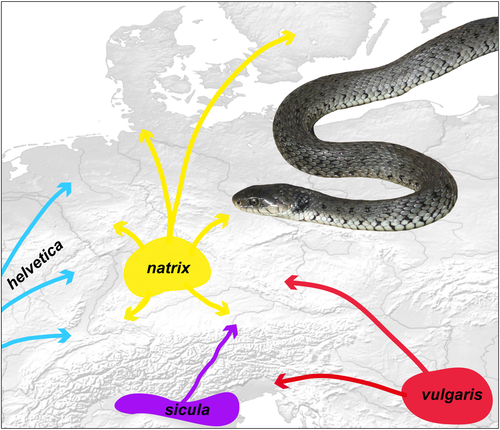
Grass snakes are widely distributed in the Palaearctic region. For decades, it was believed that these semi-aquatic snakes represent one polytypic species (Natrix natrix sensu lato), occurring from northern Africa through most of Europe to Central Asia (Kabisch, 1999; Mertens & Wermuth, 1960). Recent studies based on mitochondrial and nuclear DNA changed this picture. Due to limited gene flow across geographic contact zones, three full species are now recognized (Asztalos et al., 2020; Kindler et al., 2017; Pokrant et al., 2016; Schultze et al., 2019, 2020; Speybroeck et al., 2020). These three species were previously identified with distinct subspecies (Kabisch, 1999; Mertens & Wermuth, 1960). The red-eyed grass snake Natrix astreptophora (Seoane, 1884) occurs in the northern Maghreb region, the Iberian Peninsula, and southwestern France (Asztalos et al., 2020; Geniez, 2015; Pokrant et al., 2016). The barred grass snake Natrix helvetica (Lacepède, 1789) occurs in France, Great Britain, the Benelux countries, in the Rhine region and southern Bavaria (Germany), Switzerland, and in Italy (Geniez, 2015; Glaw et al., 2019; Kindler et al., 2017; Schultze et al., 2020). Between the two species, limited bidirectional gene flow was detected in a narrow, bimodal contact zone in southern France (Asztalos et al., 2020; Pokrant et al., 2016). The third species is the common grass snake, Natrix natrix (Linnaeus, 1758) sensu stricto, which is widely distributed from the Rhine region to Lake Baikal in Central Asia. It also occurs in Fennoscandia, northeastern Italy, in the Balkan Peninsula and Anatolia (Geniez, 2015; Kindler et al., 2017; Schultze et al., 2020). Two subspecies of N. helvetica hybridize with N. natrix. The northern subspecies Natrix helvetica helvetica (Lacepède, 1789) hybridizes with N. natrix in a narrow zone largely coinciding with the Rhine region. There, gene flow is mainly unidirectional from N. h. helvetica into N. natrix (Kindler et al., 2017; Schultze et al., 2019), and the genetic transition is paralleled by a sharp morphological transition (Thorpe, 1979). Further south another subspecies of the barred grass snake, Natrix helvetica sicula (Cuvier, 1829), hybridizes with N. natrix in northeastern Italy. The width of the Italian hybrid zone resembles that of the Rhine region, but gene flow is bidirectional (Schultze et al., 2020).
Natrix helvetica sicula is distributed across mainland Italy and Sicily. In this area, the local populations harbor several, in part deeply divergent, mitochondrial lineages with largely parapatric distribution (Schultze et al., 2020). One of them, mtDNA lineage C of Kindler et al. (2013), occurs in the Po Plain and enters from there Alpine valleys connected to the Po drainage. Barred grass snakes of lineage C are locally also known beyond the Po drainage (Valais, Switzerland; Kindler & Fritz, 2018).
The occurrence of N. h. sicula in southern Bavaria and its local contact zone with N. natrix have been discovered only recently. Although preliminary genetic evidence (mtDNA sequences corresponding to lineage C) and morphological data were published (Glaw et al., 2019), the putative hybrid zone has not yet been studied in detail. It differs from other hybrid zones because it is situated in a transition region between a high mountain chain, the Alps, and the abutting foothill region in the north. Most other contact zones between grass snake species lie in more or less open terrain, so that different genetic patterns are possible.
As far as known, except for the very south near the border to Tyrol (Austria) where Glaw et al. (2019) recorded N. h. sicula, Bavaria is inhabited exclusively by two genetic lineages of N. natrix, the “yellow” and “red lineages” of Kindler et al. (2013). These lineages have recently been identified with the subspecies Natrix natrix natrix (Linnaeus, 1758) and Natrix natrix vulgaris Laurenti, 1768, respectively (Fritz & Schmidtler, 2020). In addition, the occurrence of the nominotypical subspecies of N. helvetica seems possible in the southwest of Bavaria, near Lake Constance and in the Allgäu, and in the northwest, in the Bavarian Main river region (Lower Franconia). Thus, in southwestern Bavaria the two subspecies of N. helvetica could meet and hybridize. Previous studies have shown that each of the subspecies of N. natrix and N. helvetica corresponds to a distinct microsatellite cluster and is characterized by at least one distinct mtDNA lineage (Asztalos et al., 2020; Fritz & Schmidtler, 2020; Kindler et al., 2013, 2017; Schultze et al., 2020). Using the terminology of Kindler et al. (2013) and Schultze et al. (2020), the mtDNA lineage of N. h. helvetica is lineage E. The mtDNA lineage of N. h. sicula in the study region is lineage C. The mtDNA lineage of the nominotypical subspecies of N. natrix is lineage 3, and of N. n. vulgaris, it is lineage 4 (the “yellow” and “red lineages” of Kindler et al., 2017 and Kindler et al., 2018).
For the present study, we used extended sampling from Bavaria. Glaw et al. (2019) examined mtDNA sequences of 42 Bavarian grass snakes (new data and sequences from Kindler et al., 2013, 2017). However, mtDNA allows no direct insights into hybridization because it is inherited matrilineally. We increased the number of genetically studied samples from Bavaria to 208. To detect hybridization, we scrutinized our material using biparentally inherited nuclear genomic markers (13 microsatellite loci) in combination with mtDNA sequences (up to 1983 bp length). Beyond Bavaria, we included additional material from adjacent regions, bringing the tally of analyzed samples to 1031. Our aims were (a) to describe the distribution and hybridization of N. helvetica and N. natrix in our study region and (b) to explore the distribution and hybridization patterns of the two species and their subspecies in the northeastern Alpine region.
2 MATERIAL AND METHODS
2.1 Sampling and laboratory procedures
A total of 208 grass snakes from Bavaria, southeastern Germany, were genetically analyzed (Table S1). Data for 32 of these samples were from Kindler et al. (2013, 2017). Fourteen of our samples were already studied by Glaw et al. (2019). Eleven of these samples were resequenced and genotyped as our remaining material (162 samples), and for three samples from Glaw et al. (2019) were only previously published mtDNA sequences available because they were used up. Our samples were mainly tissues (109) and cloaca or mouth swabs (51); the remaining 13 samples were shed skins. For embedding the genetic variation within Bavaria into a broader context, data for additional 823 grass snakes from adjacent regions in Austria, the Czech Republic, Germany, northern Italy, and Switzerland were included in all calculations, among them samples from the main distribution range (northern Italy) of the mitochondrial lineage of N. h. sicula that occurs also in Bavaria (lineage C; Table S1). Except for 15 newly processed samples, these data were from previous studies (Kindler et al., 2013, 2014, 2017; Kindler & Fritz, 2018; Kindler, Graciá, et al., 2018; Schultze et al., 2019, 2020).
Two marker systems (mtDNA, microsatellite loci) were applied, which were previously used in other studies on grass snakes (Kindler et al., 2013, 2017; Kindler, de Pous, et al., 2018; Kindler & Fritz, 2018; Pokrant et al., 2016; Schultze et al., 2019, 2020). Two mtDNA fragments were sequenced, the cytochrome b (cytb) gene (1117 bp), and the partial ND4 gene plus adjacent DNA coding for tRNAs (tRNA-His, tRNA-Ser, tRNA-Leu; 866 bp). According to Kindler et al. (2013), total genomic DNA was extracted using either the DTAB method (Gustincich et al., 1991) or the innuPREP DNA Mini Kit (Analytik Jena AG). Mitochondrial DNA fragments were amplified with the primers in Table S2. When the primers of Guicking et al. (2006) did not yield PCR products, those from Kindler et al. (2013) and Schultze et al. (2020) were used to amplify shorter overlapping amplicons. For primer combinations, PCR conditions, and amplicon lengths, see Table S2. PCRs were carried out in a total volume of 25 µl containing one unit Taq polymerase (Bioron), 1× buffer as recommended by the supplier, 0.4 µM of each primer (Biomers), 0.2 mM of each dNTP (Thermo Fisher Scientific), and 10 µg BSA (Thermo Fisher Scientific). PCR products were purified with the ExoSAP-IT enzymatic cleanup (USB Europe GmbH; modified protocol: 30 min at 37°C, 15 min at 80°C) and sequenced using an ABI 3730 Genetic Analyser (Thermo Fisher Scientific) and the ABI PRISM Big Dye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific); purification of reaction setups for cycle sequencing was conducted with Sephadex (GE Healthcare). For three new samples, no mtDNA data could be obtained, and for some other samples, only one mtDNA fragment could be sequenced. For a few samples, only sequences of 475–809 bp length were obtained for ND4 + tRNAs (n = 11) and of 347–1079 bp length for cytb (n = 72), respectively (Table S1).
In addition, the samples were genotyped at 13 polymorphic microsatellite loci. Following Pokrant et al. (2016), three or four microsatellite loci were multiplexed and two loci were processed alone (Table S3). PCRs were performed using the Type-it Microsatellite PCR Kit (Qiagen). After an initial denaturation at 95°C for 5 min, 40 cycles were run with denaturation at 95°C for 30 s, followed by annealing at primer-specific temperatures (Table S3) for 90 s, extension at 72°C for 30 s, and a final extension step at 60°C for 30 min. The PCR products were diluted with water in a ratio of 1:5–1:100, depending on the multiplex reaction. Using the Gene-Scan-600 LIZ Size Standard and peak scanner 1.0 (both Thermo Fisher Scientific), fragment lengths were determined on an ABI 3730 Genetic Analyser. For one new sample, no microsatellite data could be obtained. For further information on microsatellite loci, primers, and PCR conditions for mtDNA sequences and microsatellites, see Tables S2 and S3.
2.2 Mitochondrial DNA sequences and network analyses
Cytb sequences could be generated for 110 samples, ND4 + tRNAs for 172 samples. These sequences were aligned with data from our previous studies (Kindler et al., 2013, 2014, 2017; Kindler, de Pous, et al., 2018; Kindler & Fritz, 2018; Schultze et al., 2019, 2020) using bioedit 7.0.5.2 (Hall, 1999). To identify mitochondrial haplotypes, previously characterized haplotypes (Kindler et al., 2017; Kindler, de Pous, et al., 2018; Schultze et al., 2019) were added to the alignments of each mtDNA block, resulting in a 1117-bp-long alignment of 1001 cytb sequences and an 866-bp-long alignment of 1080 ND4 + tRNAs sequences (Alignments S1 and S2). Then, parsimony networks for each mtDNA block were drawn using tcs 1.21 (Clement et al., 2000), with gaps coded as fifth character state. Using the default 95% connection limit, unconnected haplotype clusters were obtained, which is why the connection limit was arbitrarily set to 100 steps.
2.3 Microsatellite cluster analyses, inferring hybrid status, and PCAs
The microsatellites were examined for the presence of null alleles using micro-checker 2.2.3 (van Oosterhout et al., 2004), resulting in the detection of null alleles for some loci. Thus, unsupervised Bayesian cluster analyses using structure 2.3.4 (Falush et al., 2003; Pritchard et al., 2000) were adapted for the presence of null alleles. One structure analysis was performed using the admixture model and correlated allele frequencies (thereafter abbreviated as “CAF”), and another analysis was performed using the admixture model and independent allele frequencies (thereafter abbreviated as “IAF”). Since structure is known to detect only the uppermost level of population differentiation (Evanno et al., 2005), different hierarchical runs were executed: The first calculation included the whole data set. A second and a third calculation included only N. helvetica and N. natrix, respectively, but without admixed individuals (Table S1). All calculations were repeated 10 times for each K ranging from 1 to 10, using a Monte Carlo Markov chain of 1,000,000 generations and a burn-in of 250,000. The most likely number of clusters (K) was determined using the ΔK method (Evanno et al., 2005) as implemented in the software structure harvester (Earl & vonHoldt, 2012). Population structuring and individual admixture were visualized using the R package pophelper 2.2.9 (Francis, 2017).
To resolve in structure analyses whether individual genotypes represent pure taxa or admixed individuals, hybrid genotypes were modeled using hybridlab 1.0 (Nielsen et al., 2006). From the first structure run, 20 pure individuals of N. helvetica and 20 pure individuals of N. natrix were selected as parental genotypes (Table S4). Then, 20 genotypes of each hybrid class (F1, F2, two backcrosses) were modeled. To infer the threshold to distinguish pure individuals, hybrids, and backcrosses, the data of the pure parental genotypes plus the simulated hybrid data were then analyzed using structure. As a result, for pure N. helvetica, thresholds of 96% (CAF) and 97% (IAF) were determined and for pure N. natrix, 98% (both for CAF and IAF). For the hybrid threshold of the two subspecies of N. helvetica, 20 pure individuals each of N. h. helvetica and N. h. sicula were chosen as parental genotypes (Table S5). For each hybrid class (F1, F2, two backcrosses), 20 genotypes were modeled, and the simulated data were processed in structure. This resulted for N. h. helvetica in thresholds of 87% (CAF) and 93% (IAF) and for N. h. sicula, 75% (CAF) and 67% (IAF). To infer the threshold for the subspecies of N. natrix, 20 pure individuals of N. n. natrix (yellow lineage) and 20 pure individuals of N. n. vulgaris (red lineage) were selected as parental genotypes (Table S6). Then, 20 genotypes of each hybrid class (F1, F2, two backcrosses) were modeled and analyzed using structure, resulting for pure N. n. natrix in thresholds of 88% (CAF) and 92% (IAF). For pure N. n. vulgaris, thresholds of 84% (CAF) and 77% (IAF) were inferred.
structure analyses are based on population genetic presumptions (Hardy–Weinberg equilibrium, linkage equilibrium; Pritchard et al., 2000) and therefore prone to bias from uneven sample sizes (Puechmaille, 2016). To validate structure results, additional Principal Component Analyses (PCAs) were run using the R package adegenet 2.1.1 (Jombart, 2008). PCAs are independent from any population genetic presumptions and exclusively based on genetic information, making them less sensitive to different sample sizes. PCAs were run for the same data sets as used for structure analyses, and processed individual data were depicted either according to their mitochondrial or nuclear genomic identity.
3 RESULTS
3.1 Mitochondrial DNA
3.1.1 Haplotype networks
In parsimony network analyses, each mitochondrial lineage corresponded to a distinct haplotype cluster. For cytb (Figure 1, top), there were 13 haplotypes of lineage C, typical for N. h. sicula, and 46 haplotypes of lineage E, typical for N. h. helvetica. Forty-nine haplotypes of lineage 3, typical for N. n. natrix, and 70 haplotypes of lineage 4, typical for N. n. vulgaris, occurred. Haplotypes of lineages C and E differed by a minimum of 25 mutation steps. Within both lineages, the haplotypes differed by a maximum of five mutations. Lineage 4 was connected by a minimum of 61 mutation steps to lineage C and by a minimum of 53 mutation steps to lineage 3. The haplotypes of lineage 3 differed by a maximum of eight mutations, those of lineage 4 by a maximum of 14 mutations. The following haplotypes were newly identified in the present study: c13, r67, r68, r69, r70, y44, y45, y46, y47, y48, and y49 (European Nucleotide Archive accession numbers LR991913–LR991923).
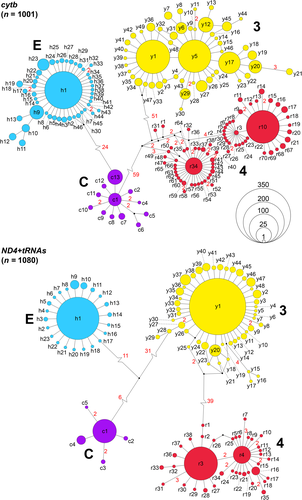
For ND4 + tRNAs (Figure 1, bottom), lineage C comprised five haplotypes, which differed by a maximum of four mutations. Lineage C was connected by a minimum of 17 mutation steps to lineage E. The latter comprised of 23 haplotypes, which differed by a maximum of two mutations. Lineages C and E were connected to lineage 3 by a minimum of 37 and 42 mutation steps, respectively. The 48 haplotypes of lineage 3 differed by a maximum of nine mutations. Lineage 4 comprised of 38 haplotypes, which differed by a maximum of 10 mutations, and was connected to lineage 3 by a minimum of 41 mutation steps. Six haplotypes were newly identified in the present study: c4, c5, r36, r37, y47, and y48 (European Nucleotide Archive accession numbers LR991924–LR991929). All currently known haplotypes and their accession numbers are listed in the Table S7.
3.1.2 Geographic distribution of mitochondrial lineages
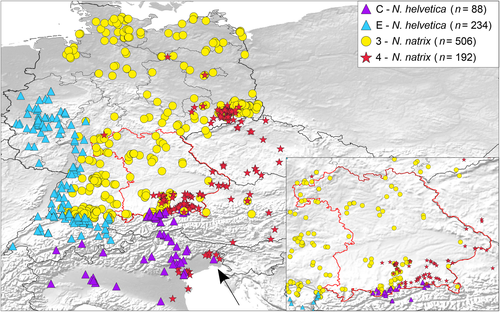
In Bavaria, mainly haplotypes of the two subspecies of N. natrix were recorded (Figure 2). While lineage 3 was distributed across whole Bavaria, lineage 4 was geographically restricted to the southeastern part of the state. In two areas in the very south of Bavaria, haplotypes of lineage C of N. helvetica and of the two lineages of N. natrix were recorded in syntopy or close proximity, sometimes separated only by 2–10 km distance. At Sachrang, haplotypes of all three lineages were found together and near Lenggries less than 3 km apart. No haplotypes of lineage E were found in Bavaria. Additional records of lineage C were found in four Austrian sites (Inn and Ziller valleys, Innsbruck, and close to the Walchsee; Table S1, ZSM DNA 427, 453, 509–514, and ZSM 55/2019).
3.2 Nuclear DNA and admixture
3.2.1 structure analyses
The 13 studied microsatellite loci were highly polymorphic, with allele numbers ranging from 11 to 29 per locus (Table S3) and a total allele number of 244, and were analyzed using structure 2.3.4. Since this software is known to reveal only the uppermost hierarchical level of genetic clustering (Evanno et al., 2005), three different data sets were processed to examine for hidden substructure. The results of the analysis using the admixture model and CAF closely resembled those using the admixture model combined with IAF. All differences between the two approaches referred to borderline cases for hybrid identity. Therefore, the figures for the IAF analysis are only presented in the Supporting Information and the figures for the CAF analysis here.
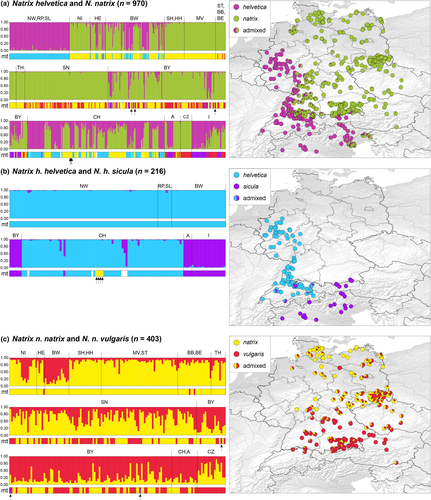
For the first structure run, the complete data set of 970 samples was included and the ΔK method identified K = 2 as the optimal number of clusters (Figure S1). One cluster corresponded to N. helvetica, and the other to N. natrix. An inspection of the structure results for K = 3 and K = 4 revealed that the additional clusters represent the individual subspecies of N. helvetica and N. natrix (Figure S2). Our analyses detected geographically restricted interspecific hybridization. In total, 352 and 311 hybrids were identified in the CAF and IAF runs, respectively (Figure 3a, Figure S3a). Most hybrids were from the previously known hybrid zones in Switzerland (Kindler et al., 2017), northern Italy (Schultze et al., 2020), and southwestern Germany (Schultze et al., 2019). However, additional hybrids were also found in Bavaria and adjacent Tyrol (Austria; Table S1). Among the samples from Bavaria, which could be genotyped using microsatellite data, 79 (CAF)/71 (IAF) were hybrids, six (CAF)/five (IAF) represented pure N. helvetica, and 118 (CAF)/127 (IAF) pure N. natrix.
The mitochondrial haplotypes of the hybrids corresponded to lineage 3 (CAF: 28 samples; IAF: 26 samples), lineage 4 (CAF: 18 samples; IAF: 12 samples), and lineage C (CAF and IAF: each 30 samples). For three samples (ZSM DNA 416, 488, ZSM 7/2020), no mtDNA could be sequenced. Five of our six hybrids from Tyrol had haplotypes of lineage C (ZSM DNA 427, 453, 511–513), and one from the border region to Germany, south of Chiemsee, had a haplotype of lineage 4 (ZSM 35/2019). In addition, four genotypically pure N. helvetica from Switzerland and three (CAF) or four (IAF) genotypically pure N. natrix from Bavaria had introgressed mitochondrial haplotypes of the other species (Table S1: DUB01-DUB04, ZSM DNA 456, 473, ZSM 262/2018, 6/2020).
As evident from the results for K = 3 and K = 4, one hybrid from the Allgäu near Immenstadt (ZSM DNA 333, mtDNA lineage 3) showed impact of N. h. helvetica (Figure S2). The same is true for six samples from the Bavarian city of Lindau at Lake Constance (MTD T 22507–22510, 22513, 22514, all mtDNA lineage 3). Another hybrid between N. h. helvetica and N. natrix was identified from the Main river in Lower Franconia (Kleinwallstadt, MTD T 20786, mtDNA lineage 3). ZSM DNA 416 (unknown mitochondrial lineage) from Burgwalden, southwest of Augsburg, had signatures for admixture with N. h. sicula (Figure S2); a second grass snake from the same site was a pure N. natrix (Table S1).
Hybridization between N. h. sicula and N. natrix occurs in both directions, as evinced by hybrids holding haplotypes of N. h. sicula (mtDNA lineage C) and N. natrix (mtDNA lineages 3 and 4; Table S1).
For the second structure run (Figure 3b, Figure S3b), all samples of pure N. natrix or with genetic impact of N. natrix were excluded to examine structuring within N. helvetica. For this data set (CAF: n = 216; IAF: n = 214), the ΔK method also revealed K = 2 as the optimal number of clusters (Figure S1). One cluster corresponded to N. h. helvetica (mitochondrial lineage E) and the other to N. h. sicula (mitochondrial lineage C). Pure individuals of the latter subspecies were, expectedly, mainly from northern Italy and southern Switzerland; some subspecies hybrids were identified from the Swiss cantons Bern, Vaud, and Zürich. As mentioned above, four introgressed snakes from Switzerland were observed (DUB01–DUB04). These were genotypically pure N. h. helvetica having mitochondrial haplotypes of N. n. natrix. The six (CAF)/five (IAF) samples from Bavaria that remained in the second run were pure N. h. sicula (ZSM DNA 368: Ferchensee; ZSM DNA 334: Isar south of Mittenwald; ZSM DNA 357: Mittenwald; ZSM DNA 330, 466: Sachrang; and only for the CAF run ZSM DNA 323: near Nußdorf am Inn; Table S1).
In the third structure analysis (Figure 3c, Figure S3c), structuring within N. natrix was examined. Samples representing pure N. helvetica or N. helvetica × N. natrix hybrids were excluded for this run. For the processed samples (n = 403, CAF; n = 445, IAF), the ΔK method revealed again K = 2 as the optimal number of clusters (Figure S1), with one cluster corresponding to N. n. natrix and the other to N. n. vulgaris. Hybridization between the two lineages was extensive and with bidirectional introgression. More or less pure representatives of the nominotypical subspecies were found mainly in the north of our study region. The genetic impact of N. n. vulgaris increased to the south. However, in southern Germany and adjacent regions, many genotypically pure N. n. vulgaris had mitochondrial haplotypes of N. n. natrix (mitonuclear discordance), while further north only nine N. n. natrix from Saxony and one from the Czech Republic had an mtDNA haplotype of N. n. vulgaris (Table S1).
3.2.2 Principal Component Analyses
The PCAs using microsatellite data supported our structure results. Snakes identified as admixed in structure analyses were also intermediate with respect to the PCA clusters. In the PCA for the whole data set, one cluster each corresponded to N. helvetica and N. natrix (Figure 4a, Figure S4a), with the above-mentioned individuals showing mitonuclear discordance being misplaced according to their nuclear genomic identity.
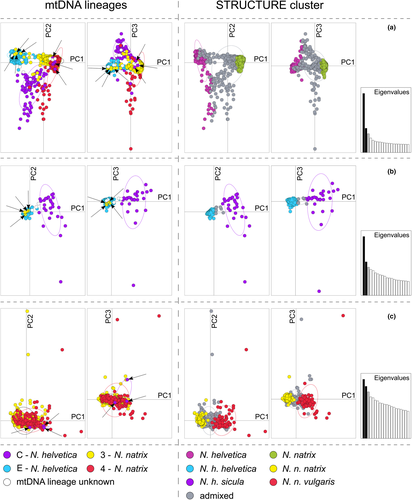
In the PCA for pure N. helvetica (Figure 4b, Figure S4b), two clusters representing N. h. helvetica and N. h. sicula occurred, with only a few admixed snakes.
The PCA for pure N. natrix (Figure 4c, Figure S4c) showed weak differentiation, reflecting massive introgression between the two subspecies in our study region.
4 DISCUSSION
Using mtDNA sequences and morphological comparisons, Glaw et al. (2019) recorded for the first time native N. h. sicula in Germany. These authors suggested that this taxon occurs in southernmost Bavaria in the regions of the Loisach and the upper Isar rivers and along the Inn river close to the Austrian border. Based on mitochondrial haplotypes and color pattern, Glaw et al. (2019) inferred that N. h. sicula occurs there in sympatry with N. natrix. Our investigation using nuclear genomic markers (microsatellite loci) in combination with mtDNA sequences confirms the presence of N. h. sicula in southernmost Bavaria. However, in contrast to Glaw et al. (2019), our marker systems allow the identification of hybrids. Accordingly, some records identified by Glaw et al. (2019) as N. h. sicula were unraveled as hybrids.
In Lower Franconia and near Lake Constance (Allgäu, Lindau), we found signatures for admixture between the nominotypical subspecies of N. helvetica and N. natrix. This is not unexpected because these Bavarian regions border the well-known hybrid zone in the Rhine region (Kindler et al., 2017; Schultze et al., 2019; Thorpe, 1979). All hybrids had relatively little genotypic impact from N. h. helvetica, suggesting that they represent the easternmost outliers of the hybrid zone.
Among our 208 grass snake samples from Bavaria, 90 were from the putative range of N. h. sicula in Bavaria. However, only five (IAF) or six (CAF) of them were genotypically pure N. h. sicula. Significantly more samples (n = 58, CAF; n = 54, IAF), among them material identified by Glaw et al. (2019) as N. h. sicula, were hybrids with N. natrix, and the remaining samples from Bavaria represented N. natrix, among them also many from southern Bavaria (n = 26, CAF; n = 21, IAF). Three or four genotypically pure N. natrix (ZSM DNA 456, 473, ZSM 262/2018, 6/2020) show mitonuclear discordance and hold an mtDNA haplotype of lineage C (for the samples ZMH R09037, ZSM DNA 320, 325, 329, and 365 no microsatellite data were available). Thus, our genetic data confirm that the two grass snake species live in southern Bavaria in sympatry, and in the same area many hybrids occur.
With a few exceptions, N. h. sicula and its hybrids are restricted to a narrow strip of less than 20 km width north of the border to Tyrol (Austria), roughly matching the region between Rosenheim and the Alps (Figure 5). We studied from the Austrian side nine samples of mitochondrial lineage C, and four of them were pure N. h. sicula and five had only slight signatures for introgression from N. natrix (Table S1). One exception refers to a sample (ZSM DNA 416) from Burgwalden, approximately 5 km southwest of Augsburg (Bavaria) in the catchment area of the Wertach river. This sample shows weak genotypic signatures of N. h. sicula. Its collection site lies approximately 90 km north of other records of N. h. sicula or its hybrids. Another grass snake from Burgwalden was a pure N. natrix (ZSM 37/2019). Yet, a photo record from Schwabstadl (approx. 15 km south of Burgwalden) resembles a barred grass snake (photo available from https://nwv-schwaben.de/naturfotografie), suggesting that Alpine grass snakes are washed from time to time by floods into this region. Further records with weak signatures of N. h. sicula are from the Munich region and the Salzach river (Figure 5; Table S1). Thus, we cannot fully exclude that the hybrid zone extends along some river courses further north. On the other hand, it is also possible that human-mediated long-distance dispersal is responsible. Cases of incidentally or intentionally translocated grass snakes have also been reported from other regions (Arnold, 2019; Böhme & Grell, 2013; Dubey et al., 2017; Kindler et al., 2017; van Riemsdijk et al., 2020; Schultze et al., 2020), and admixture with native local grass snakes was described for some sites (Asztalos et al., 2021; Dubey et al., 2017; Kindler et al., 2017).
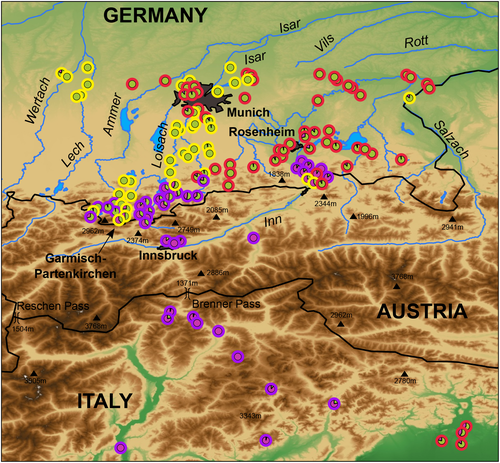
With respect to the distribution of the two subspecies of N. natrix, our results corroborate the idea that the nominotypical subspecies (the “yellow lineage” of Kindler et al., 2017) was already distributed in Central Europe when N. n. vulgaris (the “red lineage” of Kindler et al., 2017) immigrated to this region (Figure 6). Approximate Bayesian Computation results presented by Kindler, Graciá, et al. (2018) make it likely that N. n. vulgaris reached Central Europe only about 3300 years ago, while N. n. natrix survived the Last Glacial Maximum (LGM) there. According to species distribution models for the mid-Holocene and the LGM presented by Asztalos et al. (2020), N. h. helvetica also reached the middle and upper Rhine region only after the mid-Holocene (<6000 years ago), so that both the hybrid zones between N. h. helvetica and N. natrix in the Rhine region and between the two subspecies of N. natrix in Central and southeastern Europe were established only a few thousands of years ago. The differences in the extent of hybridization and gene flow as well as the different widths of the contact zones (N. h. helvetica × N. natrix: less than 50 km; N. n. natrix × N. n. vulgaris: approx. 680 km, both based on microsatellite data; Kindler et al., 2017) mirror most likely different degrees of genetic incompatibility or, in other words, different stages in the speciation process. Schultze et al. (2020) described for N. h. sicula and N. natrix a similarly narrow hybrid zone in northeastern Italy that differed, however, from that in the Rhine region in that in the Italian hybrid zone gene flow is bidirectional, while in the Rhine region gene flow is largely unidirectional from N. h. helvetica into N. natrix. Our data support that hybridization in southern Bavaria is also bidirectional (Figure 3; Table S1). Based on microsatellite data, the distribution of N. h. sicula and its hybrids extends only for less than 20 km into Bavaria, suggesting that the hybrid zone is similarly narrow like those in the Rhine region and northeastern Italy.
Currently, the distribution of N. h. sicula in the Alps is only incompletely known. It is likely that it survived the last glacial south of the Alps, in the Po Plain (Kindler et al., 2013; Kindler & Fritz, 2018; Schultze et al., 2020), and expanded its range northwards across the Alps with the Holocene warming (Glaw et al., 2019). Kindler and Fritz (2018) reported the occurrence of N. h. sicula (mtDNA lineage C) beyond the Alpine main divide in Switzerland, north of the Simplon Pass (2005 m a.s.l.), suggesting that N. h. sicula has a much wider Alpine distribution than currently known. This is supported by on-going research in Switzerland (S. Ursenbacher, pers. comm.), and we expect that unrecognized populations exist also in western Austria, especially in Tyrol. When the distribution will be better known, additional inferences about the timing of the Holocene dispersal will become possible using species distribution modeling or other approaches.
It seems likely that N. h. sicula dispersed in our study area northwards across the Alpine main divide by using the relatively low Brenner Pass (approx. 1370 m a.s.l.) and/or the Reschen Pass (approx. 1500 m a.s.l.) to reach the Inn valley of Austria and southernmost Bavaria. From the Inn valley, N. h. sicula also dispersed northward into the Alpine valleys of the Isar and Loisach rivers (Glaw et al., 2019). These valleys are narrow and surrounded by relatively high mountains (Figure 5). This geographic setting prevents in the valleys hybridization with N. natrix and thus supports N. h. sicula to maintain its genetic and morphological identity. However, to the north, when the valleys open into the pre-Alpine plains, the distribution of N. h. sicula terminates abruptly. We speculate that the pre-Alpine plains were already colonized by N. natrix before N. h. sicula extended its range northwards. Direct competition, “high-density blocking,” as defined by Waters et al. (2013), and hybridization with surrounding N. natrix prevented that a wider distribution could be established. However, the Bavarian N. h. sicula population along the Inn river could be an exception. It might have extended its range in close proximity to the river several kilometers northwards into the open plain of the Rosenheim basin, probably supported by a continuous influx of N. h. sicula from the Alpine Inn valley.
This scenario suggests that the northern Alpine hybrid zone of N. h. sicula and N. natrix has some peculiarities compared to other hybrid zones of the same species. Unlike in the Rhine region or northeastern Italy, one taxon (N. h. sicula) is effectively isolated by its occurrence in closed Alpine valleys, and contact to N. natrix can only be established where the valleys widen into the pre-Alpine plains. Populations inside the isolated Alpine valleys are protected against hybridization because the local individual densities prevent substantial immigration of N. natrix via the valley mouths. On the other hand, high-density blocking through local N. natrix populations in the pre-Alpine plains, acting in concert with intrinsic genetic factors like in the other hybrid zones of N. helvetica and N. natrix (Kindler et al., 2017; Schultze et al., 2019, 2020), counteracts the spread of N. h. sicula beyond the valleys.
With respect to N. natrix, it is remarkable that the vast majority of the Bavarian, and in general the German, populations hold mtDNA haplotypes of the “yellow lineage” of Kindler et al. (2017), corresponding to the nominotypical subspecies (Figure 1). However, their nuclear genomic identity is often admixed or, especially in southern Germany, the grass snakes represent pure N. n. vulgaris showing mitonuclear discordance (Figure 3). It is well-known that during range expansions the invader often replaces genotypically the local taxon when no strong reproductive barriers exist, but that the mitochondrial genomes of the original taxon persist (Currat et al., 2008). We suggest that the observed pattern in grass snakes reflects exactly this pattern: The resident subspecies N. n. natrix that survived the LGM in a Central European refuge (Kindler, Graciá, et al., 2018) was “genetically overrun” by the immigrating N. n. vulgaris, expanding its range from a Balkan refugium northwestward, probably using the Danube valley as a corridor to enter Bavaria and the Elbe valley to enter Saxony. To the north, the decreasing genetic impact of N. n. vulgaris reflects this on-going genetic expansion, which seems to be only possible because the two subspecies of N. natrix are genetically fully compatible. This is unlike the situation when N. helvetica is involved, where a reduced genetic compatibility seems to cause selection against hybrids, leading to the establishment of narrow hybrid zones.
5 CONCLUSIONS
Natrix helvetica colonized Central Europe via two distinct Holocene dispersal routes, one coming from the southwest and reaching the Rhine region (Kindler et al., 2017). The other dispersal route reached southernmost Bavaria and Tyrol and is a range extension from the south across the Alps (Glaw et al., 2019; this study). In the Rhine region, a narrow, but longitudinally extended, bimodal hybrid zone of N. h. helvetica and N. natrix was established (Kindler et al., 2017; Kindler, Graciá, et al., 2018; Schultze et al., 2019; Thorpe, 1979). This hybrid zone is approximately 800 km long (Thorpe, 1979) but only approximately 50 km wide (Kindler et al., 2017; Schultze et al., 2019). In Bavaria and Tyrol, the southern subspecies N. h. sicula hybridizes locally with N. natrix at the widening of a few Alpine valleys into the pre-Alpine plains, forming another bimodal hybrid zone where the parental taxa co-occur with hybrid snakes. The formation of a geographically more extended hybrid zone is prevented by the combination of geographic setting (occurrence of N. h. sicula in sheltered Alpine valleys; N. natrix in the pre-Alpine plains) and population-density-dependent blocking of immigrants of the respective other species.
Our study provides evidence that the Alps are not an impermeable biogeographic barrier as often assumed (Schmitt, 2007), even though there are only a few other lowland vertebrates known that dispersed across this mountain chain (Erinaceus europaeus, Hewitt, 2000; Bufotes viridis, Podarcis muralis maculiventris, Schmidtler, 2019). In the face of grass snake records in elevations of up to 2300 m a.s.l. in the French Alps, Piedmont (Italy) and East Tyrol (Austria; Kabisch, 1999), the transalpine dispersal of N. h. sicula is not entirely unexpected.
In contrast to N. h. sicula and N. natrix, the two subspecies of N. natrix established broad admixture across the pre-Alpine plains and further north, matching their better genetic compatibility. The nominotypical subspecies of N. natrix as a glacial northern survivor has genetically been massively introgressed by N. n. vulgaris, expanding its range from a Balkan refugium into Central Europe (Kindler, Graciá, et al., 2018). Our present study shows that this process led to a “genetic swamping” of many resident populations of N. n. natrix in southern Germany (mainly Baden-Württemberg and Bavaria). These populations have been genetically absorbed by immigrating N. n. vulgaris, and only the mtDNA of the nominotypical subspecies persists.
ACKNOWLEDGEMENTS
Many thanks go to Simon Anthofer, Otto Aßmann, Diethard Baron, Sigrid Baurmann, Jens Bohn, Bobby and Laura Bok, Alois and Susanne Breckl-Stock, Peter Dürr, Niklas and Ursula Franzen, M. and P. Frey, Jochen Fünfstück, Florian Glaser, Kathrin and Timon Glaw, Hans-Jürgen Gruber, Günter Hansbauer, Inga Hansen, Ullrich Heckes, Klaus Hertwig-Rädlein, Alfred Karle-Fendt, Jacqueline Kuhn, Heiko Liebel, Thomas Lindner, Tessy Lödermann, Adrian Neumann, Isabella Oberhammer, Alexander Pieh, Amadeus Plewnia, David Prötzel, Ursula Sauter-Heiler, Karl-Heinz Schaile, Steffen Scharrer, Manfred Schinagl, M. Schmolz, Carmen Schramm, Simon Schuster, Margarete Siering, Sebastian Swoboda, Johannes Voith, Andreas Zahn, and Jochen Zauner for donating grass snake samples, help during fieldwork or other support. This study was conducted in the Senckenberg Dresden Molecular Laboratory (SGN-SNSD-Mol-Lab). Fieldwork in Bavaria was financially supported by the PSD Bank München eG and SEA LIFE München. Open Access funding was enabled and organized by ProjektDEAL.




