Cophylogenetic relationships between Dactylogyrus (Monogenea) ectoparasites and endemic cyprinoids of the north-eastern European peri-Mediterranean region
Abstract
The study of host–parasite coevolution is one of the cornerstones of evolutionary biology. The majority of fish ectoparasites belonging to the genus Dactylogyrus (Monogenea) exhibit a high degree of host specificity. Therefore, it is expected that their evolutionary history is primarily linked with the evolutionary history of their cyprinoid fish hosts and the historical formation of the landmasses. In the present study, we used a cophylogenetic approach to investigate coevolutionary relationships between endemic Cyprinoidea (Cyprinidae and Leuciscidae) from selected regions in southern Europe and their respective Dactylogyrus species. A total of 49 Dactylogyrus species including endemic and non-endemic species were collected from 62 endemic cyprinoid species in the Balkan and Apennine Peninsulas. However, 21 morphologically identified Dactylogyrus species exhibited different genetic variants (ranging from 2 to 28 variants per species) and some of them were recognized as cryptic species on the basis of phylogenetic reconstruction. Phylogenetic analyses revealed several lineages of endemic and non-endemic Dactylogyrus species reflecting some morphological similarities or host affinities. Using distance-based and event-based cophylogenetic methods, we found a significant coevolutionary signal between the phylogenies of parasites and their hosts. In particular, statistically significant links were revealed between Dactylogyrus species of Barbini (Cyprinidae) and their hosts belonging to the genera Aulopyge, Barbus and Luciobarbus. Additionally, a strong coevolutionary link was found between the generalist parasites D. alatus, D. sphyrna, D. vistulae, and their hosts, and between Dactylogyrus species of Pachychilon (Leuciscidae) and their hosts. Cophylogenetic analyses suggest that host switching played an important role in the evolutionary history of Dactylogyrus parasitizing endemic cyprinoids in southern Europe. We propose that the high diversification of phylogenetically related cyprinoid species in the Mediterranean area is a process facilitating the host switching of specific parasites among highly diverse congeneric cyprinoids.
1 INTRODUCTION
Host–parasite coevolution plays an important role in the processes of parasite speciation and represents one of the most fascinating topics in evolutionary biology (Poulin, 2007). If the host specificity of the parasite is high (i.e., a parasite species restricted to a single host species or very few phylogenetically closely related host species), it is tempting to assume that the evolution of parasitic organisms is associated with the evolution of their hosts (Ronquist, 1997). Hence, the Fahrenholz rule (Brooks & McLennan, 1993; Stammer, 1957) states that parasite phylogeny mirrors host phylogeny and that cospeciation drives host–parasite coevolution. Congruent host–parasite phylogenies have usually been inferred when the host switching of parasites is impossible or highly improbable, such as in the case of chewing lice and pocket gophers, where parasite cospeciation likely resulted from an allopatric distribution of hosts and host switching was supported only in the case of physical contact between two gopher species (Hafner & Nadler, 1988; Hafner et al., 1994; Page, 1996). However, the whole concept of the ‘Fahrenholz rule’ has been re-evaluated and several studies have suggested that cospeciation is not always the predominant driver of parasite speciation during reciprocal host–parasite evolution. Host switching (Klassen, 1992) and parasite duplication, that is parasite speciation within a host lineage (Johnson, Adams, Page, & Clayton, 2003), play significant roles in parasite evolution, often resulting in incongruent host and parasite phylogenies (Desdevises, Morand, Jousson, & Legendre, 2002; Mendlová, Desdevides, Civáňová, Pariselle, & Šimková, 2012; Šimková, Morand, Jobet, Gelnar, & Verneau, 2004; Šimková, Serbielle, Pariselle, Vanhove, & Morand, 2013). Despite the fact that frequent host switching during the evolutionary history of parasite taxa usually results in incongruent host–parasite phylogenies, a series of multiple host switches followed by parasite speciation can generate trees with similar topologies (de Vienne, Giraud, & Shykoff, 2007). Moreover, host switching tends to occur more often between the phylogenetically close host species, what may lead to further congruence between host and parasite trees (Charleston & Robertson, 2002; de Vienne et al., 2013). Therefore, the independent estimation of the age of speciation events in host and parasite trees (e.g., extrapolated from the estimated time of host speciation) should also be taken into account when interpreting the outputs of cophylogenetic analyses.
Dactylogyrus Diesing, 1850 (Monogenea) are gill parasites generally exhibiting narrow host specificity and high morphological variability with respect to attachment organ (termed haptor), putatively reflecting adaptations to their different host species or within-host microhabitats (Gibson, Timofeeva, & Gerasev, 1996; Šimková, Desdevises, Gelnar, & Morand, 2000, 2001; Šimková & Morand, 2008; Šimková, Verneau, Gelnar, & Morand, 2006). In addition, Dactylogyrus currently represents the platyhelminth genus with the highest species diversity (more than 900 described species according to Gibson et al., 1996), certainly largely underestimated as new species have recently been described (Aydogdu, Molnár, Emre, & Emre, 2015; Benovics, Kičinjaová, & Šimková, 2017; Nitta & Nagasawa, 2016; Rahmouni, Řehulková, Pariselle, Rkhami, & Šimková, 2017). This high species richness in Dactylogyrus is associated with their narrow host specificity towards a single host species or closely related species, and with a high diversity of their host species—primarily freshwater fish of Cyprinoidea (considering recent phylogenetic studies, for example Schönhuth, Vukić, Šanda, Yang, & Mayden, 2018). Previous studies have suggested that each host species harbours at least one Dactylogyrus species (Dupont & Lambert, 1986; Galli, Stefani, Zaccara, & Crosa, 2002; Gibson et al., 1996; Moravec, 2001). In regards to host specificity, Šimková, Verneau, et al. (2006) classified five groups of Dactylogyrus species ranging from strict specialists, living on a single host species, to generalists parasitizing host species from different phylogenetic lineages. The high host specificity of Dactylogyrus (and other monogeneans) is linked with their direct life cycle, where the larva (oncomiracidium) actively searches for a suitable (specific) host and attaches directly to the gills or body surface. Oncomiracidia are sensitive to chemical cues from hosts which can either initiate the hatching of oviparous species, attract larvae or initiate larva deciliation (Buchmann & Lindenstrøm, 2002). The recognition of these signals most likely requires specific parasite adaptation (Buchmann, 1999; Whittington & Kearn, 2011).
Their narrow host specificity and expected host–parasite coevolution make monogeneans potential proxies for the study of the evolution and dispersion of their hosts. Previous studies (on Lamellodiscus Johnston & Tiegs, 1922 parasitizing Sparidae, Desdevises et al., 2002; Gyrodactylus von Nordmann, 1832 parazitizing Gobiidae, Huyse, Audenaert, & Volckaert, 2003; Huyse, Oeyen, Larmuseau, & Volckaert, 2017; Huyse & Volckaert, 2005; Cichlidogyrus Paperna, 1960 and Scutogyrus Pariselle & Euzet, 1995 parazitizing Cichlidae, Mendlová et al., 2012; and Thaparocleidus Jain, 1952 parasitizing Pangasiidae, Šimková et al., 2013) suggested that cophylogenetic patterns between monogeneans and their hosts are complex, involving less cospeciation than expected and involve putatively high number of host switches, duplications and losses. Frequent host switching in these systems may be expected because of the active dispersion of the larvae and the capacity of adults to survive without the hosts for a short period of time (Bakke, Cable, & Harris, 2007; Brooks & McLennan, 1991), potentially allowing them to infect phylogenetically closely related host species with similar ecological requirements.
In spite of the large interest in host-specific monogeneans, few phylogenetic and/or cophylogenetic studies have been performed for Dactylogyrus. In Dactylogyrus from central European cyprinoids, intra-host duplication was inferred as a more widespread diversification process than host switching (Šimková et al., 2004). Several coevolutionary scenarios were proposed by Benovics et al. (2017), Benovics, Desdevises, Vukić, Šanda, and Šimková (2018), and Šimková, Benovics, Rahmouni, and Vukić (2017) regarding Dactylogyrus and peri-Mediterranean endemic cyprinoids, the last one hypothesizing that Iberian cyprinids harbour Dactylogyrus species originating from two different colonization events.
South European freshwater fauna is extremely rich in endemic cyprinoid species (Kottelat & Freyhof, 2007). For instance, the Balkan Peninsula is considered a hotspot of endemic freshwater diversity and harbours 59% of all European cyprinoid species (Abell et al., 2008; Albrecht & Wilke, 2008; Oikonomou, Leprieur, & Leonardos, 2014; Schultheiss, Albrecht, Bossneck, & Wilke, 2008; Sušnik, Snoj, Wilson, Mrdak, & Weiss, 2007), which have recently become the common interest of ichthyologists (Buj et al., 2017; Gante, 2011; Marková et al., 2010; Perea, Vukić, Šanda, & Doadrio, 2016; Stierandová et al., 2016). The Mediterranean drainages of the Balkans were divided into several ichthyological regions based on the presence of freshwater fish species, especially of the cyprinoids (Oikonomou et al., 2014). The eastern Balkans regions in the Aegean Sea slope are characteristic by the presence of cyprinoid species of Pontocaspian origin (e.g., Abramis brama, Barbus balcanicus, Leuciscus aspius or Rutilus rutilus complex), especially in the northern and eastern part (Economidis & Banarescu, 1991; Economou et al., 2007). The conspecifity of these species with populations from the Pontocaspian region was recently genetically corroborated (Geiger et al., 2014; Levin et al., 2017; Marková et al., 2010). Genetic data also suggest affinities of some of the endemic species from this area to Pontocaspian, but also to Anatolian congeners (e.g., Alburnoides, Chondrostoma, Squalius, Barbus, Luciobarbus or Vimba; Geiger et al., 2014; Perea et al., 2010; Stierandová et al., 2016). The south-eastern part of the Balkans, that is south-western part of the Aegean Sea drainages, is on the other hand inhabited by mostly endemic cyprinoids (from genera Barbus, Rutilus, Scardinius, Telestes or Pelasgus) with affinities to congeneric species from Ionian Sea slope (Buj et al., 2017; Gante, 2011; Geiger et al., 2014; Perea et al., 2010).
The south-western and western part of the Mediterranean drainages of the Balkans is characterized by presence of almost exclusively endemic cyprinoids, both of ancient origin (from Miocene), like genera Aulopyge, Delminichthys, Pelasgus, Phoxinellus, or several species of Telestes or Squalius (Buj et al., 2017, 2019; Gante, 2011; Perea et al., 2010, 2016) as well as of more recent origin, that is species of Alburnus or Scardinius, probably from Pliocene/Pleistocene colonization events, based on much lower genetic differentiation from congeneric species outside the Balkans (Perea et al., 2010).
In comparison to the species-rich Balkan Peninsula, only several endemic cyprinoid species were described from the Apennine Peninsula (Bianco, 1995). Since most of this peninsula was below the sea level during most of the Miocene era, it is assumed that Apennine ichthyofauna is of more recent origin than ichthyofauna of other south European peninsulas (Steininger & Rögl, 1984). In general, Apennine cyprinoids, especially leuciscids, are phylogenetically more related to Balkan species than to central European or Iberian species (Perea et al., 2010). Several cyprinoid species occur both in the northern part of the Apennine peninsula (northern Adriatic river systems [Padano-Venetian ichthyologic district sensu Bianco, 1990]) and in the western-most Balkan, showing no or very low degree of molecular divergence between taxa from these two regions (Buj et al., 2010; Geiger et al., 2014; Perea et al., 2010). It is a consequence of the glacial periods, when sea level dropped considerably and rivers of the northern Adriatic were connected together, which led to the exchange of many primary native fish species between the two peninsulas (Stefani, Galli, Crosa, Zaccara, & Calamari, 2004; Waelbroeck et al., 2002). However, many of the northern and north-eastern Mediterranean drainages are heavily affected by introductions of non-native freshwater species, including numerous cyprinoids (Bianco, 1995; Piria et al., 2018; Vukić, Eliášová, Marić, & Šanda, 2019), even the endemic ones being translocated often outside the native range (Bianco, 1995; Koutsikos et al., 2019). This could lead to the simultaneous introduction of their non-native parasite species, which can subsequently infect the native fishes (such as parasite Dactylogyrus, documented in Benovics et al., 2017).
Since cophylogenetic patterns and processes between peri-Mediterranean cyprinoids and their Dactylogyrus parasites are not known, we aimed to study the cophylogeny of these two groups in selected southern European regions in the peri-Mediterranean area and to elucidate the historical dispersion of endemic cyprinoids using Dactylogyrus phylogeny. Therefore, the objectives of this study were (a) to reconstruct the coevolutionary histories of Balkan and Apennine endemic cyprinoids and their endemic Dactylogyrus parasites, (b) to investigate the speciation patterns of host-specific Dactylogyrus and (c) to assess whether parasite phylogeny is linked to host phylogeny and the historical formation of the landmass, or rather to the recent distribution and introduction of non-native species into the investigated regions.
2 MATERIAL AND METHODS
2.1 Material collection and fixation
Between 2014 and 2017, 76 cyprinoid species were sampled from 56 localities across the Balkan and Apennine Peninsulas (Table 1). A fin clip was obtained from 608 inspected fish individuals and preserved in 96% ethanol. Fishes were dissected using standard methods described by Ergens and Lom (1970). Dactylogyrus parasites were collected from the gills and nasal cavity, mounted on slides, and fixed using a mixture of glycerine and ammonium picrate (GAP, Malmberg, 1957). Species determination was performed according to the size and shape of the sclerotized hard parts of the haptor and the reproductive organs (male copulatory organ and vaginal armament) using Pugachev, Gerasev, Gussev, Ergens, and Khotenowsky (2009). Identification at the species level was performed using an Olympus BX51 microscope equipped with phase-contrast optics. Several representatives of each collected Dactylogyrus species were bisected using fine needles. A part of the body (usually the half of body containing the reproductive organs) was mounted on a slide and used for morphological identification, while the other part was individually preserved in 96% ethanol for subsequent DNA extraction.
| Host species | Country | Locality | Main river basin | Ichthyogeographic district |
|---|---|---|---|---|
| Alburnoides devolli Bogutskaya, Zupančić & Naseka, 2010 | Albania | Devoli, Maliq | Seman | Albanian |
| Alburnoides economoui Barbieri, Vukić, Šanda & Zogaris, 2017 | Greece | Sperchios, Ypati | Sperchios | Western Aegan |
| Alburnoides fangfangae Bogutskaya, Zupančić & Naseka, 2010 | Albania | Osum, Vodice | Seman | Albanian |
| Alburnoides ohridanus (Karaman, 1928) | Albania | Fani i Vogel, Reps | Mat | Albanian |
| Alburnoides prespensis (Karaman, 1924) | Greece | Aoos, Kalithea | Aoos | Albanian |
| Alburnoides strymonicus Chichkoff, 1940 | Greece | Angistis, between Alistrati and Drama | Strymon | North-eastern Aegan |
| Alburnoides thessalicus Stephanidis, 1950 | Greece | Pinios, Rongia—Valamandrio | Pinios | North-western Aegan |
| Alburnus arborella (Bonaparte, 1841) | Italy | Canale maestro de la Chiana, Chuisa dei Capannoi, Arno basis | Arno | Tuscano-Latium |
| Alburnus neretvae Buj, Šanda & Perea, 2010 | Bosnia and Herzegovina | Mušnica, Avtovac | Neretva | Central Adriatic |
| Bosnia and Herzegovina | Zagorje, Jabuke | Neretva | Central Adriatic | |
| Alburnus scoranza Bonaparte, 1845 | Albania | Skadar lake, Shiroke | Ohrid-Drin-Skadar lake system | Albanian |
| Aulopyge huegelii Heckel, 1842 | Bosnia and Herzegovina | Šujica, Duvansko Polje | Cetina | Central Adriatic |
| Barbus balcanicus Kotlík, Tsigenopoulos, Ráb & Berrebi, 2002 | Greece | Gallikos, Mandres | Gallikos | North-western Aegan |
| Barbus cyclolepis Heckel, 1837 | Greece | Macropotamos River | Filiouri | North-eastern Aegan |
| Barbus peloponnesius Valenciennes, 1842 | Greece | Neda, Gianitsochori | Neda | Ionian |
| Greece | Kokitos, Pagrati | Acheron | Ionian | |
| Barbus plebejus Bonaparte, 1839 | Croatia | Bribirske Mostine, Bribišnica | Krka | Central Adriatic |
| Barbus prespensis Karaman, 1924 | Albania | Shkumbini, Perrenjas | Shkumbini | Albanian |
| Greece | Aoos, Kalithea | Aoos | Albanian | |
| Barbus rebeli Koller, 1926 | Albania | Mat, Klos | Mat | Albanian |
| Barbus sp. | Albania | Kiri | Ohrid-Drin-Skadar lake system | Albanian |
| Barbus sperchiensis Stephanidis, 1950 | Greece | Sperchios, Ypati | Sperchios | Western Aegan |
| Barbus strumicae Karaman, 1955 | Greece | Rihios river, Stavros | Volvi lake | North-eastern Aegan |
| Barbus tyberinus Bonaparte, 1839 | Italy | Torrente Cerfone, Intoppo | Tiber | Tuscano-Latium |
| Chondrostoma knerii Heckel, 1843 | Bosnia and Herzegovina | Rečina river, near Jelim lake, Hutovo Blato | Neretva | Central Adriatic |
| Chondrostoma ohridana Karaman, 1924 | Greece | Aoos, Kalithea | Aoos | Albanian |
| Chondrostoma phoxinus Heckel, 1843 | Bosnia and Herzegovina | Šujica, Šujicko Polje | Cetina | Central Adriatic |
| Chondrostoma vardarense Karaman, 1928 | Greece | Angistis, between Alistrati & Drama | Strymon | North-eastern Aegan |
| Greece | Pinios, Rongia—Valamandrio | Pinios | North-western Aegan | |
| Delminichthys adspersus (Heckel, 1843) | Bosnia and Herzegovina | Nezdravica, Trebižat | Neretva | Central Adriatic |
| Luciobarbus albanicus (Steindachner, 1870) | Greece | Trichonis lake, Panetolio | Acheloos | Ionian |
| Luciobarbus graecus (Steindachner, 1895) | Greece | Sperchios, Ypati | Sperchios | Western Aegan |
| Pachychilon macedonicum (Steindachner, 1892) | Greece | Pinios, Rongia—Valamandrio | Pinios | North-western Aegan |
| Pachychilon pictum (Heckel & Kner, 1858) | Albania | Ohrid lake | Ohrid-Drin-Skadar lake system | Albanian |
| Greece | Aoos, Kalithea | Aoos | Albanian | |
| Pelasgus laconicus (Kottelat & Barbieri, 2004) | Greece | Evrotas, Sparti | Evrotas | Ionian |
| Pelasgus marathonicus (Vinciguerra, 1921)† | Greece | Sperchios, Ypati | Sperchios | Western Aegan |
| Pelasgus stymphalicus (Valenciennes, 1844)† | Greece | Pamissos, Vasiliko | Pamissos | Ionian |
| Pelasgus thesproticus (Stephanidis, 1939)† | Greece | Acheron, Gliki | Acheron | Ionian |
| Greece | Kokitos, Pagrati | Acheron | Ionian | |
| Phoxinellus alepidotus Heckel, 1843 | Bosnia and Herzegovina | Bosansko Grahovo, Korana River | Korana | Central Adriatic |
| Phoxinellus pseudalepidotus Bogutskaya & Zupančić, 2003 | Bosnia and Herzegovina | Lištica, Polog | Neretva | Central Adriatic |
| Phoxinus lumaireul Schinz, 1840† | Croatia | Lovinac, Ričica River | Ričica | Northern Adriatic |
| Phoxinus sp. | Bosnia and Herzegovina | Zalomka, Ribari | Neretva | Central Adriatic |
| Protochondrostoma genei (Bonaparte, 1839) | Italy | Torrente Cerfone, Le Ville | Tiber | Tuscano-Latium |
| Rutilus aula (Bonaparte, 1841) | Croatia | Baštica river, Grabovač reservoir | Baštica | Northern Adriatic |
| Rutilus basak (Heckel, 1843) | Bosnia and Herzegovina | Krenica lake, Drinovci | Neretva | Central Adriatic |
| Rutilus lacustris (Pallas, 1814) | Greece | flood pools by Struma, Lithopos | Strymon | North-eastern Aegan |
| Rutilus ohridanus (Karaman, 1924) | Albania | Skadar lake, Shiroke | Ohrid-Drin-Skadar lake system | Albanian |
| Rutilus panosi Bogutskaya & Iliadou, 2006† | Greece | Rivio, Amvrakia | Acheloos | Ionian |
| Rutilus rubilio (Bonaparte, 1837) | Italy | Torrente Cerfone, Intoppo | Tiber | Tuscano-Latium |
| Rutilus sp.† | Greece | channel near Sperchios | Sperchios | Western Aegan |
| Scardinius acarnanicus Economidis, 1991† | Greece | Trichonis lake, Panetolio | Acheloos | Ionian |
| Scardinius dergle Heckel & Kner, 1858 | Croatia | Bribirske Mostine, Bribišnica | Krka | Central Adriatic |
| Scardinius plotizza Heckel & Kner, 1858 | Bosnia and Herzegovina | Rečina river, near Jelim lake, Hutovo Blato | Neretva | Central Adriatic |
| Squalius illyricus Heckel & Kner, 1858 | Croatia | Cetina river, Kosore | Cetina | Central Adriatic |
| Squalius keadicus (Stephanidis, 1971)† | Greece | Evrotas, Sparti | Evrotas | Ionian |
| Squalius lucumonis (Bianco, 1983) | Italy | Torrente Cerfone, Intoppo | Tiber | Tuscano-Latium |
| Squalius microlepis Heckel, 1843† | Bosnia and Herzegovina | Trebižat, Klobuk | Neretva | Central Adriatic |
| Squalius orpheus Kottelat & Economidis, 2006 | Greece | Rihios river, Stavros | Volvi lake | North-eastern Aegan |
| Squalius pamvoticus (Stephanidis, 1939) | Greece | Acheron, Gliki | Acheron | Ionian |
| Squalius peloponensis (Valenciennes, 1844) | Greece | Pamissos, Vasiliko | Pamissos | Ionian |
| Squalius platyceps upančić, Marič, Naseka & Bogutskaya, 2010 | Albania | Ohrid lake | Ohrid-Drin-Skadar lake system | Albanian |
| Squalius prespensis (Fowler, 1977) | Albania | Shkumbini, Pajove | Shkumbini | Albanian |
| Greece | Aoos, Kalithea | Aoos | Albanian | |
| Squalius sp. | Greece | Trichonis lake, Panetolio | Acheloos | Ionian |
| Squalius squalus (Bonaparte, 1837) | Bosnia and Herzegovina | Donja Drežnica, Drežnica river | Neretva | Central Adriatic |
| Italy | Po, Between Verona & Modena | Po | Padano-Venetian | |
| Squalius svallize Heckel & Kner, 1858 | Croatia | Konavočica, Grude | Ljuta | central Adriatic |
| Squalius tenellus Heckel, 1843 | Bosnia and Herzegovina | Šujica, Duvansko Polje | Cetina | Central Adriatic |
| Bosnia and Herzegovina | Šujica, Šujičko Polje | Cetina | Central Adriatic | |
| Squalius vardarensis Karaman, 1928 | Greece | Sperchios, Ypati | Sperchios | Western Aegan |
| Greece | Gallikos, Mandres | Gallikos | North-western Aegan | |
| Squalius zrmanjae Karaman, 1928† | Croatia | Udbina, Krbava River | Krbava | Northern Adriatic |
| Telestes alfiensis (Stephanidis, 1971) | Greece | Erimantos, Tripotamo | Alfios | Ionian |
| Telestes beoticus (Stephanidis, 1939)† | Greece | stream in Livadia, Kifisos | Kifissos | Western Aegan |
| Telestes croaticus (Steindachner, 1866)† | Croatia | Sveti Rok, Obsenica river | Obsenica | Northern Adriatic |
| Telestes dabar Bogutskaya, Zupančić, Bogut & Naseka, 2012 | Bosnia and Herzegovina | Vrijeka, Dabarsko Polje | Neretva | Central Adriatic |
| Telestes fontinalis (Karaman, 1972) | Croatia | Krbavsko polje, Laudonov gaj | Krbava | Northern Adriatic |
| Telestes karsticus Marčić & Markovčić, 2011 | Croatia | Drežnica, Sušik river | Drežnica | Northern Adriatic |
| Telestes metohiensis (Steindachner, 1901) | Bosnia and Herzegovina | Zalomka River, Nevesinjsko polje | Neretva | Central Adriatic |
| Telestes montenigrinus (Vukovic, 1963) | Albania | Skadar lake, Shegan | Ohrid-Drin-Skadar lake system | Albanian |
| Telestes muticellus (Bonaparte, 1837) | Italy | Torrente Cerfone, Intoppo | Tiber | Tuscano-Latium |
| Telestes pleurobipunctatus (Stephanidis, 1939) | Greece | Kokitos, Pagrati | Acheron | Ionian |
| Tropidophoxinellus hellenicus (Stephanidis, 1971)† | Greece | Rivio, Amvrakia | Acheloos | Ionian |
| Tropidophoxinellus spartiaticus (Schmidt-Ries, 1943) | Greece | Neda, Gianitsochori | Neda | Ionian |
Note
- Host species without Dactylogyrus are shown by cross symbol (†).
2.2 DNA extraction, amplification and sequencing
Bisected Dactylogyrus samples preserved in ethanol were dried using a vacuum centrifuge. DNA extraction was performed following the standard protocol (DNeasy Blood & Tissue Kit; Qiagen). For molecular analyses, four genetic markers commonly applied for monogeneans were used. A section comprising a part of the 18S rRNA gene, the entire ITS1 region, and partial 5.8S rRNA gene were amplified using the primers S1 (forward, 5′-ATTCCGATAACGAACGAGACT-3′) and IR8 (reverse, 5′-GCTAGCTGCGTTCTTCATCGA-3′), which anneal to the genes for 18S and 5.8S rRNA, respectively (Šimková, Plaisance, Matějusová, Morand, & Verneau, 2003); PCR followed the protocol optimized by Benovics et al. (2018). Partial 28S rRNA gene was amplified using primers C1 (forward, 5′-ACCCGCTGAATTTAAGCA-3′) and D2 (reverse, 5′-TGGTCCGTGTTTCAAGAC-3′) following Hassouna, Michot, and Bachellerie (1984); PCR followed the protocol optimized in Šimková, Matějusová, and Cunningham (2006). The PCR products (~1,000 and ~800 bp, respectively) were checked on 1% agarose gel and purified using the ExoSAP-IT kit (Ecoli) following the standard protocol. The purified products were directly sequenced using the same primers as for PCR and BigDye Terminator Cycle Sequencing kit (Applied Biosystems). Sequencing was performed on an ABI 3130 Genetic Analyzer (Applied Biosystems).
For fish DNA extraction, fin clips were removed from the ethanol and dried, and the JETQUICK Tissue DNA Spin Kit (GENOMED) was applied following the manufacturer's instructions. The complete mtDNA cytochrome b gene (1,140 bp) was amplified using primers GluF (forward, 5′-AACCACCGTTGTATTCAACTACAA-3′) and ThrR (reverse, 5′-ACCTCCGATCTTCGGATTACAAGACCG-3′) according to Machordom and Doadrio (2001a). The PCR reaction settings, amplification protocol and PCR product purification followed Šanda et al. (2008). The sequencing was carried out by the Macrogen Service Centre (Seoul, South Korea) using the amplification primers.
The new DNA sequences for parasites and hosts obtained during this study were deposited in GenBank (see Tables S1 and S2 for accession numbers).
2.3 Phylogenetic reconstruction
DNA sequences of hosts and parasites were aligned using fast Fourier transform in MAFFT (Katoh, Misawa, Kuma, & Miyata, 2002). The new sequences of Dactylogyrus were trimmed to concur with the length of sequences obtained from GenBank.
Gaps and ambiguously aligned regions were removed from the alignment of Dactylogyrus sequences using GBlocks v. 0.91 (Talavera & Castresana, 2007). The most appropriate DNA evolutionary model was determined using the Bayesian information criterion (BIC) with jModelTest 2.1.10 (Darriba, Taboala, Doallo, & Posada, 2012; Guindon & Gascuel, 2003). Phylogenetic trees were inferred by means of Bayesian inference (BI) and Maximum Likelihood (ML) using MrBayes 3.2 (Ronquist et al., 2012) and RaxML v8.1.X (Stamatakis, 2014), respectively. BI trees were constructed using the Metropolis-coupled Markov chain Monte Carlo algorithm, with two parallel runs of one cold and three hot chains, 107 generations, and trees sampled every 100 generations. 30% of all saved trees were discarded as burn-in after checking that the standard deviation split frequency value fell below 0.01. Convergence was assessed using Tracer v.1.7.1 (Rambaut, Drummond, Xie, Baele, & Suchard, 2018). Posterior probabilities (PP) were calculated as the frequency of samples recovering any particular clade. The clade support for ML trees (bootstrap support, BS) was assessed by 1,000 bootstrap pseudoreplicates.
The phylogenetic reconstruction of the relationship between 49 Dactylogyrus species was based on combined parts of the genes for 18S and 28S rRNA. The resulting phylogram was rooted by Dactylogyrus species from Carassius gibelio (Bloch, 1782) and Cyprinus carpio L., following Šimková et al. (2004). Data were treated as partitioned and the optimal evolutionary model was selected for each marker individually, including the alpha parameter of the gamma distribution (G) accounting for rate heterogeneity across sites and/or the proportion of invariable sites (I). The phylogenetic reconstruction of the relationship between 76 cyprinoid species based on the complete cytochrome b gene was rooted following Mayden et al. (2009), using the outgroup comprising four representatives of the family Cobitidae (Cobitis jadovaensis Mustafić & Mrakovčić, 2008 [KP208162], C. illyrica Freyhof & Stelbrink, 2007 [KJ487484], C. narentana Karaman, 1928 [KP208170] and C. elongata Heckel & Kner, 1858 [EF672382]). Host sequence data were treated as codon partitioned, and optimal evolutionary models were selected independently for each position within the codon, including both gamma distribution and the proportion of invariable sites.
2.4 Cophylogenetic analyses
The tanglegram connecting host and parasite phylogenetic trees via host–parasite associations was built with TreeMap 3.0b (Charleston, 2012). From many existing methods to investigate the congruence between parasite and host phylogenies (de Vienne et al., 2013), a distance-based method and an event-based method were used in the present study. ParaFit (Legendre, Desdevises, & Bazin, 2002), implemented in CopyCat (Meier-Kolthoff, Auch, Huson, & Göker, 2007), was used with patristic distances calculated for each host and parasite phylogeny, and 999 permutations to assess the statistical significance of global and individual coevolutionary links. The event-based analysis was performed with Jane 4.0 (Conow, Fielder, Ovadia, & Libeskind-Hadas, 2010), which allows different costs to be set for each of the five coevolutionary events (i.e., cospeciation, duplication, duplication followed by host switch, loss, and failure to diverge where host speciation is not followed by parasite speciation). Eleven models with different event cost schemes were applied, using 500 generations and a population size of 50 as parameters of the genetic algorithm to assess the influence of each type of evolutionary event. The Jane 4.0 default model, TreeMap default model (Charleston, 1998) and TreeFitter default model (Ronquist, 1995) were included in our analyses following Deng et al. (2013). Each of these default models assumes that cospeciation has the lowest cost (i.e., is the most common evolutionary event). Several additional models were included in the cophylogenetic analyses: TreeFitter models adjusted for host switch and codivergence, respectively; a model with equal weights for coevolutionary events following Mendlová et al. (2012); and five models where each event is alternatively extremely penalized (cost of specific event set to 10 and all others to 1, following Deng et al., 2013). To statistically test whether the global reconstruction cost was significantly lower than expected by chance, 500 randomizations were performed with the use of random parasite trees.
3 RESULTS
3.1 Parasite phylogeny
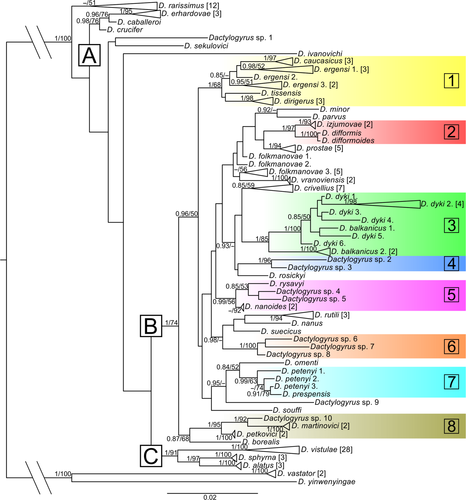
Dactylogyrus parasites were collected from 62 cyprinoid species (Table 1). A total of 49 Dactylogyrus species (Table 2) were identified on the basis of morphological markers (Pugachev et al., 2009). Genetic variability was observed among individuals of Dactylogyrus species collected from multiple host species and, therefore, all genetic variants were included in the final sequence alignment. The final 1,177 base-pair-long alignment of the 49 putative Dactylogyrus species included 138 sequences of partial gene for 18S rRNA combined with partial gene for 28S rRNA (see Supporting Information S3 for alignment). The following optimal evolutionary models were selected: TrNef+I for the 441 bp-long sequence alignment of partial gene for 18S rRNA and TVM+I+G for the 736 bp-long sequence alignment of partial gene for 28S rRNA. BI and ML analyses generated trees with identical topologies (the BI tree is shown in Figure 1). Morphological and molecular data suggested the presence of 10 potentially new species, labelled from Dactylogyrus sp. 1 to Dactylogyrus sp. 10. The phylogenetic reconstruction divided Dactylogyrus species into several groups, of which three were well-supported (A, B and C in Figure 1). The D. rarissimus group, which displayed a high level of intraspecific variability (12 genetic variants), formed a sister group to these three large clades, but this group was not supported (PP = 0.49, BS = 51, respectively). The first clade (group A, PP = 0.98, BS = 76) included D. erhardovae, D. cabelleroi and D. crucifer. These three species are common parasites of Rutilus spp. The second group (group B, PP = 1, BS = 74) comprised the majority of Dactylogyrus species. Within this group, Dactylogyrus species were divided into number of lineages of which eight were moderate to well-supported. Different genetic variants of D. ergensi collected from six host species from three genera clustered with D. dirigerus (a parasite of Chondrostoma spp.), D. caucasicus and D. tissensis (both parasites of Alburnoides spp., lineage 1). All four above-mentioned species share a similar shape of male copulatory organ (see Pugachev et al., 2009 for morphology). Each of the four species D. balkanicus, D. dyki, D. folkmanovae and D. petenyi contains morphologically similar but genetically different individuals (different genetic forms of the given Dactylogyrus species parasitized different host species). However, all different genetic forms of each above-mentioned morphologically identified species did not form monophyletic groups. The well-supported lineage 3 (PP = 1, BS = 100) comprised all genetic variants of D. dyki, a common parasite of Barbus spp. in Europe, but also included individuals of D. balkanicus resulting in the paraphyly of both species. Both Dactylogyrus species from Luciobarbus (Dactylogyrus sp. 2 and Dactylogyrus sp. 3) formed the well-supported lineage 4. Two potentially new species collected from C. knerii and S. tenellus (Dactylogyrus sp. 4 and Dactylogyrus sp. 5, respectively) clustered with D. nanoides from Squalius spp. and D. rysavyi, a known parasite of Alburnoides spp. (but collected only from A. thessalicus in this study). The phylogenetic proximity of the four above-mentioned species (lineage 5) was well-supported by BI, but only weakly by ML (PP = 0.99, BS = 56). Lineage 6 exclusively comprised potentially new Dactylogyrus species collected from Telestes spp. (Dactylogyrus sp. 6, Dactylogyrus sp. 7 and Dactylogyrus sp. 8). The monophyly of D. petenyi was not supported (lineage 7) because D. prespensis clustered with one of the genetic variants of D. petenyi. Lineage 8 within group B was formed by Dactylogyrus species from Pachychilon spp. (PP = 1, BS = 95). The third well-supported group (group C, PP = 1, BS = 91) included D. alatus, D. sphyrna and D. vistulae. All 28 genetic variants of D. vistulae collected from 25 cyprinoid species from seven genera formed a well-supported clade (PP = 1, BS = 100).
| Dactylogyrus species | Host species |
|---|---|
| D. alatus Linstow, 1878 | Alburnus arborella |
| Alburnus neretvae | |
| D. balkanicus Dupont & Lambert, 1986 | Barbus plebejus |
| Barbus prespensis | |
| Barbus rebeli | |
| D. borealis Nybelin, 1937 | Phoxinus sp. |
| D. caballeroi Prost, 1960 | Rutilus ohridanus |
| D. caucasicus Mikailov & Shaova, 1973 | Alburnoides devolli |
| Alburnoides fangfangae | |
| Alburnoides prespensis | |
| D. crivellius Dupont & Lambert, 1986 | Barbus balcanicus |
| Barbus peloponnesius | |
| Barbus plebejus | |
| Barbus prespensis | |
| Barbus rebeli | |
| Barbus sp. | |
| Barbus tyberinus | |
| D. crucifer Wagener, 1857 | Rutilus lacustris |
| D. difformis Wagener, 1857 | Scardinius plotizza |
| D. difformoides Glaeser & Gussev, 1967 | Scardinius plotizza |
| D. dirigerus Gussev, 1966 | Chondrostoma ohridana |
| Chondrostoma vardarense | |
| D. dyki Ergens & Lucky, 1959 | Barbus balcanicus |
| Barbus cyclolepis | |
| Barbus peloponnesius | |
| Barbus prespensis | |
| Barbus rebeli | |
| Barbus sperchiensis | |
| Barbus strumicae | |
| D. ergensi Molnar, 1964 | Chondrostoma knerii |
| Chondrostoma ohridana | |
| Chondrostoma vardarense | |
| Protochondrostoma genei | |
| Squalius lucumonis | |
| Squalius squalus | |
| D. erhardovae Ergens, 1970 | Rutilus aula |
| Rutilus basak | |
| Rutilus ohridanus | |
| D. folkmanovae Ergens,1956 | Squalius sp. |
| Squalius orpheus | |
| Squalius platyceps | |
| Squalius prespensis | |
| Squalius squalus | |
| Squalius vardarensis | |
| D. ivanovichi Ergens, 1970 | Pachychilon pictum |
| D. izjumovae Gussev, 1966 | Scardinius dergle |
| Scardinius plotizza | |
| D. martinovici Ergens, 1970 | Pachychilon pictum |
| D. minor Wagener, 1857 | Alburnus scoranza |
| D. nanoides Gussev, 1966 | Squalius prespensis |
| Squalius squalus | |
| D. nanus Dogiel & Bychowsky, 1934 | Rutilus rubilio |
| D. omenti Benovics et al., 2017 | Aulopyge huegelii |
| D. parvus Wegener, 1910 | Alburnus scoranza |
| D. petenyi Kastak, 1957 | Barbus balcanicus |
| Barbus cyclolepis | |
| Barbus peloponnesius | |
| D. petkovici Ergens, 1970 | Pachychilon pictum |
| D. prespensis Karaman, 1924 | Barbus prespensis |
| D. prostae Molnar, 1964 | Squalius sp. |
| Squalius lucumonis | |
| Squalius pamvoticus | |
| Squalius prespensis | |
| Squalius squalus | |
| D. rarissimus Gussev, 1966 | Alburnus arborella |
| Alburnus neretvae | |
| Pelasgus laconicus | |
| Rutilus basak | |
| Rutilus lacustris | |
| Rutilus ohridanus | |
| Rutilus rubilio | |
| Telestes alfiensis | |
| Telestes dabar | |
| Telestes fontinalis | |
| Telestes metohiensis | |
| D. rosickyi Ergens, 1970 | Pachychilon pictum |
| D. rutili Glaeser, 1965 | Rutilus basak |
| Rutilus lacustris | |
| Rutilus ohridanus | |
| D. rysavyi Ergens, 1970 | Alburnoides thessalicus |
| D. sekulovici Ergens, 1970 | Pachychilon pictum |
| D. soufii Lambert, 1977 | Telestes montenigrinus |
| Dactylogyrus sp. 1 | Delminichthys adspersus |
| Dactylogyrus sp. 2 | Luciobarbus graecus |
| Dactylogyrus sp. 3 | Luciobarbus albanicus |
| Dactylogyrus sp. 4 | Chondrostoma knerii |
| Dactylogyrus sp. 5 | Squalius tenellus |
| Dactylogyrus sp. 6 | Telestes karsticus |
| Dactylogyrus sp. 7 | Telestes muticellus |
| Dactylogyrus sp. 8 | Telestes montenigrinus |
| Dactylogyrus sp. 9 | Tropidophoxinellus spartiaticus |
| Dactylogyrus sp. 10 | Pachychilon macedonicum |
| D. sphyrna Linstow, 1878 | Rutilus basak |
| Rutilus ohridanus | |
| Rutilus rubilio | |
| D. suecicus Nybelin, 1937 | Rutilus lacustris |
| D. tissensis Zachvatkin, 1951 | Alburnoides thessalicus |
| D. vastator Nybelin, 1924 | Aulopyge huegelii |
| Barbus plebejus | |
| D. vistulae Prost, 1957 | Alburnoides ohridanus |
| Alburnoides strymonicus | |
| Alburnoides thessalicus | |
| Chondrostoma ohridana | |
| Chondrostoma phoxinus | |
| Chondrostoma vardarense | |
| Phoxinellus alepidotus | |
| Phoxinellus pseudalepidotus | |
| Protochondrostoma genei | |
| Rutilus rubilio | |
| Squalius illyricus | |
| Squalius lucumonis | |
| Squalius peloponensis | |
| Squalius platyceps | |
| Squalius prespensis | |
| Squalius squalus | |
| Squalius svallize | |
| Squalius tenellus | |
| Squalius vardarensis | |
| Telestes fontinalis | |
| Telestes karsticus | |
| Telestes metohiensis | |
| Telestes montenigrinus | |
| Telestes muticellus | |
| Telestes pleurobipunctatus | |
| D. vranoviensis Ergens, 1956 | Squalius squalus |
| Squalius vardarensis | |
| D. yinwenyingae Gussev, 1962 | Squalius lucumonis |
3.2 Host phylogeny
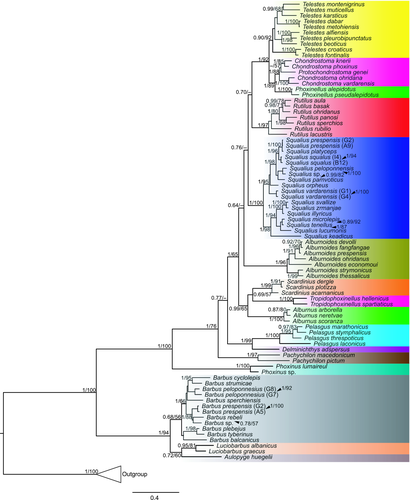
The alignment of complete cytochrome b sequences was used for phylogenetic analyses of cyprinoid hosts. All investigated cyprinoid species were included in the phylogenetic reconstruction. Five species (Barbus peloponnesius, B. prespensis, S. prespensis, S. squalus and S. vardarensis) showed interpopulation variability (each cyprinoid species was collected from two localities). One haplotype from each locality for each of these five species was included in the analyses. Additionally, five species (Alburnus neretvae, Chondrostoma vardarense, Pachychilon pictum, Pelasgus thesproticus and S. tenellus) exhibited no interpopulation variability, even though they were collected from more than one locality, and therefore, only one haplotype from each of these species was included in the analyses, as well as for all other species, which were collected from only one locality. The final alignment contained 85 sequences with 1,140 unambiguous nucleotide positions (see Supporting Information S4 for alignment). GTR+I+G was selected as the best evolutionary model for each position within the codon. Both BI and ML analyses yielded trees with congruent topologies and therefore, only phylogram resulting from BI was used for subsequent analyses (Figure 2). In general, phylogenetic relationships between the respective leuciscid clades (genera) were in congruence with the molecular phylogenies proposed by Perea et al. (2010) and Schönhuth et al. (2018) (e.g., Telestes formed well-supported monophyletic group with Phoxinellus Heckel, 1843 and Chondrostoma s.l. Agassiz, 1832; Delminichthys Freyhof, Lieckfeldt, Bogutskaya, Pitra & Ludwig, 2006 and Pelasgus Kottelat & Freyhof, 2007 formed well-supported group, and Phoxinus Rafinesque, 1820 clade displayed a sister position to other leuciscids). Tribus Barbini (Cyprinidae) formed a strongly supported group in the sister position to the leuciscids. However, the clade of the genus Barbus was only weakly supported by both analyses (PP = 0.68, BS = 56). Using the present data set, A. huegelii appears in sister position to Luciobarbus spp.
3.3 Cophylogeny
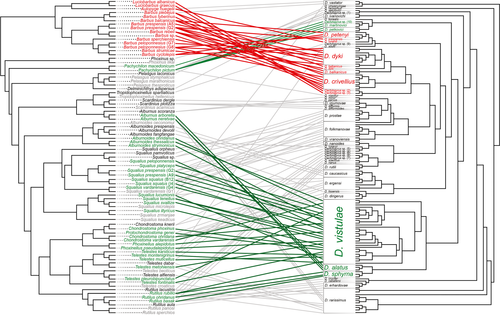
BI phylogenetic reconstructions were used for cophylogenetic analyses (Figure 3). The distance-based analysis using ParaFit yielded a highly significant (p < .001) overall cophylogenetic structure. Out of 138 host–parasite individual links, 65 contributed significantly to the global cophylogenetic structure (p < .05). Significant links (p < .05) were inferred between the representatives of group C (D. alatus, D. sphyrna and D. vistulae or their genetic variants, Figure 1) and their leusiscid host species, and between Dactylogyrus representatives belonging to lineage 8 (D. martinovici, D. petkovici and Dactylogyrus sp. 10) and their Pachychilon hosts. Highly significant individual links (p < .001) were found between representatives of the cyprinid genera Barbus and Luciobarbus and the monotypic Aulopyge and their Dactylogyrus spp. (or genetic forms of these Dactylogyrus): ‘D. balkanicus’, D. crivellius, ‘D. dyki’, ‘D. petenyi’, ‘D. prespensis’ from Barbus, undescribed Dactylogyrus sp. 2 and Dactylogyrus sp. 3 from Luciobarbus spp., and D. omenti from A. huegelii. Subsequent analysis performed using the same number of permutations (999) and focussed only on this group supported the initial significant cophylogenetic structure (p < .05).
Applying different cost schemes, Jane produced reconstructions with similar proportions of coevolutionary events (Table 3). Global costs using each scheme were all statistically significant (p < .01). In general, it appears that Dactylogyrus speciation is primarily driven by duplication followed by host switching, which was an important component in 8 of the 11 models tested. The lowest total cost was produced by the host switch-adjusted TreeFitter model. The duplication-prohibited model and host switch-prohibited model resulted in a high number of loss events and represented the scenarios with the highest total costs (also suggesting the importance of host switching in the evolution of Dactylogyrus). Setting the duplication cost to zero and equalizing the costs of the other events (codivergence adjusted TreeFitter model) or extremely penalizing cospeciation cost (cospeciation-prohibited model) resulted in a higher occurrence of duplication events compared to cospeciation events in contrast to a relatively low occurrence of duplication events within each of the other models. Additionally, no losses were inferred in these models (models 4, 6 and also 9, Table 3). A high number of cospeciations were inferred in models with the cospeciation cost set to zero or in models with a high penalization of duplication, host switching or failure to diverge. A low occurrence of duplication events was found either when cospeciation was not penalized (TreeMap default model), or when failure to diverge or duplication were highly penalized (FTD prohibitive model and duplication prohibitive models, respectively). In the latter model, a remarkably high number of losses were inferred (such as in the case of the host switch-prohibited model).
| Model | Event costs | Total cost | Cospeciation | Duplication | Duplication and Host switch | Loss | Failure to diverge |
|---|---|---|---|---|---|---|---|
| Jane default† | 0 1 2 1 1 | 220 | 58 | 15 | 64 | 77 | – |
| TreeMap default† | 0 1 1 1 1 | 120 | 46 | 7 | 84 | 29 | – |
| TreeFitter default† | 0 0 2 1 1 | 183 | 39 | 18 | 80 | 23 | – |
| Codivergence adjusted TreeFitter model† | 1 0 1 1 1 | 116 | – | 21 | 116 | – | – |
| Host switch-adjusted TreeFitter model† | 0 0 1 1 1 | 100 | 29 | 14 | 94 | 6 | – |
| Cospeciation prohibitive† | 10 1 1 1 1 | 137 | – | 21 | 116 | – | – |
| Duplication prohibitive† | 1 10 1 1 1 | 399 | 72 | 10 | 55 | 172 | – |
| Host switch prohibited† | 1 1 10 1 1 | 588 | 70 | 56 | 11 | 352 | – |
| Sorting prohibited† | 1 1 1 10 1 | 137 | 18 | 17 | 102 | – | – |
| FTD prohibitive† | 1 1 1 1 10 | 142 | 28 | 14 | 95 | 5 | – |
| Equal weights† | 1 1 1 1 1 | 144 | 30 | 14 | 93 | 7 | – |
Note
- Total costs represent the sum of inferred numbers of each evolutionary event multiplied by their respective costs. Values in columns represent frequency of the specific evolutionary event in the reconstruction resulting from applied scheme. Statistically significant scenarios are marked by cross symbol (†). Dashes (–) represent null values.
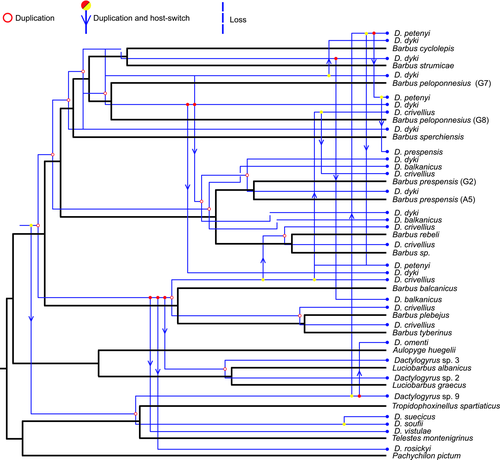
Applying the same cost schemes with the same number of generations and population size on a selected subgroup of cyprinids belonging to the Barbini tribe and their respective specific Dactylogyrus spp., between which a strong cophylogenetic signal was initially detected, resulted in only five schemes yielding cophylogenetic scenarios with statistically significant global costs (tested on 500 randomizations, Table 4). Three of these five models (schemes 1, 3 and 6) were set to expect duplication followed by host switching as the least probable coevolutionary event simulating the allopatric speciation of hosts where the host switching of parasites between new lineages is unlikely (an example of a cophylogenetic scenario from this subsequent data set is presented in Figure 4). Nevertheless, in the majority of scenarios, duplication followed by host switching was the most common coevolutionary event. This event was omitted only in the case of its extremely high penalization, modelling the scenario where physical contact between congeneric host species should be completely excluded. Equalizing all event costs, or highly penalizing other coevolutionary events when compared to duplication followed by host switching resulted in the same proportions of coevolutionary events. However, the results of all models with these cost schemes were not statistically significant.
| Model | Event costs | Total cost | Cospeciation | Duplication | Duplication and Host switch | Loss | Failure to diverge |
|---|---|---|---|---|---|---|---|
| Jane default† | 0 1 2 1 1 | 37 | 13 | 1 | 16 | 4 | – |
| TreeMap default† | 0 1 1 1 1 | 21 | 12 | 1 | 17 | 3 | – |
| TreeFitter default† | 0 0 2 1 1 | 36 | 13 | 2 | 15 | 6 | – |
| Codivergence adjusted TreeFitter model | 1 0 1 1 1 | 26 | – | 4 | 26 | – | – |
| Host switch-adjusted TreeFitter model† | 0 0 1 1 1 | 20 | 12 | 1 | 17 | 3 | – |
| Cospeciation prohibitive | 10 1 1 1 1 | 30 | – | 2 | 28 | – | – |
| Duplication prohibitive | 1 10 1 1 1 | 30 | – | 2 | 28 | – | – |
| Host switch prohibited† | 1 1 10 1 1 | 90 | 17 | 13 | – | 60 | – |
| Sorting prohibited | 1 1 1 10 1 | 30 | – | 2 | 28 | – | – |
| FTD prohibitive | 1 1 1 1 10 | 30 | – | 2 | 28 | – | – |
| Equal weights | 1 1 1 1 1 | 30 | – | 2 | 28 | – | – |
Note
- Total costs represent the sum of inferred numbers of each evolutionary event multiplied by their respective costs. Values in columns represent frequency of the specific evolutionary event in the reconstruction resulting from applied scheme. Statistically significant scenarios are marked by cross symbol (†). Dashes (–) represent null values.
4 DISCUSSION
4.1 Phylogeny of Dactylogyrus
Following the former phylogenetic study by Benovics et al. (2018) focussed on 53 Dactylogyrus species parasitizing endemic cyprinoids in the Balkans, this work is the first wide-ranging study focussing on the cophylogenetic relationships between endemic cyprinoids of the north-eastern European peri-Mediterranean and their specific parasites. In the present study, a large data set of 76 endemic cyprinoid species covering 95% of the known cyprinoid diversity of the whole north-eastern European peri-Mediterranean region (Balkan and Apennine Peninsulas) was used. A total of 49 morphologically identified Dactylogyrus species were recognized, representing 139 genetic variants. In the majority of host–parasite associations, Dactylogyrus species were specific to a single cyprinoid species or to a group of congeneric cyprinoids. For many Dactylogyrus species parasitizing several cyprinoid species, that is generalists, different genetic variants of morphologically identical Dactylogyrus species were observed. In the majority of cases, even these genetic variants exhibited host specificity—unique genetic variant was found in a single host species.
The phylogenetic position of D. rarissimus is in congruence with the findings of Benovics et al. (2018), where this species represented a sister group to other Dactylogyrus species from leuciscids. However, the monophyly of this taxon was only weakly supported by ML analysis and unsupported by BI. In contrast to the previous study by Benovics et al. (2018), our results suggest the monophyly of three Dactylogyrus species common to Rutilus spp. (D. caballeroi, D. crucifer and D. erhardovae, group A). The monophyly of the former two species was also suggested by Šimková et al. (2004).
Group B, recognized from phylogenetic reconstruction, contained several well-to-moderately supported clades. However, several Dactylogyrus species, formerly recognized on the basis of morphology, were not phylogenetically supported as monophyletic. These species include D. ergensi, D. folkmanovae, D. dyki, D. balkanicus and D. petenyi. The monophyly of D. ergensi was not supported, as D. caucasicus collected from Alburnoides spp. was included in a well-supported group comprising all D. ergensi individuals. However, two well-supported groups that follow the biogeographical distribution of leuciscid hosts were formed by D. ergensi individuals (Figure 1). Dactylogyrus ergensi lineage 1, a sister group to D. caucasicus, included individuals found on Protochondrostoma genei, S. lucumonis and S. squalus, all leuciscid species native to the central/northern Adriatic and neighbouring Tyrrhenian ichthyogeographic districts (Bianco, 1990). The other clade, D. ergensi lineage 3, contained the genetic forms of D. ergensi collected from C. ohridana and C. vardarense, both endemic to the southern Balkans, specifically to the Albanian and north-eastern Aegan ichthyogeographic districts (Kottelat & Freyhof, 2007). The present data suggest that D. ergensi encompasses several species. In fact, the morphometric variability in the shape and size of the male copulatory organ of D. ergensi from the Chondrostoma spp. in different regions of Europe was reported in its original description by Gussev (1966). Later, Lambert (1977) proposed the splitting of D. ergensi by separating D. toxostomi (parasitizing Parachondrostoma toxostoma (Vallot, 1837)), but its taxonomic status was not considered valid since measurements of the sclerotized parts of the attachment organ and male copulatory organ overlapped with D. ergensi individuals (Pugachev et al., 2009). Therefore, on the basis of the present molecular data, we can conclude that D. ergensi, originally described as a parasite of Chondrostoma spp. (although its presence was also documented on Squalius spp. in the Apennines), is in fact a species complex. Our results also suggest that D. caucasicus evolved from D. ergensi by host switching to the phylogenetically distant Alburnoides Jeitteles, 1861 species (Perea et al., 2010; Schönhuth et al., 2018), moreover both of these Dactylogyrus species have a similar shape with respect to the male copulatory organs (Pugachev et al., 2009).
The previous phylogenetic reconstruction of Dactylogyrus performed by Šimková et al. (2004) was focussed on the species parasitizing central European cyprinoids. Our study confirmed most of the phylogenetic relationships between Dactylogyrus species previously suggested in their study. For example, the sister species D. minor and D. parvus parasitizing A. alburnus L. in Central Europe were also found on A. scoranza in the Balkans. Dactylogyrus izjumovae, D. difformis and D. difformoides, all parasites of Scardinius erythrophthalmus L. in Central Europe, formed a monophyletic group also reported in the phylogenetic reconstruction of Dactylogyrus parasitizing endemic Balkan leuciscids, more specifically S. plotizza and S. dergle. Congruency was also reported in the sister position of D. prostae of the clade formed by Dactylogyrus from Scardinius Bonaparte, 1837. The present results suggest that D. nanoides is phylogenetically closer to the new Dactylogyrus species from Chondrostoma knerii and S. tenellus (Dactylogyrus sp. 4 and sp. 5 respectively) and to D. rysavi rather than to D. folkmanovae (as was shown in the phylogenetic reconstruction of Dactylogyrus parasitizing Central European cyprinoids by Šimková et al., 2004). However, D. folkmanovae collected from seven Squalius species appears to be paraphyletic, as its representatives clustered with other Dactylogyrus from leuciscids including D. prostae and D. vranoviensis parasitizing Squalius, which also suggests the existence of a D. folkmanovae morphotype species complex. The phylogenetic position of D. borealis is very interesting, as this species is host-specific only for representatives of the genus Phoxinus in the Balkans and Central Europe. According to Šimková et al. (2004), D. borealis is phylogenetically proximal to D. amphibothrium Wagener, 1857 and D. hemiamphibothrium Ergens, 1956, both parasitizing Gymnocephalus cernuus L. (Percidae) in the Czech Republic. However, considering only Dactylogyrus of cyprinoids (more specifically only leuciscids in our study), D. borealis clusters together with Dactylogyrus spp. of Pachychilon Steindachner, 1882 (Dactylogyrus lineage 8), which is endemic in the Balkans and represents the ancient leuciscid lineage in this region.
The high molecular diversity among Dactylogyrus individuals collected from three Telestes species (T. karsticus, T. muticellus and T. montenigrinus) suggests the existence of three unknown Dactylogyrus species (Dactylogyrus sp. 6, sp. 7 and sp. 8 respectively, representing Dactylogyrus lineage 6). Extrapolating from the branch lengths and molecular similarity, we can postulate that these species diverged probably by cospeciation with the Telestes genus (see phylogeny in Buj et al., 2017). On the basis of the shape and size of sclerotized elements of the haptor and copulatory organs, these three potentially new species greatly resemble D. nanus and D. suecicus, belonging together with D. rutili to the clade which is sister to the clade including three new Dactylogyrus species parasitizing Telestes. Dactylogyrus nanus, D. rutili and D. suecicus are common parasites of Rutilus, the leuciscid species which is phylogenetically related to Telestes (Perea et al., 2010; Schönhuth et al., 2018, and also supported by our results, see below).
The group C, also recognized in previous phylogenetic studies (Benovics et al., 2018; Šimková et al., 2004), was strongly supported in the present study. It comprises D. alatus, D. sphyrna and D. vistulae, which all possess large haptoral anchor hooks (‘sphyrna’ morphotype) and miss a ventral connective bar except for D. alatus, which has a thin ‘phoxini’ type ventral connective bar (Pugachev et al., 2009). Šimková et al. (2004) also suggested that Dactylogyrus similis Wagener, 1909, morphologically close to D. sphyrna and D. vistulae, is included in this group, but this species was not found on endemic cyprinoids of the north-eastern peri-Mediterranean region. While D. alatus and D. sphyrna were collected from two Alburnus Rafinesque, 1820 and three Rutilus Rafinesque, 1820 species, D. vistulae used a wide range of host species representing different genera and exhibiting a wide biogeographical distribution. However, the true origin of this generalist species is unknown and to investigate it we suggest that the representatives from Central European cyprinoids (e.g., Squalius cephalus L. or Chondrostoma nasus L.), in which molecular variability was also observed (Šimková et al., 2004), should be included in future studies, based on population genetic markers (necessary to be developed).
4.2 Phylogeny of Cyprinoidea
The phylogenetic reconstruction of the north-eastern peri-Mediterranean leuciscids obtained in this study is in general agreement with the molecular phylogenies proposed by Perea et al. (2010) and Schönhuth et al. (2018). Observed differences in the resulting generic phylogenies are most probably due to different taxon sampling, limited in the case of our study to the Balkan and Apennine representatives, and in comparison with Schönhuth et al. (2018) also in different markers used (multilocus study). Basically, all genera were resolved in our study as monophyletic, with exception of Chondrostoma, which in our study include Protochondrostoma. This is the most probably a result of limited taxon sampling in our study. Genus Protochondrostoma was defined by Robalo, Almada, Levy, and Doadrio (2007), together with Achondrostoma, Iberochondrostoma, Parachondrostoma and Pseudochondrostoma (all from Iberian peninsula), which are not included in our phylogenetic reconstruction.
Our study supports the phylogenetic grouping of Alburnus, Scardinius and Tropidophoxinellus, which was previously hypothesized (Briolay, Galtier, Brito, & Bouvet, 1998; Brito, Briolay, Galtier, Bouvet, & Coelho, 1997; Perea et al., 2010; Zardoya & Doadrio, 1999). Interestingly, all three genera harbour Dactylogyrus from different evolutionary lineages. While Alburnus spp. are parasitized by D. alatus, D. minor, D. parvus and D. rarissimus (the last is a common species on Rutilus spp. and Telestes spp. and rare on Pelasgus spp.), Scardinius and Tropidophoxinellus harbour host-specific Dactylogyrus spp. (D. difformis, D. difformoides, D. izjumovae and Dactylogyrus sp. 9). The phylogenetic relationships within the Alburnoides clade follow the biogeographical distribution of Alburnoides species: a clade formed by A. ohridanus, A. prespensis, A. devolli and A. fangfangae comprises species distributed in the Albanian ichthyogeograpical district (Kottelat & Freyhof, 2007), and a second clade is formed by A. strymonicus and A. thessalicus from the Aegan district.
Regarding the cyprinids, in our phylogenetic reconstruction, the genus Barbus was supported only weakly, however, it formed a monophyletic clade. In the present study, A. huegelii seems to be phylogenetically closer to the Luciobarbus clade, although this relationship is only moderately supported. The phylogenetic position of A. huegelii appears generally uncertain. Yang et al. (2015) suggested that A. huegelii occupied the sister position to Barbus lineage, while Gante (2011) showed its sister position to clade comprising both Barbus and Luciobarbus genera.
4.3 Cophylogenetic host–parasite relationships
In spite of their direct life cycle and narrow host specificity, previous cophylogenetic studies of monogeneans and their fish hosts suggested that cospeciation is a rare event, much less common than host switching and intra-host speciation (Desdevises et al., 2002; Huyse et al., 2003; Mendlová et al., 2012; Messu Mandeng et al., 2015; Šimková et al., 2004, 2013; Zietara & Lumme, 2002).
It has been hypothesized that during evolutionary time monogeneans developed very specialized haptors specifically to attach to (generally one) well-defined host species (Jarkovský, Morand, Šimková, & Gelnar, 2004; Sasal, Trouvé, Müller-Graf, & Morand, 1999; Šimková, Desdevises, Gelnar, & Morand, 2001). For example, Šimková et al. (2001) found a positive correlation between the size of Dactylogyrus anchor hooks and the size of their host species. Such highly adapted attachment organs would make the switch to a different host species very difficult, and even unlikely (but that may depend on the intraspecific variability of the sclerified pieces in this organ, see Kaci-Chaouch, Verneau, & Desdevises, 2008). However, some Dactylogyrus species, such as D. vistulae, parasitize phylogenetically distant hosts, from small-sized (e.g., Alburnoides spp. or Phoxinellus spp.) to large-sized species (e.g., Chondrostoma spp. or Squalius spp.), displaying only minor morphological variability in their haptoral sclerites (M. Benovics, unpublished data). This species clusters among the largest Dactylogyrus species (see Pugachev et al., 2009 for morphology), exhibiting also large anchor hooks, which suggests that monogenean species developing large attachment structures as an adaptation to large-sized hosts can host switch to smaller-size hosts.
According to our results, host switching clearly appears to be the main coevolutionary event inferred from the cophylogenetic reconstructions of Dactylogyrus and their hosts, followed by cospeciation (Table 3). Host switches likely result here from the sympatric distribution of phylogenetically distant cyprinoid species linked to the historical shift of the landmass and/or from the more recent human-induced introduction of non-native cyprinoid species into the Balkans and Apennines. In the present study, intra-host speciation (i.e., duplication) is suggested to be a rather rare coevolutionary event. This is in contrast to previous cophylogenetic studies on dactylogyrids, where intra-host duplication was the most commonly inferred coevolutionary event (e.g., Dactylogyrus by Šimková et al., 2004, Cichlidogyrus and Scutogyrus on cichlids by Mendlová et al., 2012, or Thaparocleidus on pangasiids by Šimková et al., 2013). This may be explained by the fact that these studies included either a limited number of host species from the investigated area or a high number of representatives from phylogenetically distant host species where host switching was highly improbable, in contrast to our study where highly diversified groups of phylogenetically close and/or sympatric cyprinoid species were included. This suggests that host switching is the primary cause of speciation in Dactylogyrus, followed by intra-host speciation only if host switching is not possible due to geographical isolation or phylogenetic divergence (then presenting too large differences in parasites’ microhabitat) among fish species living in sympatry.
In the present study, a statistically significant overall cophylogenetic structure was inferred among Dactylogyrus and their Cyprinoidea hosts. The significant global fit computed with ParaFit relies on 47% significant individual host–parasite links. Among these individual associations, the most significant were found between cyprinids of the Barbini tribe and their Dactylogyrus spp. All these Dactylogyrus species are genus-specific and their phylogenetic relationships followed the evolutionary history of barbels. However, this Dactylogyrus group is potentially subjected to cospeciation, as suggested in testing different cost schemes and reconstructing scenarios from phylogenetic trees topologies. Cophylogenetic analyses considering only fish in Barbini and their Dactylogyrus species confirmed this significant cophylogenetic structure and suggested scenarios strongly implying duplication events in the evolutionary history of Dactylogyrus from Barbini. This intimate coevolutionary history between ‘barbels’ and their specific Dactylogyrus lineages could be related to the fact that Barbini belong to another group, Cyprinidae (Machordom & Doadrio, 2001b; Schönhuth et al., 2018; Yang et al., 2015). We can hypothesize that during evolution several Dactylogyrus species (i.e., D. balkanicus, D. dyki, D. crivellius) specialized on barbels, as is supported also by their specific distribution on European Barbus and the strong cophylogenetic structure between Dactylogyrus and Barbini in the Balkan and Apennine Peninsulas (Figure 4). However, two species, D. petenyi and D. prespensis (representatives of Dactylogyrus lineage 7 in our phylogenetic reconstruction), likely colonized their host via a recent host switching from phylogenetically distant cyprinoid taxa, followed by fast speciation on endemic barbels.
In addition to D. vistulae, a strong cophylogenetic signal was also inferred between D. alatus and D. sphyrna, each with their respective hosts. In central Europe, these two species parasitize hosts from two or more leuciscid genera (Moravec, 2001), while in southern European peninsulas, they use only Alburnus spp. and Rutilus spp., respectively. Frequent host switching in the evolutionary history of these Dactylogyrus species, inferred by the event-based analyses in Jane, suggest that these species originally parasitized Alburnus and Rutilus, and subsequently switched to other leuciscid genera in central Europe.
The cophylogenetic history of Pachychilon and their Dactylogyrus parasites reconstructed in this study is noteworthy. Despite the fact that all Dactylogyrus species are genus or species-specific, they in this case do not form a monophyletic group. Three of the six Dactylogyrus species from Pachychilon spp. found in this study formed a clade within group B (lineage 8), and a strong cophylogenetic signal was observed exclusively between these species and their representative Pachychilon hosts. This suggests that D. petkovici and the common ancestor of D. martinovici and Dactylogyrus sp. 10 originated from an intra-host duplication during the evolutionary history of Pachychilon, and that Dactylogyrus sp. 10 with D. martinovici originated from cospeciation during the divergence of Pachychilon species. Additionally, D. rosickyi is phylogenetically close to Dactylogyrus species from Barbus spp., which suggests a more recent host switch of parasites between these phylogenetically distant cyprinoid taxa. Dactylogyrus rosickyi was collected only from P. pictum in the Aoos River (north-western Greece, a tributary of the Adriatic Sea), where the occurrence of Barbus species (B. prespensis) was also documented, and this Dactylogyrus species was not present on P. pictum in Lake Ohrid. Dactylogyrus rosickyi was originally described by Ergens (1970) from Lake Skadar. Both lakes are part of Ohrid-Drin-Skadar system. This system potentially represents the area within a range of D. rosickyi where the initial transfer between ancestral Barbus lineages and Pachychilon spp. took place.
ACKNOWLEDGEMENTS
We are grateful to Kateřina Čermáková, Jaroslav Červenka, Milan Gelnar, Kristýna Hejlová, Maria Lujza Červenka Kičinja, Tomáš Pakosta, Eva Řehulková and Petra Zahradníčková for their help with fish dissection and parasite collection. Moreover, we are thankful to Stefano Porcelloti, Stamatis Zogaris, Spase Shumka, Denik Ulqini, Dario Marić, Ivan Bogut, Ivana Buj and Zoran Marčić for arranging permissions and help with the field work. We kindly thank Matthew Nicholls for English revision of the final draft. Computational resources were supplied by the Ministry of Education, Youth and Sports of the Czech Republic under the Projects CESNET (Project No. LM2015042) and CERIT-Scientific Cloud (Project No. LM2015085) provided within the program Projects of Large Research, Development and Innovations Infrastructures. This study was funded by the Czech Science Foundation (project number 15-1938S).
Open Research
DATA AVAILABILITY STATEMENT
All new sequences of Dactylogyrus obtained during this study were deposited in NCBI GenBank under accession numbers MK434927–MK434965, MK455795 and MK455801. New sequences of cyprinoid species obtained during this study were deposited in NCBI GenBank under accession numbers MK482020–MK482050 (Table S1). Appropriate accession numbers according to Dactylogyrus species and specific genes coding rRNA regions are presented in Table S2. Since whole fish specimens were completely processed during parasitological dissection, additional specimens of each analysed host species were collected from the same locality and fish vouchers were deposited in the ichthyological collection of the National Museum in Prague (Czech Republic). Voucher specimens of the sequenced Dactylogyrus species (excluding undescribed species) are deposited in the Finnish Museum of Natural History in Helsinki.




