Ancient DNA from an extinct Mediterranean micromammal—Hypnomys morpheus (Rodentia: Gliridae)—Provides insight into the biogeographic history of insular dormice
Abstract
The dormice (Gliridae) are a family of rodents represented by relatively few extant species, though the family was much more species-rich during the Early Miocene. Intergeneric phylogenetic relationships among glirids in some cases remain unresolved, despite extensive molecular and morphological analyses. Uncertainty is greatest with respect to the relationships among fossil taxa and how extinct lineages are related to modern species. The fossil genus Hypnomys from the Balearic Islands (western Mediterranean Sea) includes the Late Pleistocene–Holocene species Hypnomys morpheus, which has variously been considered a close relative or subgenus of the extant Eliomys. In the present study, we sequenced ancient mitochondrial DNA from H. morpheus, which suggests a sister relationship with the extant members of Eliomys. In addition, the pairwise sequence variation between Hypnomys and Eliomys is higher than that observed between congeneric glirid species (e.g., many Graphiurus spp.), which allows us to reject the hypothesis that Hypnomys is a subgenus of Eliomys. Our molecular dating analyses suggest that Hypnomys and Eliomys diverged 13.67 million years ago (95% highest posterior density [HPD] = 7.39–20.07). The relatively early split between these genera together with the molar morphology of early representatives of Hypnomys points to a Middle-Late Miocene origin from a continental glirid with a complex molar pattern, such as Vasseuromys or a closely related genus.
1 INTRODUCTION
The dormice (Gliridae) are a family of rodents whose interspecific phylogenetic relationships have been widely discussed. In some cases, these remain unresolved despite analyses using extensive molecular datasets (e.g., Bentz & Montgelard, 1999; Montgelard, Matthee, & Robinson, 2003; Nunome, Yasuda, Sato, Vogel, & Suzuki, 2007) or morphological characters (e.g., Freudenthal & Martín-Suárez, 2013; Storch, 1995; Wahlert, Sawitzke, & Holden, 1993). Within extant Gliridae, Holden (2005) recognizes three subfamilies: Graphiurinae (single genus Graphiurus Smuts, 1832, including three subgenera and 15 species), Glirinae (monotypic genera Glis Brisson, 1762 and Glirulus Thomas, 1906), and Leithiinae (including Chaetocauda Wang, 1985, Dryomys Thomas, 1906, Eliomys Wagner, 1840, Muscardinus Kaup, 1829, Myomimus Ognev, 1924, and Selevinia Belosludov and Bashanov, 1939, with a total of 12 species). A number of glirids have been recorded in the Pliocene–Holocene fossil record of the Mediterranean Islands (van der Geer, Lyras, de Vos, & Dermitzakis, 2010) several of which have been assigned to the subfamily Leithiinae based on morphological characters, including the genus Hypnomys Bate, 1918 from the Balearic Islands. However, the phylogenetic relationships of these extinct dormice have not been tested using molecular data, which may provide new evidence to clarify uncertainties about their taxonomy and biogeographical origin.
The extinct genus Hypnomys (Rodentia: Gliridae) was originally erected by Bate (1918) to accommodate two species of Pleistocene dormouse discovered in the Balearic Islands: H. mahonensis Bate, 1918 and H. morpheus Bate, 1918. de Bruijn (1966) initially described an additional species—H. gollcheri—from the Pleistocene of Malta, though H. gollcheri was ultimately transferred to the newly erected genus Maltamys Zammit-Maempel & de Bruijn, 1982 (see Zammit-Maempel & de Bruijn, 1982). Similarly, while Esu and Kotsakis (1980) recorded putative Hypnomys remains in the Early Pleistocene deposit of Nuraghe Su Casteddu (Sardinia), this material was later included in Tyrrhenoglis Engesser, 1976, an endemic genus from Sardinia (Zammit-Maempel & de Bruijn, 1982). Alcover and Agustí (1985) mentioned remains of a species of Gliridae from Cova de ca na Reia on Eivissa (Pityusic Islands, western Group of the Balearic Islands; presumably from the Lower Pleistocene/Upper Pliocene) that has often been considered to belong to Hypnomys, but this material has never been properly studied.
According to Wahlert et al. (1993), members of the subfamily Leithiinae share four morphological characters: posterior emargination or a foramen in the posterior part of the squamosal bone, fenestra in the angle of the mandible, low inclination of the coronoid process relative to the occlusal surface, and one complete transverse valley in the second lower molar. Hypnomys possess all of these diagnostic morphological characters (Figure S1), though the fenestra in the angle of mandible is not strictly present in all Hypnomys—or other Leithiinae such as Eliomys—but in most of them (see Yuste & Calzada, 2009, and pers. obs.). More specifically, a close relationship with Eliomys was suggested in the original description of Hypnomys (Bate, 1918), on the basis of the general plan of the skull, mandible, and limb bones, and a fenestra in the angle of the mandible. Subsequent studies also suggested that the closest relative of Hypnomys was Eliomys (Petronio, 1970), Leithia Lydekker, 1895 (Mills, 1976), or Tyrrhenoglis (Chaline & Mein, 1979). Several authors have since suggested that the Western Mediterranean insular fossil glirids—Hypnomys, Leithia, Tyrrhenoglis, Maltamys, and Eivissia Alcover & Agustí, 1985—all descended from Eliomys (Alcover & Agustí, 1985; Alcover, Moyà-Solà, & Pons-Moyà, 1981; Daams & de Bruijn, 1995; Zammit-Maempel & de Bruijn, 1982). Indeed, Agustí (1980) suggested that Eliomys should be considered the most likely ancestor of Hypnomys, and Zammit-Maempel and de Bruijn (1982) considered Hypnomys (and other insular genera as Tyrrhenoglis and Maltamys) as a subgenus of Eliomys, which is a view that has been widely adopted in the literature (e.g., Alcover & Agustí, 1985; Reumer, 1982, 1994). However, no consensus exists regarding the taxonomy of these fossil glirid taxa.
Though it has never been directly tested, it is generally assumed that the ancestor of Hypnomys likely dispersed to the Balearic Islands while they were connected by land to the European mainland during the Late Miocene Messinian Salinity Crisis (MSC) (e.g., Agustí, 1980, 1986; Alcover et al., 1981; Bover et al., 2014; Mas et al., 2018; Moyà-Solà & Pons-Moyà, 1980). The fossil record in Mallorca is consistent with this biogeographical hypothesis, with extensive evidence that a radiation of this endemic clade had occurred by the Pliocene: Hypnomys/Eliomys sp. [Early Pliocene (Bover et al., 2014)], Hypnomys sp. [Zanclean (Bover et al., 2014)], H. waldreni [Piazencian (Reumer, 1979)], H. onicensis [formerly H. intermedius, Early Pleistocene (Reumer, 1981, 1994)], and H. morpheus [Middle Pleistocene-Holocene Bate, 1918)]. Establishing the age of the divergence between this Mallorcan dormouse lineage and its nearest living continental relatives may help in narrowing down its phylogenetic origins by constraining the range of fossil taxa from which it could possibly have descended.
Ancient DNA sequences have been successfully used for phylogenetic analyses of small extinct species (see review in Woods, Marr, Brace, & Barnes, 2017) and can provide information about phylogenetic relationships in situations that are challenging for morphological analyses (e.g., fossil insular species where taxonomic position is frequently obscured by autapomorphies acquired during isolation). Although a close relationship between Hypnomys and Eliomys based on morphology has been widely accepted, major discrepancies in the attribution of several genera to different Gliridae subfamilies using morphological (e.g., Daams & de Bruijn, 1995; Freudenthal & Martín-Suárez, 2013; Storch, 1995; Wahlert et al., 1993) or molecular characters (e.g., Bentz & Montgelard, 1999; Fabre, Hautier, Dimitrov, & Douzery, 2012; Montgelard et al., 2003; Nunome et al., 2007) suggest that the taxonomic position of Hypnomys requires confirmation using genetic data. In this paper, we generate the first DNA sequences for Hypnomys and use these to infer its phylogenetic relationship to extant glirids. We also conduct molecular dating analysis to test hypotheses about the biogeographic history of Hypnomys—specifically that the temporal origin of the Hypnomys lineage coincides with the Messinian Salinity Crisis—and to identify potential ancestral taxa in the fossil record.
2 MATERIALS AND METHODS
2.1 Samples
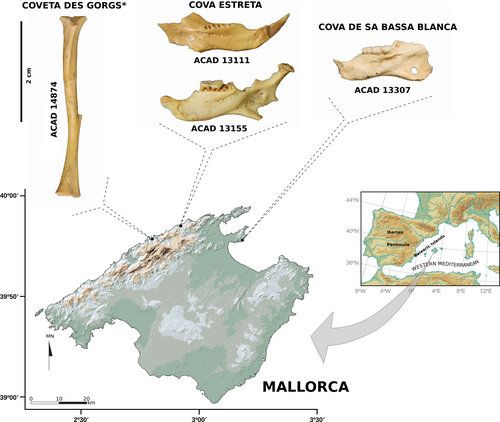
In this study, we attempted to extract DNA from four Hypnomys morpheus samples curated at the vertebrate public collection of the Mediterranean Institute for Advanced Studies (IMEDEA, Balearic Islands, Spain) and from three different caves (Figure 1): a pool of two bones [left (ACAD 13111) and right (ACAD 13155) mandibles from Cova Estreta (Pollença) (Encinas & Alcover, 1997)], a right mandible (ACAD 13307) from Cova de sa Bassa Blanca (Alcúdia) (Ginés & Ginés, 1974), and a left tibia (ACAD 14874) from Coveta des Gorgs (Escorca). The exact chronology of these samples could not be established as the specimens were entirely consumed during DNA extraction and could not be radiocarbon dated. Nevertheless, up to three radiocarbon dates have been obtained from remains obtained in the same stratigraphic level of Cova Estreta: H. morpheus bone [UtC-5175, 6,357 ± 44 BP, 5,469–5,288 (86.3%) 5,272–5,227 (9.1%) calBC] (Encinas & Alcover, 1997), and a bone [UtC-5171, 5,720 ± 60 BP, 4,716–4,449 calBC] (Encinas & Alcover, 1997) and coprolite [Wk-33010, 4,950 ± 38 BP, 3,798–3,650 calBC] (Rivera et al., 2014) of the extinct bovid Myotragus balearicus Bate, 1909. Radiocarbon dates of several bones of M. balearicus from the same level (surface) of the Coveta des Gorgs indicate a chronology range from 4,456 ± 33 BP [RICH-21771, 3339–3205 (45.2%) 3,197–3,014 (50.2%) calBC] to 9,164 ± 42 BP [RICH-21974, 8,533–8,516 (2.5%) 8,480–8,285 (92.9%) calBC] (Bover & Alcover, 2003; Bover et al., 2016, 2018; Lalueza-Fox, Shapiro, Bover, Alcover, & Bertranpetit, 2002). No chronology was available for the sample from Cova de sa Bassa Blanca. Although several rodents are currently living in Mallorca, differences in size and anatomy between them and Hypnomys are distinctive enough to clearly discriminate genera in terms of dental, skull, and postcranial morphology (Agustí, 1980; Bover, Alcover, Michaux, Hautier, & Hutterer, 2010; Mills, 1976; Reumer, 1979, 1981, 1982). Up to six rodents currently live on the island as a result of historical introductions (e.g., Alcover, 2010; Bover & Alcover, 2008), including the glirid Eliomys quercinus (Linnaeus, 1766), and murids Mus musculus Linnaeus, 1758, Mus spretus Lataste, 1883, Apodemus sylvaticus Linnaeus, 1758, Rattus rattus Linnaeus, 1758, and Rattus norvegicus Berkenhout, 1769. The first human settlers to the island (around 4,300 years ago, Bover et al., 2016) introduced E. quercinus and A. sylvaticus, which were putatively involved in the extinction of the only pre-human rodent H. morpheus (Bover & Alcover, 2008). The only H. morpheus sample that yielded endogenous DNA, tibia ACAD 14874 (see below, sections 2.2 and 3), displays enough diagnostic traits to identify it as unquestionably belonging to the fossil species: The cross-section of the distal half of the diaphysis and the extent of the tibia-ulna synostosis allow the discrimination of Gliridae from Muridae tibiae. In addition, the position and relative size of the trochlear process of the calcaneum and thus its corresponding structure in the tibia are as in other glirids and not expanded distally as in murids (Stains, 1959), and the lateral groove of the tibia resembles that of Eliomys, not weakly developed as in murids (Mills, 1976). However, differences in size between the Mallorcan E. quercinus and H. morpheus allow each to be discriminated from the other (Bover et al., 2010). The tibiae of H. morpheus have been illustrated by Alcover and Roca (1975), Alcover et al. (1981) and Bover et al. (2010).
2.2 Extraction, library preparation, enrichment, and sequencing
Sample processing, DNA extraction, PCR preparation, and library construction were performed at the facilities of the Australian Centre for Ancient DNA (ACAD) at the University of Adelaide (Australia). The samples were cleaned with surgical blades to remove surface contamination and dirt, irradiated with UV for 30 min on each side, wiped with 3% sodium hypochlorite, soaked for 2 min in 80% ethanol to fully remove sodium hypochlorite, air-dried, and finally irradiated again for 15 min on each side. Each sample was placed in a sterilized stainless steel container with an 8-mm tungsten ball and powdered using a Braun Mikrodismembrator U (B. Braun Biotech International, Berlin, Germany) for 5 s at 3,000 rpm. We obtained 190 mg for the pool of ACAD 13111 and 13155, 150 mg of bone powder for sample ACAD 13307, and 180 mg for sample ACAD 14874. The bone powder for each sample was decalcified and digested overnight at 55°C on a rotary wheel in 4 ml 0.5 M EDTA (pH 8.0) (Life Technologies, Carlsbad, CA, USA), 200 µl of 10% SDS (Life Technologies), and 40 µl of 20 mg/ml Proteinase K (Life Technologies). DNA extraction was performed using a modified QG buffer [15.5 ml QG buffer (Qiagen, Valencia, CA, USA), 1.3% Triton X-100 (Sigma-Aldrich, Saint Louis, MO, USA), 25 mM NaCl (Sigma-Aldrich), and 0.17 M sodium acetate (Sigma-Aldrich)] and suspended in 100 µl of silicon dioxide solution (Brotherton et al., 2013). Samples were then purified using 80% ethanol, and bound DNA was eluted in 200 µl TLE buffer (10 mM Tris, 0.1 mM EDTA, pH 8). A negative control was included for all extractions. No other glirids have ever been processed in the ancient DNA laboratory at ACAD before.
A PCR was performed to screen for the presence of DNA using primer pairs Mamm_12S_E and Mammal_12S_H (Macqueen, Seddon, Austin, Hamilton, & Goldizen, 2010) to amplify a ~95 bp fragment of mitochondrial 12S ribosomal RNA gene (12S). Two microliters of template was used in the PCR (final volume 25 μl), which contained: 1 × Platinum Taq High Fidelity Buffer (Invitrogen, Carlsbad, CA, USA), 3 mM MgSO4, 0.4 μM each primer, 0.25 mM each dNTP, 1.25 U Platinum Taq HiFi (Invitrogen), 2 mg/ml rabbit serum albumin (RSA, Sigma-Aldrich). PCR cycling conditions were as follows: initial denaturation at 94°C for 2 min; 50 cycles of denaturation at 94 ºC for 20 s (s), primer annealing at 55°C for 15 s, elongation at 68°C for 30 s; and a final elongation step at 68°C for 10 min. PCR products were visualized under UV light on a 3.5% agarose gel stained with Gel-Red (Jomar Bioscience, Kensington, Australia). PCR products were purified using Agencourt AMPure XP magnetic beads (Beckman Coulter, Brea, CA, USA) according to the manufacturer's protocol. Both strands were sequenced using the BigDye 3.1 Terminator Kit (Applied Biosystems, Foster City, CA, USA). Dye terminators were removed using the Agencourt CleanSEQ magnetic particle solution (Beckman Coulter), and DNA sequencing was performed on 3130xl and 3730xl Genetic Analyzers (Applied Biosystems).
Of the three H. morpheus samples analyzed, only ACAD 14874 (Coveta des Gorgs) yielded a positive PCR amplification. Sanger sequencing of the purified amplicon from ACAD 14874 produced a 95-bp fragment after primer trimming. The first BLASTn (Altschul et al., 1997) hit of this sequence against the NCBI nucleotide database (accessed January 10, 2019) was the garden dormouse Eliomys quercinus (coverage 98%, identity 95%, E-value 6e-32), whereas a second hit was the Asian garden dormouse Eliomys melanurus (Wagner, 1840) (coverage 100%, identity 93%, E-value 4e-29).
We constructed a double-stranded DNA sequencing library from ACAD 14874 following the protocol described by Meyer and Kircher (2010) using modifications as in Llamas et al. (2016), which uses truncated Illumina adapters with a P5 5-mer barcode and Platinum Taq HiFi (Invitrogen) for post-Bst amplification. Enrichment of this library for mtDNA was performed using the protocol described by Mitchell et al. (2016). We performed two parallel enrichment reactions of the DNA library. Following amplification with full-length Illumina sequencing adapters, the molecules retained after enrichment were sequenced on an Illumina HiSeq (Fast Run 2x100 PE), an Illumina MiSeq (2x150 PE), and a NextSeq (2x75 PE) runs.
2.3 Sequencing data processing and sequence assembly
Resulting reads were demultiplexed according to P5 barcode sequences using Sabre v.1.0 (https://github.com/najoshi/sabre) allowing one mismatch (option -m 1), adapter sequences were removed using AdapterRemoval v.2.1.7 (Schubert, Lindgreen, & Orlando, 2016), and paired reads were collapsed in a single read when overlapping by 11 nucleotides. A concatenated file of 2,136,646 collapsed reads from the three sequencing runs was used to iteratively map to different references. To date (April 2019), the only available complete mitochondrial genomes from glirids available in GenBank are from Glis glis (Linnaeus, 1766) and Graphiurus kelleni (Reuvens, 1890), and in general, mitochondrial sequences for the Gliridae are scarce, with cytochrome b (CYTB) and 12S genes as the most represented genes across the family. For this reason, we mapped our sequencing reads to the putatively closest relative of Hypnomys, Glis glis (complete mitochondrial genome, GenBank accession number NC_001892), and the longest available CYTB and 12S sequences for genus Eliomys (E. quercinus: CYTB accession number GQ453668, 12S accession number Y16896; E. melanurus: CYTB assembly of sequences HE614010 and KF422705, 12S accession number AJ536350; see Table 1 for details on references and mapping results) using BWA v.0.7.17 (Li & Durbin, 2009) with the recommended parameters for ancient DNA (aln -l 1,024, -n 0.01, -o 2). Reads with a mapping quality Phred score above 25 were filtered using SAMtools v.1.8 (Li et al., 2009), and duplicates removed using FilterUniqueSAMCons.py (Kircher, 2012). Mapping results were visualized using Geneious v.11.1.4 (Biomatters, http://www.geneious.com, Kearse et al., 2012), with 75% majority intermediate consensus sequences generated in Geneious using the reference to call nucleotides in positions with coverage read-depth < 3. This consensus was then used as reference for a new round of mapping. The process was iterated until no more unique reads were mapped to the reference. We generated final 75% majority consensus sequences with nucleotides only called in positions with coverage read-depth ≥ 3x. Nucleotide misincorporation and DNA fragmentation patterns were assessed using mapDamage v.2.0.2 (Jónsson, Ginolhac, Schubert, Johnson, & Orlando, 2013).
| Reference data | Mapping results | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | Sequence | GenBank # | Length (bp) | Iterations | Unique reads | Coverage (%) | Coverage depth (x) | Mean Fragment length (bp) |
| Glis glis | Mitogenome | NC_001892 | 16,602 | 18 | 1,066 | 23.5 | 4.8 | 74.3 |
| Eliomys quercinus | CYTB | GQ453668 | 1,140 | 14 | 143 | 33.3 | 9.2 | 73.0 |
| Eliomys quercinus | 12S | Y16896 | 963 | 5 | 314 | 99.7 | 24.2 | 74.0 |
| Eliomys melanurus | CYTB | HE614010 + KF422705 | 1,003 | 13 | 197 | 61.8 | 15.0 | 76.7 |
| Eliomys melanurus | 12S | AJ536350 | 967 | 5 | 279 | 94.5 | 21.3 | 73.7 |
We recovered up to 3,501 bp of the Hypnomys morpheus mitochondrial genome after 18 mapping iterations to Glis glis mitogenome (Table 1), including nine complete transfer RNA genes [Cysteine (Cys), Glutamine (Gln), Glutamic acid (Glu), Isoleucine (Ile), two Leucines (Leu1, Leu 2), Methionine (Met), Tyrosine (Tyr), Valine (Val)], fragments of six protein coding genes [NADH dehydrogenase subunit 1 (ND1) (65 bp, two fragments), NADH dehydrogenase subunit 2 (ND2) (17 bp, one fragment), NADH dehydrogenase subunit 5 (ND5) (40 bp, one fragment), NADH dehydrogenase subunit 6 (ND6) (43 bp, one fragment), cytochrome c oxidase subunit I (COX1) (259 bp, two fragments), and cytochrome b (CYTB) (318 bp, one fragment)], and fragments of the two rRNA genes [12S (697 bp, four fragments) and 16S ribosomal RNA (16S) (1,221 bp, five fragments)]. Sequences obtained for 12S and CYTB genes were aligned to the corresponding sequences from the iterative mapping to isolated 12S and CYTB genes of Eliomys quercinus (942 and 361 bp of each gene recovered, respectively) and E. melanurus (848 and 590 bp of each gene recovered, respectively). The sequences for each gene overlapped and were identical, and thus, the longest sequence for each gene was selected for the phylogenetic analysis. Despite the low number of unique reads mapping to each reference, mapDamage analyses (Figure S2) displayed damage patterns consistent with ancient samples for unrepaired libraries (Briggs et al., 2007).
2.4 Phylogenetic analyses
We aligned the Hypnomys morpheus 12S (942 bp, GenBank accession number MN153772) and CYTB (590 bp, GenBank accession number MN164630) sequences with available data from other glirid and outgroup species (Table 2 for GenBank accession numbers) using MUSCLE (Edgar, 2004) in Geneious. We adjusted the size of each gene in all the 18 species to the length of these genes obtained for H. morpheus, and ambiguous regions in the 12S gene alignment were removed using stringent default parameters in Gblocks v.0.91b (Castresana, 2000), which kept 739 out of the 1,014 bp of the full 12S alignment (73%). Our final alignment (see Alignment S1) comprised 1,330 bp (591 bp for CYTB and 739 bp for 12S). Partitioning schemes and substitution models (Table 3) in the 12S region and codon positions in the CYTB region were estimated in PartitionFinder v.2.1.1 (Lanfear, Frandsen, Wright, Senfeld, & Calcott, 2016). We inferred a maximum likelihood tree in RAxML v.8.2.11 (Stamatakis, 2014), with node support values estimated by performing 1,000 bootstrap replicates. We also performed a MrBayes v.3.2.3 analysis (Ronquist et al., 2012) with four separate runs of four Markov chains each using default priors. Each chain ran for 108 generations sampling trees and parameter values every 104 generations. Sampled trees were summarized as a majority-rule consensus tree after discarding the first 10% of trees as burn-in (Figure S3).
| Species | 12S | CYTB |
|---|---|---|
| Dryomys laniger Felten and Storch, 1968 | AJ536349 | |
| Dryomys nitedula (Pallas, 1778) | D89005 | KJ739702 |
| Eliomys melanurus (Wagner, 1840) | AJ536350 | HE614010 + KF422705 |
| Eliomys quercinus (Linnaeus, 1766) | Y16896 | AJ225030 |
| Glirulus japonicus (Schinz, 1845) | D89007 | D89001 |
| Glis glis (Linnaeus, 1766) | NC_001892 | NC_001892 |
| Graphiurus kelleni (Reuvens, 1890) | HE978360 | HE978360 |
| Graphiurus lorraineus Dollman, 1910 | AJ536356 | |
| Graphiurus microtis (Noack, 1887) | AJ536352 | |
| Graphiurus murinus (Desmarest, 1822) | AJ536351 | AJ225115 |
| Graphiurus ocularis (Smith, 1829) | AJ536355 | |
| Graphiurus parvus (True, 1893) | AJ536353 | |
| Graphiurus platyops Thomas, 1897 | AJ536354 | |
| Muscardinus avellanarius (Linnaeus, 1758) | D89006 | AJ225117 |
| Myomimus roachi (Bate, 1937) | AJ536348 | |
| Glaucomys volans (Linnaeus, 1758) (outgroup) | AF038020 | AF157921 |
| Sciurus aestuans Linnaeus, 1766 (outgroup) | AJ012746 | AJ389530 |
| Partition | Substitution model | ||
|---|---|---|---|
| RAxML | MrBayes | BEAST | |
| 12S, CYTB_1 | GTR + G | GTR + G | GTR + G |
| CYTB_2 | GTR + G | HKY + I | HKY + I |
| CYTB_3 | GTR + G | HKY + G | HKY + G |
We implemented a birth-death tree prior and a single relaxed uncorrelated lognormal clock model (with rate multipliers for each of the three partitions, see Table 3) to estimate phylogeny and divergence times using BEAST v.1.8.4 (Drummond, Suchard, Xie, & Rambaut, 2012). To calibrate our analysis, we followed previous studies (e.g., Montgelard et al., 2003; Nunome et al., 2007; Mouton et al., 2017) and constrained the age of the divergence between Sciuridae and Gliridae according to a uniform distribution with a minimum of 50 million years ago (Mya) and a maximum of 55 Mya, corresponding to the earliest known fossil representatives of these families (Hartenberger, 1998). We repeated our analysis four times with different starting trees created using Mesquite v.3.04 (Maddison & Maddison, 2018) based on an ML tree created using IQTREE v.1.6.6 (Nguyen, Schmidt, von Haeseler, & Minh, 2015). Each analysis comprised a chain of 108 iterations, sampling every 104 iterations. Parameter convergence and sampling was assessed using Tracer v.1.6.1. The first 10% of trees from each chain were removed as burn-in, with the remainder from each chain combined using LogCombiner v.1.8.4 and summarized using TreeAnnotator v.1.8.4 (Rambaut & Drummond, 2010).
We graphically depicted the pairwise distances between different Gliridae sequences using the heatmap similarity (%) implemented in Geneious (Figure S4) for the two different mitochondrial genes available (16 species for the 12S gene and nine for the CYTB and a combination of CYTB and 12S).
We tested the position of Hypnomys morpheus in relation to the variation within the Eliomys genus using a 370-bp CYTB fragment obtained for 48 individuals of E. quercinus (accession numbers AJ225030, FM164278, FR848957, FR848958, GQ453668, GQ453669, HE611090-HE611093, HE613976-HE614008, JX457812-JX457816), eight individuals of E. melanurus (accession numbers FM164279, FM164280, FR848955, FR848956, HE614009-HE614012), and Graphiurus kelleni (accession number HE978360) as outgroup using an unpartitioned IQTREE analysis. The same alignment without the outgroup was used in the haplotype network analysis using Fitchi (Matschiner, 2015). Eliomys clades obtained in both IQTREE and Fitchi analyses (Figure S5) have been named following Perez, Libois, and Nieberding (2013).
3 RESULTS
The mapDamage analysis (Figure S2), which shows the expected damage pattern of degraded ancient DNA, and negative PCR results on both extraction blank controls and other extracts from Hypnomys samples rule out the possibility of any introduction of contaminants or cross-contamination during laboratory work.
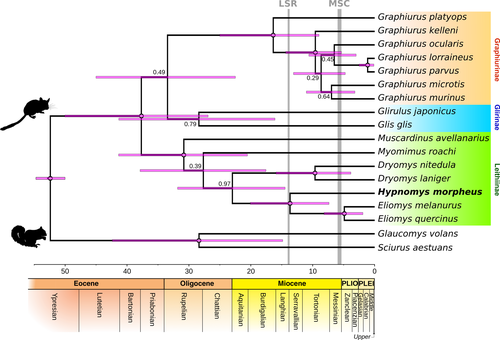
Overall, the results of our phylogenetic analyses are consistent with the published molecular phylogenies of Gliridae (e.g., Fabre et al., 2012; Montgelard et al., 2003; Nunome et al., 2007). While we did not find strong support for the previously established Glis-Glirulus clade, our results agree with Holden (2005)’s classification of glirid subfamilies—Graphiurinae (including Graphiurus), Glirinae (including Glis and Glirulus), and Leithiinae (including Eliomys, Dryomys, Muscardinus, and Myomimus)—which we find to be reciprocally monophyletic. Both maximum likelihood (ML) and MrBayes Bayesian inference (BI) analyses supported the monophyly of Graphiurinae (maximum likelihood bootstrap value [MLB] = 100, posterior probability [PP] = 1) and Leithiinae (sensu Holden, 2005; MLB = 79, PP = 0.99, Figure S3). The monophyly of the Leithiinae node has been previously well supported just using a 952-bp fragment of the 12S by Montgelard et al. (2003). However, our results do not recapitulate support for Glirinae (Glis-Glirulus node) or the basal position of Muscardinus and Myomimus within Leithiinae, which were observed in published molecular phylogenies based on nuclear genes or a combination of nuclear and mitochondrial genes (Fabre et al., 2012; Montgelard et al., 2003; Nunome et al., 2007). Importantly, we obtained high support for a clade comprising Hypnomys morpheus and Eliomys (MLB = 100, PP = 1) and for the monophyly of Eliomys (MLB = 100, PP = 1) and Dryomys (MLB = 99, PP = 1). The clade formed by these three genera (Dryomys, Eliomys, and Hypnomys) received moderate support (MLB = 78, PP = 0.95).
The time-calibrated tree (Figure 2) displayed a similar topology to the uncalibrated Bayesian tree, that is, monophyly with similarly high posterior probability values of clades Gliridae (PP = 1), Graphiurinae (PP = 1), Leithiinae (PP = 1), Dryomys (PP = 1), and Eliomys (PP = 1), a Hypnomys-Eliomys clade (PP = 1), and a Dryomys-Hypnomys-Eliomys clade (PP = 0.97). The main difference between the trees was the unresolved relationship of Glirinae with Leithiinae (PP = 0.68, uncalibrated tree) or with Graphiurinae (PP = 0.49, calibrated tree). In general, the 95% highest posterior densities (HPD) of node ages were wide, though they overlapped with those observed in previously published glirid phylogenies (Table 4). The split between the fossil Hypnomys and its putative sister taxon Eliomys was 13.67 Mya (95% HPD = 7.39–20.07).
| Node | This paper | Montgelard et al. (2003) | Nunome et al. (2007) | Mouton et al. (2012) | Mouton et al. (2017) |
|---|---|---|---|---|---|
| (E. quercinus, E. melanurus) | 1.91–8.19 | 7.0 ± 0.9 | na | 4.87–8.88 | 5.56–7.49 |
| (D. nitedula, D. laniger) | 3.85–15.96 | 16.7 ± 1.8 | na | na | na |
| (Eliomys, Dryomys) | 14.47–31.84 | 28.5 ± 2.8 | 14.5 ± 2.4 | na | 13.08–24.40 |
| (Eliomys, Dryomys), Myomimus) | 17.56–37.89 | 38.1 ± 3.6 | na | na | na |
| Leithiinae | 20.55–41.36 | 40.8 ± 3.8 | 22.3 ± 2.8 | 9.85–54.36 | na |
| (Glis, Glirulus) | 16.12–41.34 | 27.7 ± 3 | 27.0 ± 2.9 | na | na |
| Graphiurinae | 9.00–25.04 | 8.7 ± 1 | na | na | na |
| Gliridae | 26.91–50.08 | 50.0 | 28.6 ± 2.9 | na | na |
| (Sciuridae, Gliridae) | 50.00–54.72 | na | 52.7 ± 1.4 | na | na |
Our ML analysis using only the 370 bp CYTB sequences of Eliomys, Hypnomys morpheus, and the outgroup Graphiurus kelleni (Figure S5b) resulted in moderate support for the monophyly of Eliomys (MLB = 88) with H. morpheus as its sister taxon. Our haplotype network (Figure S5a) further illustrates the distinction between H. morpheus and extant Eliomys variation. Both analyses indicated that sequences from the fossil H. morpheus do not fall within the variability of the modern E. quercinus and E. melanurus. As expected, pairwise comparisons of sequence similarity displayed higher values when species from the same genus were analyzed (Figure S4). For the 591 bp of CYTB, similarity values >93% and >90% were observed between congeneric species within Eliomys and Graphiurus, respectively. For this same CYTB fragment, H. morpheus displayed a similarity of 84% and 85% with E. quercinus and E. melanurus, respectively, comparable to the similarity values observed between members of other glirid genera, for example, Muscardinus-Glis (85.2%), Glirulus-Glis (84.2%), or Graphiurus kelleni-Glis (84%). Similar patterns were observed in our pairwise analysis of the 739 bp of the 12S gene. The highest similarity values were displayed by pairwise comparison between congeneric species within Eliomys (96.6%), Dryomys (95.4%), and Graphiurus (93%–99.6%). Values around 93% were also observed for pairwise comparison between Hypnomys-E. quercinus (93.8%), Hypnomys-E. melanurus (93.1%), and Glirulus-Glis (93.5%). Finally, where data were available for both CYTB and 12S, the highest values were again observed between congeneric species within Eliomys (95.2%) and Graphiurus (93%), followed by Hypnomys-E. quercinus (89.4%), Hypnomys-E. melanurus (89.5%), and Glirulus-Glis (89.4%).
Our phylogenetic analyses clearly place our H. morpheus within Gliridae and as sister clade of Eliomys (Figure 2 and S3). For this reason, we can confidently discard the hypothesis that the tibia belongs to a murid, especially to similar-sized rodents of the genus Rattus Fischer de Waldheim, 1803. Furthermore, the position of H. morpheus sequences outside the genetic variability of Eliomys CYTB gene (Figure S5) clearly shows that there is no reason to interpret our data as a result of a misidentification of an Eliomys bone.
4 DISCUSSION
Our molecular data are fully consistent with a close relationship between Hypnomys and Eliomys, as suggested by past morphological analyses (e.g., Agustí, 1980, 1981; Bate, 1918; Mills, 1976; Zammit-Maempel & de Bruijn, 1982). The basal placement of H. morpheus outside the variability of the modern Eliomys species and the pairwise similarity (Figure S4) between the fossil and each of two Eliomys species studied here (which display equivalent levels of similarity as that between Glis and Glirulus; but see Graphiurus platyops Thomas, 1897 in comparison with other Graphiurus species using 12S data) suggest that Hypnomys should not be considered as a subgenus of Eliomys, and the generic status established by Bate (1918) should be retained.
In general, the lowest values of the 95% HPD intervals for node ages (Table 4) are similar to the values obtained by Nunome et al. (2007), whereas the highest values of 95% HPD intervals are similar to those obtained by Montgelard et al. (2003). However, Freudenthal and Martín-Suárez (2013) suggested that the base age of Gliridae (50 Mya) used as a calibration age by Montgelard et al. (2003) was too old and should be replaced by 16 Mya, a view that has subsequently not been followed (e.g., Mouton et al., 2017). Despite the uncertainties of the node ages of our (and other published) calibrated trees, the available Hypnomys-Eliomys divergence estimate of 13.67 Mya (95% HPD = 7.39–20.07 Mya) allows us to identify three possible palaeobiogeographic scenarios for the origin of the Hypnomys lineage in the Balearic Islands.
The first possible origin for Hypnomys, which is the most commonly accepted hypothesis (e.g., Agustí, 1980; Alcover et al., 1981; Bover et al., 2014; Colom, 1978; Mas et al., 2018; Moyà-Solà & Pons-Moyà, 1980), involves a split from a continental ancestor during the Late Tortonian/Early Messinian (Figure 2) and its arrival into the Balearic Islands during the Messinian Salinity Crisis (MSC, 5.97–5.33 Mya; Krijgsman, Hilgen, Raffi, Sierro, & Wilson, 1999; Manzi et al., 2013). According to Agustí (1986) and Bover, Quintana, and Alcover (2008), the putative continental ancestor would be the fossil species Eliomys intermedius Priant, 1953 or E. truci Mein and Michaux, 1970, both representatives of the lineage E. truci-E. yevesi-E. intermedius-E. quercinus (Mansino, García-Álix, Ruiz-Sánchez, & Montoya, 2015). However, preliminary morphological analysis of the earliest known representative of the Hypnomys lineage (currently under analysis) obtained from the Early Pliocene Zanclean site of Na Burguesa-1 (NB-1) on Mallorca conflicts with this hypothesis. The NB-1 glirid shows an unexpectedly complex dental pattern (Figure 3), whereas proposals that Hypnomys descends from fossil Eliomys species (e.g., Agustí, 1980, 1981, 1986; Alcover & Agustí, 1985; Bate, 1918; Zammit-Maempel & de Bruijn, 1982) suggest that members of the Hypnomys lineage evolved increasing dental pattern complexity through time from a relatively dentally simple Eliomys-like ancestor. Reumer (1982) likewise observed a contrary trend toward a simplification of the dental pattern in the Hypnomys lineage (from H. waldreni to H. morpheus). Thus, while the results of our molecular dating analyses are consistent with this hypothesis, it conflicts with available morphological evidence.
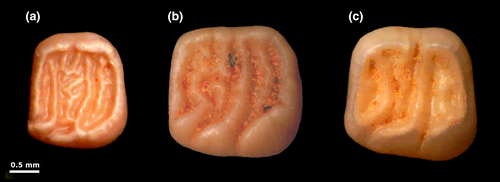
An alternative and more morphologically plausible origin for Hypnomys is its descent from a Middle-Late Miocene glirid with a high dental complexity, such as Vasseuromys Baudelot and de Bonis, 1966, or some closely related genus. Vasseuromys spans the latest Oligocene to Late Miocene (Sinitsa & Nesin, 2018) while the older Microdyromys de Bruijn, 1966 and Bransatoglis Hugueney, 1967, which also possess high dental complexity, span the Early Oligocene to the Middle Miocene and the Late Oligocene to the Middle Miocene, respectively (Freudenthal & Martín-Suárez, 2013). Under this scenario, the simplified dental pattern displayed by more recent Hypnomys species (Figure 3) would be an effect of insular evolution (Reumer, 1982).
A final possibility for the origin of Hypnomys, consistent with the upper bounds of our node age 95% HPDs, is an early split from mainland ancestors and pre-MSC arrival to the Balearic Islands during the Langhian–Serravalian regression (Moyà-Solà, Quintana, Alcover, & Köhler, 1999; Riba, 1981). However, the fossil record of mammals of this age from Mallorca and Menorca is restricted to the ochotonid Gymnesicolagus gelaberti Mein & Adrover, 1982, and the glirids Carbomys sacaresi Mein & Adrover, 1982, Margaritamys llulli Mein & Adrover, 1982 and Peridyromys ordinasi Mein & Adrover, 1982 in Mallorca (Adrover, Agustí, Moyà-Solà, & Pons-Moyà, 1985; Mein & Adrover, 1982), and Margaritamys adroveri Quintana & Agustí, 2007 in Menorca (Quintana & Agustí, 2007). All of the glirids from this faunal episode have lower dental complexity than NB-1 glirid, making them unlikely ancestors (as for Eliomys).
Although the resolving of phylogenetic relationships of all extinct and extant Gliridae subfamilies is beyond the scope of this paper, the dental morphology and the genetics of Hypnomys clearly support its inclusion within Leithiinae. Our node age estimates, the chronological range of Vasseuromys, and the original complex dental pattern of Hypnomys (see Figure 3a in this paper, and Vasseuromys tectus Sinista and Nesin, 2018 depicted in Figure 6 in Sinista & Nesin, 2018) suggest that Vasseuromys, or some close relative, could be considered as the potential ancestor of Hypnomys.
Ultimately, obtaining additional genetic data from extinct and extant dormouse species as well as a systematic review of extinct genera could contribute to further illuminating the evolution, taxonomy, and palaeobiogeography of Gliridae.
ACKNOWLEDGEMENTS
This research was supported by a Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme (project MEDITADNA, PIOF-GA-2011-300854, FP7-PEOPLE), and it is included in the Research Project CGL2016-79795, [“Cambios holocénicos en la biodiversidad animal de las islas de la Macaronesia y de Baleares. II,” CGL2016-80000-P (Dirección General de Investigación Científica y Técnica, Ministerio de Economía y Competitividad)] and SGR2017-859 (AGAUR, Gencat).




