Evolutionary history of the European free-tailed bat, a tropical affinity species spanning across the Mediterranean Basin
Funding information
This work was funded by Fundação para Ciência e Tecnologia (FCT) [project LTER/BIA-BEC/0004/2009] and EDP—Energias de Portugal Biodiversity Chair. FCT also supported F. Amorim [grant PD/BD/52606/2014], V.Mata [grant PD/BD/113462/2015] and H. Rebelo [IF/00497/2013]. O. Razgour was funded through a Natural Environment Research Council (NERC) [Independent Research Fellowship NE/M018660/1].
Abstract
The Mediterranean Basin is a global biodiversity hotspot, hosting a number of native species belonging to families that are found almost exclusively in tropical climates. Yet, whether or not these taxa were able to survive in the Mediterranean region during the Quaternary climatic oscillations remains unknown. Focusing on the European free-tailed bat (Tadarida teniotis), we aimed to (a) identify potential ancient populations and glacial refugia; (b) determine the post-glacial colonization routes across the Mediterranean; and (c) evaluate current population structure and demography. Mitochondrial and nuclear markers were used to understand T. teniotis evolutionary and demographic history. We show that T. teniotis is likely restricted to the Western Palearctic, with mitochondrial phylogeny suggesting a split between an Anatolian/Middle East clade and a European clade. Nuclear data pointed to three genetic populations, one of which is an isolated and highly differentiated group in the Canary Islands, another distributed across Iberia, Morocco, and France, and a third stretching from Italy to the east, with admixture following a pattern of isolation by distance. Evolutionary and demographic reconstruction supports a pre-Last Glacial Maximum (LGM) colonization of Italy and the Anatolian/Middle East, while the remaining populations were colonized from Italy after the Younger Dryas. We also found support for demographic expansion following the Iberian colonization. The results show that during the LGM T. teniotis persisted in Mediterranean refugia and has subsequently expanded to its current circum-Mediterranean range. Our findings raise questions regarding the physiological and ecological traits that enabled species with tropical affinities to survive in colder climates.
1 INTRODUCTION
The Mediterranean Basin is a global biodiversity hotspot (Blondel, Aronson, Bodiou, & Boeuf, 2010; Myers, Mittermeier, Mittermeier, da Fonseca, & Kent, 2000). Despite being presently located in temperate latitudes, this region was mainly covered by tropical climates during the Tertiary (Blondel & Mourer-Chauviré, 1998). Nowadays, Europe still hosts a number of members belonging to several vertebrate groups that are almost exclusively associated with the tropics (defined here as tropical affinities), including reptiles such as geckos and chameleons, and birds such as rollers and bee-eaters (Ammerman, Lee, & Tipps, 2012; Blondel & Mourer-Chauviré, 1998; Carranza & Arnold, 2006; Townsend & Larson, 2002). However, the diversity of tropical species present in Europe is lower than that of other Holarctic areas like North America or eastern Asia (Blondel & Mourer-Chauviré, 1998). The reason for such pattern is that both North America and eastern Asia remained connected to the tropics over the whole Tertiary–Quaternary. In contrast, large geographical barriers (mountain ranges, seas, and desert-belts) prevented the Palearctic tropical biota from expanding their range to tropical regions further south during glacial periods, and tropical species from colonizing northern regions during interglacial periods (Blondel & Mourer-Chauviré, 1998). Altogether, these led to a progressive decline of the tropical species during the Pleistocene (Blondel et al., 2010). Under such circumstances, it is remarkable that some of these species were able to persist in the western Palaearctic, although mostly restricted to the circum-Mediterranean area. The population history of such lineages during periods of glaciation is poorly understood, and it is not known whether these taxa were able to survive in the Mediterranean region during the climatic oscillations of the Quaternary.
Among non-flying mammals, only a small number of species in the western Palaearctic have tropical affinities (Dobson, 1998). Although in some cases, this was the result of a long-standing human-mediated introductions across the Strait of Gibraltar, in others, such as the Egyptian mongoose (Herpestes ichneumon), and this was the result of natural dispersal into the Iberian Peninsula during the Late Pleistocene (Gaubert et al., 2011). In bats, which are likely to be able to disperse over greater distances, there are a higher number of species shared between north-west Africa and Iberia (Dobson, 1998; García-Mudarra, Ibáñez, & Juste, 2009), but even for these mammals the number of species with tropical affinities occurring in temperate regions is relatively low. The European free-tailed bat (Tadarida teniotis Rafinesque, 1814) is the only European representative of the Molossidae family that comprises more than 110 species (Ammerman et al., 2012). All the remaining molossids are restricted to tropical regions, apart from the Mexican free-tailed bat (Tadarida brasiliensis) and the big free-tailed bat (Nyctinomops macrotis), which reach similar Northern latitudes in the American continent. Molossidae is an ancient bat family that split into Old and New World molossids ca. 29 million years ago (Ammerman et al., 2012), and fossil records of the genus Tadarida in Europe date from the late Eocene ca. 25 million years ago (De Bonis et al., 1973).
Understanding phylogeographic patterns shaping the distributions and expansion of species is a powerful tool for predicting how future climatic changes will shape regional biodiversity (Hickerson et al., 2010). During the Quaternary ice ages, Europe experienced dramatic climatic fluctuations between glacial and interglacial cycles contributing to the contemporary distribution and genetic composition of biodiversity (Hewitt, 2000). The distributions of many animal species have been severely restricted to refugia to escape the harsh conditions of the glacial periods. The Last Glacial Maximum (LGM 18–20 ka BP), and the Younger Dryas (11.7–12.9 ka BP), correspond to the latest episodes where the ice sheets and cold temperatures reached their extremes. The Mediterranean region encompasses a high habitat diversity combined with topographic and geographic variability. Together with a dynamic palaeogeographic and climatic history, these features contributed to marked environmental gradients (Blondel et al., 2010), strongly shaping current species and biodiversity spatial patterns, population structure and demography (Hewitt, 1999). Despite the increasing number of studies focusing on the phylogeography of species native to temperate environments, to the best of our knowledge, representatives from tropical families living in such environments have been seldom studied (but see Paulo, Pinto, Bruford, Jordan, & Nichols, 2002; Rato, Carranza, & Harris, 2011).
The European free-tailed bat is widespread throughout the Mediterranean and occurs in a variety of environments and habitats from the colder Alps to the border of the Sahara desert (Amorim, Jorge, Beja, & Rebelo, 2018; Arlettaz et al., 2000; Bendjeddou, Bakhouche, & Bouslama, 2014). However, during the Late Glacial Maximum (LGM), large parts of Europe had colder and drier habitats (Frenzel, Pécsi, & Velichko, 1992) with warmest month temperature being 10°C cooler than present, and coldest month temperature 20°C colder (Kageyama et al., 2006). These harsh conditions were likely unsuitable for most bat species (Bilgin et al., 2016; Kerth et al., 2008; Razgour et al., 2013; Rossiter, Benda, Dietz, Zhang, & Jones, 2007), thus raising the question of how species with tropical affinities were able to survive. Here we focus on the evolutionary history of T. teniotis, which belongs to a taxonomical family almost exclusively associated with the tropics and shows shorter duration of torpor bouts, and higher minimal body temperature in torpor than other temperate bats (Arlettaz et al., 2000). The high mobility and fast flight of these bats (Mata et al., 2016; McCracken et al., 2008) allows them to respond fast to environmental changes by shifting to more suitable areas. These features render T. teniotis a suitable model species to understand how species with topical affinity reacted to the climatic oscillations of the Quaternary in temperate and subtropical regions. Therefore, our main aims were to (a) identify the location of potential ancient populations and glacial refugia; (b) determine the post-glacial colonization routes across the Mediterranean; and (c) evaluate current population structure and demography in light of the post-glacial colonization history.
2 METHODS
2.1 Sample collection
A total of 154 genetic samples collected across the Western and Central Palearctic were obtained from researchers and museum collections. Samples spanned the entire range although coverage was uneven with few samples available from some regions, particularly from Asia, Eastern Mediterranean, and North Africa. For a complete list of samples, origin, and providers see Appendix 1 (GenBank accession numbers MK817165 to MK817272).
2.2 DNA extraction
Due to the different nature of the samples obtained (old museum specimens and recently collected wing tissue), we used different DNA extraction methods. For older museum specimens, we followed the ancient DNA extraction protocol described in Rohland and Hofreiter, (2007) with modifications described in Dabney et al. (2013). For recent tissue samples, we used DNA Micro Kits (Qiagen) following the manufacturer's instructions.
2.3 Validation of species identity and mitochondrial genotyping
Given the poorly resolved taxonomic status of Tadarida teniotis (Mata, Amorim, Guillén-Servent, Beja, & Rebelo, 2017), the identity of all samples was verified using mitochondrial markers prior to microsatellite genotyping. Due to taxonomic uncertainties (Mata et al., 2017), verification was considered to be especially important for putative T. teniotis samples obtained from the eastern part of the distribution (Kyrgyzstan and China). Additionally, samples from Laos previously identified as T. latouchei were also checked.
Four mitochondrial primer pairs were specifically designed using Geneious v9.1.7 (http://www.geneious.com, Kearse et al., 2012) based on an alignment of 37 mitogenomes covering the species range. The primers were designed to amplify the most variable regions of the mitogenomes (Supporting Information Table S1) and corresponded to three coding regions (COI—cytochrome c oxidase subunit I, ATP6—ATP synthase subunit 6, and CytB—cytochrome b) and one non-coding region (D-loop). While designing the primers took extra precautions and carefully examined the mitogenomic data to avoid the amplification of nuclear copies covering almost the entire mitogenome. We did this by comparing the sequences containing nuclear copies (identified by the high prevalence of stop codons) to those without nuclear copies and selecting the regions that did not amplify nuclear copies. This way the primers designed assure that only the mitochondrial haplotype was amplified, allowing the genotyping of samples through Sanger sequencing. For highly degraded museum samples that did not amplify using the regular primers, we further developed internal primers for the COI (COI-mini) and D-loop (D-loop-mini) regions targeting key SNPs that enable to differentiate T. teniotis and its different haplogroups from T. latouchei (Supporting Information Table S1).
The PCR reactions were carried in volumes of 10 µl, comprising of 5 µl of Multiplex PCR Master Mix (qiagen), with 0.4 µl of each 10 µM primer, and 1 µl of DNA extract. Cycling conditions for COI, ATP6, CytB, and D-loop used initial denaturing at 95°C for 15 min, followed by 40 cycles of denaturing at 94°C for 30 s, annealing at 59°C for 45 s and extension at 72°C for 45 s, with a final extension at 72°C for 10 min. For COI-mini and D-loop-mini, the cycling conditions were the same except the annealing temperature that was 52ºC and the number of cycles was increased to 45. Successful amplifications were enzymatically purified, sequenced following the BigDye Terminator v3.1 Cycle sequencing protocol (Applied Biosystems), and sequencing products were separated using an automated Sequencer ABI3130xl Genetic Analyzer. Sequences were aligned and compared in the software seqscape 3.0 (Applied Biosystems).
2.4 Microsatellite genotyping
A custom microsatellite library was developed through 454 GS-FLX Titanium pyrosequencing of enriched DNA libraries based on 12 individuals along the distribution range of T. teniotis (Malausa et al., 2011). This process was developed by GenoScreen (http://www.pasteur-lille.fr/fr/recherche/plateformes/tordeux_plat.html) and included sequence data quality control, assembly and analyses, and primer design.
From the 159 candidate microsatellite loci, we selected 26 microsatellites with different numbers of repeat units, compatible allelic ranges and melting temperatures for multiplexing. We first tested the genotyping performance on four T. teniotis samples and discarded microsatellites that (a) showed no amplification, (b) had multiple bands, and (c) had excessive slippage (many stutter bands). Those remaining were combined into two multiplex panels according to their allele size range and compatibility among primers, which was checked using Auto-Dimer (Vallone & Butler, 2004).
The optimization of PCR conditions for multiplex loci and polymorphism detection was performed using 16 samples. From the 26 loci initially checked, a total of 12 di- and 2 tetra-nucleotides polymorphic markers (with more than two alleles) were selected and genotyped for 129 individuals in two multiplex panels with seven markers each. PCR fragments were fluorescent labeled following Schuelke (2000) but with FAM, VIC, NED, and PET dyes. A pig tail (GTTT) was added to the 5′ end of the primer reverse in order to reduce stutter and drive the reaction to the “plusA” band (Brownstein, Carpten, & Smith, 1996). For additional details on microsatellite primers, see Table S2 (Supporting Information).
PCR amplifications were conducted as for mitochondrial fragments except that 1 µl of primer mix was used per reaction. The PCR cycling profile was divided in four main steps: denaturation at 95°C for 15 min; 13 cycles with denaturation at 95°C for 30 s, annealing at 58°C for 90 s with a touchdown of 0.5°C per cycle, and extension at 72°C for 45 s; 27 cycles with denaturation at 95°C for 30 s, annealing at 52°C for 60 s, and extension at 72°C for 45 s; and a final extension at 60°C for 30 min. PCR products were later separated by capillary electrophoresis on the same automatic sequencer ABI3130xl Genetic Analyzer (AB Applied Biosystems). Fragments were scored using genemapper v4.0 (Applied Biosystems) and checked independently by two people.
2.5 Genetic data analysis
2.5.1 Mitochondrial data
Sequences from the four mitochondrial markers were concatenated and standard molecular diversity statistics calculated in arlequin 3.5 (Excoffier & Lischer, 2010). To test for geographical genetic structure, analyses of molecular variance (AMOVA) were carried out with 10,000 permutations and diversity measures were reported for geographic groups and assessed according to the degree of differentiation between regions (ΦCT), between populations within regions (ΦSC) and between all populations (ΦST). A median-joining (MJ) haplotype network was build using popart (Leigh & Bryant, 2015) for each marker and for the concatenated sequences. Mitochondrial diversity was assessed considering seven geographic populations based on the common population structure of European bats (Bilgin et al., 2016; Razgour et al., 2013): (a) Canary Islands; (b) Iberian Peninsula (Portugal and Spain, excluding Canary Islands); (c) Morocco; (d) France; (e) Italy; (f) Greece; (g) Anatolia; and (h) Middle East (Lebanon, Israel, and Palestine).
Phylogenetic reconstruction was performed on the CIPRES Science Gateway V. 3.3 (Miller, Pfeiffer, & Schwartz, 2010) using Bayesian inference implemented in beast v1.8.4 (Drummond, Suchard, Xie, & Rambaut, 2012) considering unique haplotypes only (n = 65) from the concatenated sequences and with inclusion of T. latouchei as out-group (Mata et al., 2017, GenBank Accession numbers: NC_036331 and KY581662). The best substitution model scheme was determined using partitionfinder v2.1.1 (Lanfear, Frandsen, Wright, Senfeld, & Calcott, 2016). We used a coalescent tree prior under constant population. Three independent runs of 108 generations sampled every 1,000 were combined in tracer v1.7 (Rambaut, Drummond, Xie, Baele, & Suchard, 2018) to confirm convergence on the same posterior distribution in the MCMC runs. The first 107 runs (10%) were discarded as burn-in.
2.5.2 Microsatellite data
To test for departures from Hardy–Weinberg and linkage equilibrium, both across the whole samples and within populations, we used the “pegas” R package (Paradis, 2010). Loci that violated Hardy–Weinberg equilibrium in more than two populations were excluded from further analysis (Table S2). Allele frequencies and number of private alleles were estimated in genetix v4.05 (Belkhir, Borsa, Chikhi, Raufaste, & Bonhomme, 2004) and mean allele frequency across all loci was calculated for each population. Estimates of expected heterozygosity (He), observed heterozygosity (Hobs) and allelic richness within populations, and differentiation (Fst) among populations were all calculated using the “PopGenReport” R package (Adamack & Gruber, 2014). Relatedness among individuals was measured using the triadic maximum likelihood estimator (TrioML; Wang, 2007) implemented in “related” R package (Pew, Muir, Wang, & Frasier, 2015). This estimator was chosen because it allows for inbreeding and accounts for genotyping errors in the data.
Population genetic structure was first examined using the principal component analysis in “PopGenReport” R package (Adamack & Gruber, 2014) followed by the Bayesian clustering analysis implemented in structure 2.3.4 (Pritchard, Stephens, & Donnelly, 2000) with all genotyped samples. We performed 10 replicate runs of structure for each value of K, from K = 1 to 10, and we applied the admixture model with a burn-in of 5 x 105 and a run length of 106 with and without the prior population information (LOCPRIOR). The later can often provide accurate inference of population structure and individual ancestry in datasets where the signal of structure is too weak to be found using the standard models (Hubisz, Falush, Stephens, & Pritchard, 2009). We used structure harvaster v0.6.94 to visualize likelihood and detect the number of genetic groups that best fit the data (Earl & VonHoldt, 2012). The Greedy algorithm of clumpp (Jakobsson & Rosenberg, 2007) was used to derive symmetric similarity coefficients (SSC) among replicate runs within each value of K. Groups of runs with an SSC ≥0.8 were then combined and their outputs for each value of K were graphically displayed.
Spatial structuring was further analyzed using multivariate analyses of spatial genetic patterns in “adegenet” (Jombart, 2008). Spatial analysis of principal components (sPCA) allows to find the individual scores that maximize the product of variance and spatial autocorrelation (Jombart, Devillard, Dufour, & Pontier, 2008). Isolation by distance (IBD) across all individuals within the species range was tested for in the R using the package “ade4” (Bougeard & Dray, 2018) and using a Mantel test.
2.6 ABC inference of evolutionary and demographic history
2.6.1 General overview
The evolutionary and demographic history of T. teniotis was reconstructed using approximate Bayesian computation (ABC) approach implemented in diyabc v2.1 (Cornuet et al., 2014). We carried out two sets of analyses and aimed to (a) infer the source population and patterns of range colonization from putative refugia in the Western Palearctic and (b) infer demographic history in the western range (Iberia, Morocco, and France). In the first step, we modeled the probability of different scenarios considering 122 individuals from six populations (Iberia, Morocco, France, Italy, Greece, and Anatolia/Middle East) and combining information from 12 microsatellites loci and two mitochondrial sequences (COI and D-loop). Multiple scenarios were compared representing a comprehensive range of alternative phylogeographic hypothesis and permuting the six geographic groups at the tips (Supporting Information Figure S1 and Table S3).
Using the scenario topology identified in the first step, we carried out a demographic history analysis of the western range to determine changes in population size during colonization. We compared a null model of no change in population size (Scenario 1) to a model of colonization and expansion in all populations (Scenario 2), and two models of recent change with increase or decrease in Iberian population size (Scenario 3 and Scenario 4 respectively). For a schematic representation of the different scenarios, see Supporting Information Figure S2.
Each scenario was tested using the combined microsatellite and mtDNA datasets and running 106 simulations. The posterior probability of scenarios was then estimated using a weighted polychotomous logistic regression. Due to the criticism of ABC model choice outlined in Robert, Cornuet, Marin, and Pillai (2011), we empirically evaluated the power of the model to discriminate among scenarios by simulating pseudo-observed datasets and calculating false allocation rates (type 1 and 2 errors, Cornuet, Ravigné, & Estoup, 2010). Further details on the methods, model specifications, and run parameters are presented in the following sections and in Supporting Information Table S3.
2.6.2 Specific model parameters
Microsatellite loci were assumed to follow a generalized stepwise mutation model (GSM), and mean mutation rate was bounded between 10–3 and 10–4 (Balloux & Lugon-Moulin, 2002; Storz & Beaumont, 2002). For mtDNA, we only considered COI and D-loop due to computational requirements and sequence completeness. We used the best substitution model scheme determined using partitionfinder v2.1.1 (Lanfear et al., 2016) as follows: HKY for the coding region (COI) and K80 for non-coding region (D-loop). Generation time was set at three years, a value in between the age of first breeding for different bat families that can go from 1 to 5 years (Crichton & Krutzsch, 2000) which meets our expectations for T. teniotis. We considered a mean mutation rate (per site per generation) between 5.25E-8 and 7.2E-8 for COI (Juste et al., 2004; Ruedi & Mayer, 2001) and between 9.45E−8 and 3.75E−7 for D-loop (Petit, Excoffier, & Mayer, 1999).
In the colonization analysis, uniform priors were assumed for all demographic parameters. Effective population size (Ne) was kept as equal for all populations, ranging between 1E3 and 1E6. Population divergence time priors were bounded between 1E3 and 2E5 generations and varied depending on model analysis. Divergence times between source populations were set at either pre-LGM (1E4-2E5) or flexible pre–post-LGM (1E3-2E4). Priors for admixture rates were bounded between 0.01 and 0.99. In the demographic history analysis, we used variable effective population size ranging from 10 to 1E6. Population divergence time priors were bounded to post-LGM (10 and 1E4) and varied depending on model analysis.
In each ABC analysis, we used 269 summary statistics. For the microsatellite loci, we used three single sample statistics (mean number of alleles, mean Nei's genetic diversity index, and mean allele size variance) and five between-sample statistics (Fst, mean number of alleles, mean genic diversity, mean allele size variance, and shared allele distance). For the mtDNA sequence, we used seven single sample statistics (number of distinct haplotypes, number of segregating sites, mean pairwise differences, variance of pairwise distance, Tajima's D statistics, private segregating sites, and mean of numbers of the rarest nucleotide at segregation site) and four between-sample statistics (Fst, number of haplotypes, number of segregating sites, and mean within sample pairwise differences and number of segregating sites). The demographic history analysis included only 47 summary statistics due to the small number of groups compared.
The complete list of parameters used in the ABC analysis, respective priors, and estimated results for the most supported colonization scenario (SC2) and the most supported demographic history scenario (SC2) can be found in Supporting Information Table S4.
2.6.3 Colonization analysis
This analysis included the potential range colonization from an ancient unsampled population with unknown origin. For a schematic representation of the different scenarios, see Supporting Information Figure S1.
Scenario 1 considered an Iberian colonization from an ancient unsampled population before the LGM, and a long-range colonization of the Eastern Mediterranean through an admixture event from Iberia and the ancient unsampled population. The Iberian population later colonized Morocco and the later colonized Italy. Admixture events between Iberia and Italy and between the Eastern Mediterranean and Italy resulted in the French and Greek populations, respectively.
Scenario 2 considered an Italian colonization from an ancient unsampled population before the LGM, and a colonization of the Eastern Mediterranean through an admixture event from Italy and the ancient unsampled population. The Italian population then colonized Morocco and France, while Iberia and Greece were colonized through admixture events between France and Morocco and Italy and Eastern Mediterranean, respectively.
Scenario 3 considered a colonization of the Eastern Mediterranean from an ancient unsampled population before the LGM, and a colonization of the Greek population through an admixture event between the Eastern Mediterranean population and the ancient unsampled population. Italy was later colonized from Greece, while Morocco and France were both be colonized from Italy. Finally, Iberia was colonized through an admixture event between the Moroccan and French populations.
Scenario 4 considered an Italian colonization from an ancient unsampled population before the LGM, and a colonization of the Greek population from an admixture event between the Italian population and the ancient unsampled population. Eastern Mediterranean was then be colonized from Greece, while Italy colonized both Morocco and France. Finally, Iberia was colonized through an admixture event between the French and Moroccan populations.
Scenario 5 considered a colonization of the Eastern Mediterranean from an ancient unsampled population before the LGM, and an Italian colonization through an admixture event between the Eastern Mediterranean and the ancient unsampled populations. Greece was also colonized through an admixture event, this time between the Italian and the Eastern Mediterranean populations. The Italian population then colonized France, while the Eastern Mediterranean population colonized Morocco. Finally, Iberia was colonized through an admixture event between the Moroccan and French populations.
3 RESULTS
3.1 Mitochondrial data
We were able to amplify DNA from 136 samples. Samples from Kyrgyzstan were sequenced using COI-mini marker and showed a high mitochondrial divergence from T.teniotis (ca. 13%) and aligned with sequences belonging to T. latouchei from Laos (99% similarity, Mata et al., 2017). Additionally, from the four samples from China identified as T. teniotis in museum collections (Appendix 1), we were able to sequence two, both aligning with Chaerephon plicatus (vouchers: MVZ:Mamm:192,571 and MVZ:Mamm:193,379). According to the available information, the four samples were collected in the same event at a bat cave in Southern China and thus assumed to belong to the same species. According to the International Union for Conservation of Nature (IUCN), the species has a highly fragmented distribution in central and eastern Asia (Benda & Piraccini, 2016), and our results suggest that T. teniotis could be absent or rare in this region. Therefore, samples from Kyrgyzstan eastwards were excluded from further analysis.
A total of 120 samples belonging to T. teniotis were successfully sequenced for COI (566 bp final alignment) and D-loop (307 bp final alignment), 114 for CytB (509 bp final alignment) and 109 for ATP6 (639 bp final alignment). The number of unique haplotypes ranged from 17 for APT6 to 33 for D-loop. After concatenation, the length of the resulting sequences was between 873 and 2,020 bp (average = 1,937 bp, Alignment Data S1 and S2) and included 56 unique haplotypes (N = 109, 2,020 bp). The Bayesian phylogenetic tree showed maximum posterior probability support (>0.9) for the split of two main lineages, Anatolian/Middle East clade (AMh) and a European clade (EUh) further splitting into two subgroups but in this case with low support (EUh-A and EUh-B) (Figure 1).

The haplotype network divided the haplotypes into three separate groups, of which one was exclusive to Iberia and Morocco (EUh-A) and one was distributed elsewhere in central and western Mediterranean (EUh-B) (Figure 1 and Figure S3). The third group comprised all the haplotypes from Anatolia and Middle East and one additional haplotype from eastern Crete, broadly supporting the phylogenetic tree. The most common haplotypes from EUh-A and EUh-B were separated by only one mutational step (percent differences <0.05%), while AMh shows a divergence of 0.70% from EUh-A and 0.59% from EUh-B.
Despite the split between the eastern and western clades, the phylogenetic tree and haplotype network based on mtDNA showed low levels of geographic structuring within each haplogroup. Mitochondrial haplotype diversity was highest and equal to one in the Middle East (N = 7), France (N = 4), and Morocco (N = 6), while nucleotide diversity was highest in Anatolia (Pi = 0.0040, N = 3) and the Middle East (Pi = 0.0036, N = 7) (Table 1). The lowest values for both haplotype and nucleotide diversity were found in the Canary Islands.
| Mean Allele frequency | Mean Allelic richness | Number of private alleles | He | Hobs | Haplotypic diversity | Nucleotide diversity (Pi) | |
|---|---|---|---|---|---|---|---|
| Canary (5) | 0.34 ± 0.10 | 2.62 ± 0.45 | 0 | 0.58 | 0.63 | 0.40 | 0.0004 |
| Morocco (6) | 0.19 ± 0.04 | 3.74 ± 0.45 | 1 | 0.76 | 0.81 | 1.00 | 0.0022 |
| Iberia (60) | 0.1 ± 0.020 | 3.91 ± 0.37 | 14 | 0.80 | 0.78 | 0.92 | 0.0013 |
| France (7) | 0.19 ± 0.03 | 3.72 ± 0.31 | 1 | 0.76 | 0.78 | 1.00 | 0.0011 |
| Italy (16) | 0.12 ± 0.03 | 4.00 ± 0.40 | 3 | 0.80 | 0.77 | 0.83 | 0.0010 |
| Greece (5) | 0.22 ± 0.06 | 3.55 ± 0.47 | 3 | 0.73 | 0.73 | 0.90 | 0.0011 |
| Anatolia (3) | 0.25 ± 0.08 | 3.56 ± 0.57 | 0 | 0.71 | 0.83 | 0.67 | 0.0040 |
| Middle East (7) | 0.15 ± 0.03 | 4.00 ± 0.44 | 2 | 0.79 | 0.78 | 1.00 | 0.0036 |
Note
- Sample sizes in brackets.
- Mean allelic richness and mean allele frequency across all loci (± SD).
- He, expected heterozygosity; Hobs, observed heterozygosity.
Genetic differentiation at mitochondrial DNA was seen between all populations (χ2 = 532.49, p < 0.001, overall θST = 0.57), with Anatolia and Middle East being genetically differentiated from all populations except for each other (Table S4). The general pattern showed a higher mitochondrial diversity in Anatolia/Middle East and equally low diversity in all the three peninsula.
3.2 Microsatellite data
A total of 128 individuals were successfully genotyped. Of the 14 microsatellite loci, two markers (TAD5 and TAD9) were removed due to violation of Hardy–Weinberg equilibrium (Table S2). After removing these markers, all populations and markers were overall in Hardy–Weinberg. Our final dataset contained a total of 146 alleles, with an average number of 12.17 ± 2.44 alleles per locus (range 7–15) and 24 private alleles.
Genetic diversity in terms of allelic richness was highest in Anatolia and the Middle East, followed by Italy and the Iberian Peninsula (Table 1 and Figure S4). Expected heterozygosity was high in all populations with the exception of the Canary population, where the relatedness was particularly high (mean TrioML = 0.40). Overall population differentiation was low, suggesting a meaningful gene flow. Canaries showed the highest FST values with some degree of differentiation with Greek and Anatolian populations (Table S5).
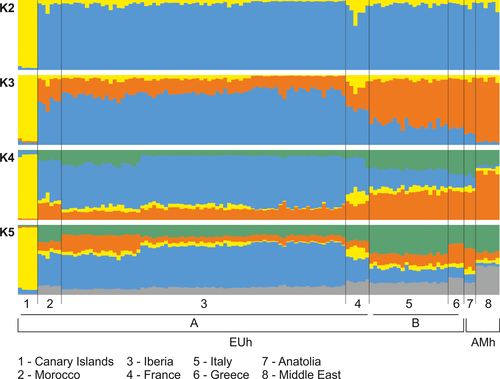
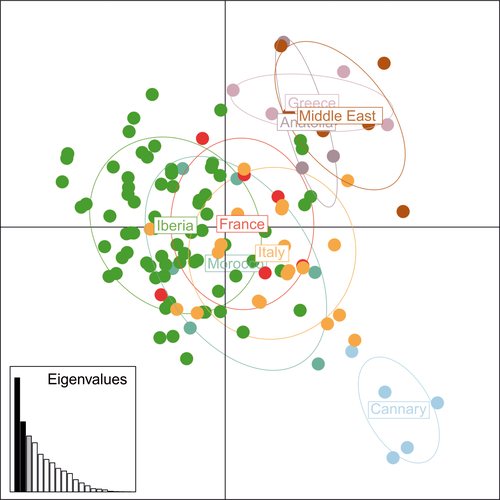
Model-based clustering method implemented in structure without prior population information did not identify any population structure (Supporting Information Figure S5). However, when using this prior, models revealed three main genetic populations (Supporting Information Figure S6 and Table S6). Individuals from the Canary Islands formed a separate population, while all individuals from the Iberian Peninsula, Morocco, and France showed a higher estimated membership fraction to a second inferred cluster, and individuals from Italy eastwards consistently showed higher estimated membership fraction to a third inferred cluster (Figure 2). The three-cluster topology was further supported by the spatial analysis of principal components (sPCA), although the pattern was not significant (Monte Carlo test, p = 0.082) (Figure 3). Both analyses showed that, except for the Canary population, most individuals had high levels of admixture, and only a west to east geographic gradient was evident. An overall observed pattern of isolation by distance was significant (Monte-Carlo test, p = 0.001) (Figure S7).
3.3 ABC inference of evolutionary and demographic history
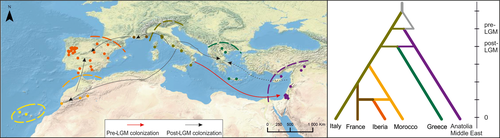
Model-based inference showed high support (86%) for a pre-LGM colonization of Italy from an unsampled population (Figure S1), while the Anatolian/Middle East population was also colonized pre-LGM from an admixture event between Italy and the unsampled population, with a similar contribution from both (proportion of admixture from unsampled population 0.46). The remaining European populations were colonized from Italy after the Younger Dryas, either directly or via a stepping-stone manner with admixture (Figure 4). However, the Greek population showed some level of admixture between Italy and Anatolia/Middle East (Figure 4 and Table S3). Overall, our models identified two glacial refugia, in Italy and the Anatolia/Middle East with high confidence and low error rates (type I = 0.04; type II = 0.05).
Within the western edge of the range, ABC inference indicated a colonization and population expansion in Iberia with a generation time similar to that of the colonization analysis (Table S4). This scenario received high support (99%) (Figure S2), and error rates were estimated at 0.19 and 0.17 for type I and II errors, respectively.
4 DISCUSSION
We reconstructed the evolutionary history of a European bat species with tropical affinities. We show that T. teniotis populations were able to survive in Italy and Anatolia/Middle East during the LGM and have subsequently colonized the current species range. The species has experienced a strong population expansion during the post-glacial colonization of its western range. Our results also point to the occurrence of another population in the Anatolian/Middle East area. Yet, the high haplotype diversity and network pattern found suggests that our samples did not cover the eastern refugium, which is likely located further east (Rossiter et al., 2007) or perhaps toward the Caucasus as suggested for the bat Myotis bechsteinii (Kerth et al., 2008).
4.1 Post-glacial colonization and demographic expansion
Our inferences of demographic history indicate two main refugia during the LGM, one in the Italian Peninsula and another further east in the Anatolian/Middle East region. During this period, the species may have been extinct throughout the rest of southern Europe, with subsequent recolonization from the Italian Peninsula. Although the origin of the ancestral population is unclear, ABC indicates some degree of gene flow between Europe and Anatolia/Middle East before the LGM. Central and western Mediterranean areas were subsequently colonized in a stepping-stone manner, and through gene flow between populations originating from North Africa and France leading to an admixed population in the Iberian Peninsula. Although samples obtained provided a good coverage of the species range in the western Palaearctic, only a limited number of samples were available from North Africa. This is a common caveat of phylogeographic studies (Husemann, Schmitt, Zachos, Ulrich, & Habel, 2014), and we stress that our models do not negate the possibility of north African or Asian glacial refugium. While such a refugium could be the origin of the unknown ancestral population inferred in this study, our evolutionary history models show that a species with tropical affinities was able to survive in Italy during the LGM, from where it expanded across its current European range.
The inferred scenario of an Italian refugium and post-glacial European recolonization concurs with the widely accepted phylogeographic paradigms for the western Palearctic (Hewitt, 1999). Among bats, Italy has been identified as a glacial refugium for Myotis myotis (Ruedi et al., 2008) and a possible refugium for Rhinolophus ferrumequinum (Rossiter et al., 2007). In a recent paper, Bogdanowicz et al. (2015) suggested that this pattern might be widespread among bat species. Focusing on Miniopterus schreibersii, Bilgin et al. (2016) suggested a new paradigm of European colonization from Anatolian populations, and although we identified an ancient population in Anatolia/Middle East, our results do not support the hypothesis of a European recolonization from this region, a similar pattern to R. ferrumequinum (Rossiter et al., 2007). In fact, samples from Anatolia and the Middle East formed a distinct clade at the mitochondrial level (AMh), with no haplotypes shared with Europe. Interestingly, the high haplotype diversity (nine haplotypes in 10 samples) and the absence of a star-like pattern in the haplotype network for this region suggest that the eastern refugium could be located further east.
High levels of relatedness and reduced genetic diversity in the Canary Islands likely reflect inbreeding in an isolated population. Increased inbreeding relative to mainland populations has been described for different taxa in insular populations (Frankham, 2008), including bats. Our results suggest that Canary Islands were colonized following a model of long-distance dispersal and establishment with limited subsequent gene flow from the parent population (Crisp, Trewick, & Cook, 2011). A general pattern of continental dispersion to the Canary Islands driven by stochastic events such as storms was described by Juan, Emerson, Oromı́, and Hewitt (2000).
The star-like topology in the European mitochondrial groups (EUh-A and EUh-B) indicates population expansion (Slatkin & Hudson, 1991). This hypothesis was further supported by the ABC inference, which shows a demographic expansion following the Iberian colonization. Such expansion could be the result of a natural process (Bilgin et al., 2016; Razgour et al., 2013) or might be mediated by human activity, such as through increased roost availability from tall buildings and other structures including bridges, many of which were built during the 20th century (Amorim, Alves, & Rebelo, 2013; Russo & Ancillotto, 2014).
Post-glacial population growth appears to be common in taxa with that underwent the same climatic changes since the LGM (Branco, Monnerot, Ferrand, & Templeton, 2002; Korsten et al., 2009) and was also suggested for another fast-flying bat species, Nyctalus noctula (Petit et al., 1999). Microsatellites have a fast mutation rate when compared to other molecular markers, but it has been questioned whether this rate is fast enough to detect recent population changes (Barrett & Schluter, 2008). Therefore, it is difficult to ascertain if these populations, especially the ones located in the western edge of the species’ range are still expanding.
4.2 Barriers to gene flow
Our results show high differentiation at mitochondrial markers between the populations from the Anatolia and Middle East region and those from central and western Mediterranean. We also found evidence of genetic differentiation within the European clade, whereby populations from Canary Islands, Morocco, and Iberia seemed to form a distinct group from Central Mediterranean populations (Italy, France, and Greece). Genetic structuring at the mitochondrial level suggests that, once established, females will not disperse freely, supporting some degree of philopatry, a common trait among several bat species (reviewed in Burland & Worthington Wilmer, 2001). In fact, the Iberian Peninsula seems to have been colonized following a first-come, first-served pattern, as indicated by the presence of haplotypes from both the central Mediterranean and North African haplogroups. Even though T. teniotis females are physically capable of crossing geographical barriers (e.g., mountain ranges and large bodies of water), philopatric behavior may have a strong effect on female dispersal, thus explaining the absence of Iberian/north African haplotypes in central Mediterranean. Contrary to mtDNA, at the nuclear level we confirmed some degree of gene flow between Europe and the Anatolia/Middle East. We also found high levels of gene flow within the European range and North Africa, whereas the Gibraltar strait does not act as a barrier to current or even past gene flow (García-Mudarra et al., 2009). Yet, the Canaries show high levels of isolation from mainland Africa. Combined, these results reflect a typical pattern of male-mediated gene flow (Castella, Ruedi, & Excoffier, 2001).
Gene flow inferred from nuclear markers seemed to be solely restricted by geographic distance, showing a clear pattern of isolation by distance and the absence of strong geographic barriers to dispersal. T. teniotis performs fast and direct flights while foraging with median speeds of 50 km/h and covering linear distances of up to 70 km (Marques, Rainho, Carapuço, Oliveira, & Palmeirim, 2004). Although flight altitudes have not been reported for T. teniotis, the species is known to prey on large moths that migrate at high altitudes (Mata et al., 2016). Indeed, the smaller congeneric species, T. brasiliensis (approx. 12 g compared to 30 g of T. teniotis), can fly up to 1 km above ground level (McCracken et al., 2008). Thus, the absence of geographic barriers to gene flow in our focal taxa is not surprising.
4.3 Implications for the phylogeography of Western Palearctic species with tropical affinity
The importance of refugia for conservation planning has been widely recognized because they can facilitate the persistence of biodiversity under changing climates (Keppel et al., 2012), and their relevance is even greater in the face of anthropogenic climate change. Common refugia in the Western Palearctic has been widely acknowledged for a number of species (Hewitt, 1999; Husemann et al., 2014); however, of the 914 studies focusing on taxa that occur in the western Palearctic (Keppel et al., 2012), only very few focus on species with tropical affinities (but see Rato et al., 2011). The location of refugia is often similar between species sharing climatic and environmental requirements, though it has been shown that species may respond differently to changes in habitat availability resulting from climatic changes at the end of the LGM (Taberlet, Fumagalli, Wust-Saucy, & Cosson, 1998). In a recent paper, Carstens, Morales, Field, and Pelletier (2018) showed that species’ traits in bats can influence the response to climatic oscillations. Most importantly, they found that heavier bat species and those with longer wings were more likely to suffer a bottleneck at the LGM, and although this was mostly driven by frugivorous species from the neotropics, it highlights the importance of phylogeographic studies on species showing different traits in similar environments.
In this study, we show that a species with tropical affinities was able to survive in the harsh environments of glacial Europe when a large area of the Western Palearctic was covered in ice sheets and permafrost, and temperatures were 10–20°C cooler than today (Kageyama et al., 2006). Yet, these results raise new questions regarding how these species survived in colder climates where the environment carrying capacity was lower (Frenzel et al., 1992). Moreover, free-tailed bats, such as T. teniotis, are thought to be poor hibernators. Although Arlettaz et al. (2000) found that in the Swiss Alps T. teniotis can go through torpor bouts that can last up to 8 days, average body temperature during hibernation and mean arousal frequency was much higher than in other temperate bat species.
This study contributes to understanding the evolutionary history of species with tropical affinities living in temperate regions and raises questions regarding the physiological, behavioral and ecological traits that enabled them to survive in colder climates. The lack of phylogeographic studies focusing on these species highlights the importance of such studies for informing their population management and conservation, in particular under future environmental changes.
ACKNOWLEDGEMENTS
We are grateful to all the people, NGOs, museums and private companies who helped with the collection of samples: T. Guillén, C. Dietz, E. Levin, C. Baby, P. Georgiakakis, L. Ancillotto, S.J. Puechmaille, Groupe chiroptères corse, Le Groupe Chiroptères de Provence, Hungarian Natural History Museum, Muséum d'histoire naturelle Bourges, Musée des Confluences, Field Museum, Muséum National d'Histoire Naturelle, Museum of Vertebrate Zoology, Natural History Museum of the American University of Beirut, Senckenberg Museum, Plecotus—Estudos Ambientais, Bioinsgiht, Ecosativa—Consultoria Ambiental as well as to those who gave invaluable suggestions on some of the analyses, D.V. Gonçalves, S. Ferreira, S. Barbosa, S.J. Puechmaille. The intensive laboratory work was only possible due to the amazing CIBIO-InBIO CTM staff.
APPENDIX 1
| Sample Code | Provider [voucher] | Lat/ Long | COI | D-Loop | ATP6 | CytB | Micros | Country/ Region |
|---|---|---|---|---|---|---|---|---|
| FG814 | HNHM [HNHM2000.46.1] | 41.41/ 22.24 | MK817186 | MK817240 | MK817225 | MK817216 | Yes | Macedonia/ Demir Kapija |
| FG815 | Eran Levin | 31.58/ 34.88 | MK817179 | MK817264 | MK817230 | MK817198 | Yes | Israel/ Yoav |
| FG816 | Eran Levin | 31.58/ 34.88 | MK817180 | MK817261 | MK817229 | MK817199 | Yes | Israel/ Yoav |
| FG817 | Eran Levin | 32.03/ 34.77 | MK817183 | MK817262 | MK817228 | MK817198 | Yes | Israel/ Tel-Aviv |
| FG818 | Eran Levin | 31.45/ 35.37 | MK817185 | MK817265 | MK817227 | MK817194 | Yes | Israel/ Be'er Tuvia Regional |
| FG819 | Eran Levin | 32.17/ 34.9 | MK817182 | MK817260 | MK817229 | MK817199 | Yes | Israel/ Kfar Sava |
| FG820 | Groupe chiroptères corse | 41.67/ 8.9 | MK817186 | MK817271 | MK817219 | MK817216 | Yes | France/ Propriano (Corsica) |
| FG821 | Muséum d'histoire naturelle Bourges | 41.92/ 8.74 | n/a | n/a | n/a | n/a | n/a | France/ Ajaccio (Corsica) |
| FG822 | Muséum d'histoire naturelle Bourges | 41.92/ 8.74 | n/a | n/a | n/a | n/a | n/a | France/ Ajaccio (Corsica) |
| FG823 | Muséum d'histoire naturelle Bourges | 43.7/ 7.27 | n/a | n/a | n/a | n/a | n/a | France/ Nice |
| FG824 | Muséum d'histoire naturelle Bourges | 43.7/ 7.27 | MK817186 | MK817267 | n/a | MK817215 | Yes | France/ Nice |
| FG825 | Muséum d'histoire naturelle Bourges | 43.7/ 7.27 | n/a | n/a | n/a | n/a | Yes | France/ Nice |
| FG826 | Christian Dietz | 40.64/ 22.94 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Greece/ Thessaloniki |
| FG827 | Christian Dietz | 40.64/ 22.94 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Greece/ Thessaloniki |
| FG828 | Christian Dietz | 31.54/ −5.92 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Morocco/ Gorges du Dadès |
| FG829 | Musée des Confluences [40001656] | - | mini | n/a | n/a | n/a | n/a | France/ Villebois |
| FG830 | FMNH [FMNH79280] | 30.07/ 31.09 | n/a | n/a | n/a | n/a | n/a | Egypt/ Memphis |
| FG831 | FMNH [FMNH79283] | 30.07/ 31.09 | n/a | n/a | n/a | n/a | n/a | Egypt/ Memphis |
| FG832 | FMNH [FMNH79284] | 30.07/ 31.09 | n/a | n/a | n/a | n/a | n/a | Egypt/ Memphis |
| FG833 | FMNH [FMNH79286] | 30.07/ 31.09 | n/a | n/a | n/a | n/a | n/a | Egypt/ Memphis |
| FG834 | FMNH [FMNH79288] | 30.07/ 31.09 | n/a | n/a | n/a | n/a | n/a | Egypt/ Memphis |
| FG835 | FMNH [FMNH79757] | 30.01/ 31.21 | n/a | n/a | n/a | n/a | n/a | Egypt/ Memphis |
| FG836 | FMNH [FMNH96280] | 40.88/ 14.2 | n/a | n/a | n/a | n/a | Yes | Italy/ Naples |
| FG837 | FMNH [FMNH99291] | 34.00/ 36.21 | n/a | n/a | n/a | n/a | n/a | Lebanon/ Baalbek |
| FG838 | FMNH [FMNH99292] | 34 0.00/ 36.21 | n/a | n/a | n/a | n/a | n/a | Lebanon/ Baalbek |
| FG839 | FMNH [FMNH99569] | 34.00/ 35.83 | MK817181 | MK817261 | MK817229 | MK817199 | n/a | Lebanon/ Farayya |
| FG840 | FMNH [FMNH111598] | 27.13/ 57.09 | n/a | n/a | n/a | n/a | n/a | Iran/ Minab |
| FG841 | Catherine Baby | 43.72/ 7.26 | MK817186 | MK817240 | MK817219 | MK817200 | Yes | France/ Nice |
| FG842 | Catherine Baby | 43.72/ 7.26 | MK817186 | MK817267 | MK817219 | MK817215 | Yes | France/ Nice |
| FG843 | Musée des Confluences [40002154] | 47.08/ 9.33 | n/a | n/a | n/a | n/a | n/a | Swiss/ Gruyère |
| FG844 | MNHM [ZM-MO−1984–369] | - | n/a | n/a | n/a | n/a | n/a | Italy |
| FG845 | MNHM [ZM-MO−1999–762] | - | n/a | n/a | n/a | n/a | n/a | - |
| FG846 | MNHM [ZM-MO−1999–765] | - | n/a | n/a | n/a | n/a | n/a | Spain |
| FG847 | MNHM [ZM-MO−1983–1423] | - | MK817179 | MK817259 | MK817231 | MK817198 | yes | Palestine |
| FG848 | MNHM [ZM-MO−1996–447] | - | n/a | mini | n/a | n/a | n/a | France |
| FG849 | MNHM [ZM-MO−1984–1207] | - | n/a | n/a | n/a | n/a | n/a | Palestine |
| FG850 | Panagiotis Georgiakakis | 35.37/ 23.63 | MK817171 | MK817240 | MK817219 | MK817216 | Yes | Greece/ Kissamos (Crete) |
| FG851 | Panagiotis Georgiakakis | 35.34/ 25.13 | MK817182 | MK817272 | MK817229 | MK817193 | Yes | Greece/ Heraklion (Crete) |
| FG852 | Leonardo Ancillotto | 40.97/ 14.21 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Italy/ Aversa |
| FG853 | Leonardo Ancillotto | 41.93/ 12.52 | MK817170 | MK817271 | MK817222 | MK817216 | Yes | Italy/ Rome |
| FG854 | Leonardo Ancillotto | 41.93/ 12.52 | MK817186 | MK817240 | n/a | MK817216 | Yes | Italy/ Rome |
| FG855 | Leonardo Ancillotto | 41.93/ 12.52 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Italy/ Rome |
| FG856 | Leonardo Ancillotto | 41.93/ 12.52 | MK817170 | MK817271 | MK817222 | MK817216 | Yes | Italy/ Rome |
| FG857 | Leonardo Ancillotto | 41.93/ 12.52 | MK817186 | MK817240 | MK817221 | MK817216 | Yes | Italy/ Rome |
| FG858 | Leonardo Ancillotto | 43.56/ 10.32 | MK817186 | MK817240 | MK817221 | MK817216 | Yes | Italy/ Livorno |
| FG859 | Leonardo Ancillotto | 43.72/ 10.4 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Italy/ Pisa |
| FG860 | Leonardo Ancillotto | 43.87/ 10.25 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Italy/ Viareggio |
| FG861 | Leonardo Ancillotto | 41.88/ 12.57 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Italy/ Rome |
| FG359 | CIBIO | 41.45/ −6.59 | MK817176 | MK817240 | MK817235 | MK817203 | Yes | Portugal/ Mogadouro |
| FG373 | CIBIO | 41.29/ −6.87 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Portugal/ Alfândega da Fé |
| FG389 | CIBIO | 41.33/ −7.36 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Portugal/ Carrazeda de Ansiães |
| FG432 | CIBIO | 41.55/ −6.98 | MK817166 | MK817248 | MK817219 | MK817216 | Yes | Portugal/ Macedo de Cavaleiros |
| FG451 | CIBIO | 41.18/ −7.06 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Portugal/ Torre de Moncorvo |
| FG530 | CIBIO | 41.55/ −6.98 | MK817186 | MK817270 | MK817226 | MK817216 | Yes | Portugal/ Macedo de Cavaleiros |
| FG739 | CIBIO | 41.33/ −7.36 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Portugal/ Carrazeda de Ansiães |
| FG793 | CIBIO | 41.28/ −6.89 | MK817186 | MK817240 | MK817235 | MK817216 | Yes | Portugal/ Alfândega da Fé |
| FG862 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817257 | MK817219 | MK817214 | Yes | Spain/ Cadiz |
| FG863 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817257 | MK817219 | MK817214 | Yes | Spain/ Cadiz |
| FG864 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Spain/ Granada |
| FG865 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Spain/ Granada |
| FG866 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Spain/ Granada |
| FG867 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817251 | MK817235 | MK817213 | Yes | Spain/ Granada |
| FG868 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817235 | MK817216 | Yes | Spain/ Granada |
| FG869 | Javier Juste & Carlos Ibáñez | - | MK817169 | MK817269 | MK817235 | MK817212 | Yes | Spain/ Jaen |
| FG870 | Javier Juste & Carlos Ibáñez | - | MK817175 | MK817250 | MK817235 | MK817211 | Yes | Spain/ Huesca |
| FG871 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817271 | MK817219 | MK817216 | Yes | Spain/ Huesca |
| FG872 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817270 | MK817235 | MK817216 | Yes | Spain/ Teruel |
| FG873 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Spain/ Teruel |
| FG874 | Javier Juste & Carlos Ibáñez | - | MK817167 | MK817240 | MK817223 | MK817216 | Yes | Spain/ Teruel |
| FG875 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Spain/ Teruel |
| FG876 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817271 | MK817219 | MK817216 | Yes | Spain/ Teruel |
| FG877 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817271 | MK817219 | MK817216 | Yes | Spain/ Teruel |
| FG878 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817270 | MK817234 | MK817216 | Yes | Spain/ Tenerife (Canary) |
| FG879 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817235 | MK817216 | Yes | Spain/ Tenerife (Canary) |
| FG880 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | n/a | n/a | Yes | Spain/ La Palma (Canary) |
| FG881 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817235 | MK817216 | Yes | Spain/ La Palma (Canary) |
| FG882 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817235 | MK817216 | Yes | Spain/ La Palma (Canary) |
| FG883 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817235 | MK817216 | Yes | Spain/ La Palma (Canary) |
| FG884 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817235 | MK817216 | Yes | Spain/ Ciudad Real |
| FG885 | Javier Juste & Carlos Ibáñez | - | MK817173 | MK817240 | MK817235 | MK817213 | Yes | Spain/ Guadalajara |
| FG886 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817235 | MK817210 | Yes | Spain/ Guadalajara |
| FG887 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Spain/ Guadalajara |
| FG888 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817232 | MK817216 | Yes | Spain/ Guadalajara |
| FG889 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817243 | MK817235 | MK817216 | Yes | Spain/ Guadalajara |
| FG890 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817235 | MK817216 | Yes | Spain/ Guadalajara |
| FG891 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817241 | MK817235 | MK817209 | Yes | Spain/ Sevilla |
| FG892 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817251 | MK817219 | MK817216 | Yes | Spain/ Caceres |
| FG893 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817270 | MK817235 | MK817216 | Yes | Spain/ Caceres |
| FG894 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817253 | MK817219 | MK817216 | Yes | Spain/ Caceres |
| FG895 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Spain/ Caceres |
| FG896 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817251 | MK817235 | MK817216 | Yes | Spain/ Caceres |
| FG897 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817251 | MK817235 | MK817216 | Yes | Spain/ Caceres |
| FG898 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817248 | MK817219 | MK817216 | Yes | Spain/ Caceres |
| FG899 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Spain/ Caceres |
| FG900 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817249 | MK817219 | MK817216 | Yes | Spain/ Caceres |
| FG901 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817251 | MK817235 | MK817216 | Yes | Spain/ Caceres |
| FG902 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Italy/ Samo |
| FG903 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Italy/ Samo |
| FG904 | Javier Juste & Carlos Ibáñez | - | MK817177 | MK817245 | MK817219 | MK817216 | Yes | Italy/ Samo |
| FG905 | Javier Juste & Carlos Ibáñez | - | MK817178 | MK817240 | MK817219 | MK817208 | Yes | Italy/ Samo |
| FG906 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817220 | MK817216 | Yes | Italy/ Samo |
| FG907 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Italy/ Samo |
| FG908 | Javier Juste & Carlos Ibáñez | - | MK817178 | MK817240 | MK817219 | MK817208 | Yes | Italy/ Samo |
| FG909 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817244 | MK817219 | MK817216 | Yes | Italy/ Samo |
| FG910 | Javier Juste & Carlos Ibáñez | - | MK817178 | MK817240 | MK817219 | MK817208 | Yes | Italy/ Samo |
| FG911 | Javier Juste & Carlos Ibáñez | - | MK817177 | MK817245 | MK817219 | MK817216 | Yes | Italy/ Samo |
| FG912 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817233 | MK817207 | Yes | Spain/ Valencia |
| FG913 | Javier Juste & Carlos Ibáñez | - | MK817175 | MK817249 | MK817235 | MK817216 | Yes | Spain/ Valencia |
| FG914 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | n/a | n/a | Yes | Spain/ Valencia |
| FG915 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | n/a | n/a | Yes | Spain/ Valencia |
| FG916 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | n/a | MK817216 | Yes | Spain/ Valencia |
| FG917 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | n/a | n/a | Yes | Spain/ Valencia |
| FG918 | Javier Juste & Carlos Ibáñez | - | n/a | n/a | n/a | n/a | n/a | Spain/ Valencia |
| FG919 | Javier Juste & Carlos Ibáñez | - | MK817168 | MK817256 | n/a | n/a | Yes | Spain/ Valencia |
| FG920 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817235 | MK817206 | Yes | Spain/ Valencia |
| FG921 | Javier Juste & Carlos Ibáñez | - | MK817174 | MK817240 | MK817219 | MK817216 | Yes | Spain/ Valencia |
| FG922 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Spain/ La Rioja |
| FG923 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817252 | MK817219 | MK817216 | Yes | Spain/ La Rioja |
| FG924 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817240 | n/a | MK817196 | Yes | Spain/ La Rioja |
| FG925 | Javier Juste & Carlos Ibáñez | - | MK817184 | MK817263 | MK817229 | MK817197 | Yes | Turkey/ Şanlıurfa |
| FG926 | Javier Juste & Carlos Ibáñez | - | MK817185 | MK817266 | MK817227 | MK817195 | Yes | Turkey/ Adıyaman |
| FG927 | Javier Juste & Carlos Ibáñez | - | MK817184 | MK817263 | MK817229 | MK817197 | Yes | Turkey/ Şanlıurfa |
| FG928 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817251 | MK817219 | MK817205 | Yes | Morocco/ Agadir-Ida |
| FG929 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817255 | MK817235 | MK817216 | Yes | Morocco/ Agadir-Ida |
| FG930 | Javier Juste & Carlos Ibáñez | - | MK817172 | MK817240 | MK817219 | MK817202 | Yes | Morocco/ Agadir-Ida |
| FG931 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817247 | MK817224 | MK817204 | Yes | Morocco/ Agadir-Ida |
| FG932 | Javier Juste & Carlos Ibáñez | - | MK817186 | MK817246 | MK817235 | MK817201 | Yes | Morocco/ Errachidia |
| FG01 | Plecotus | 40.84/ −8.19 | MK817186 | MK817268 | MK817219 | MK817216 | Yes | Portugal/ São Pedro do Sul |
| FG02 | Plecotus | 40.64/ −8.13 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Portugal/ Tondela |
| FG11 | CIBIO | 41.39/ −7.45 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | Portugal/ Tinhela |
| FG64 | CIBIO | 41.45/ −6.59 | MK817186 | MK817240 | MK817235 | MK817216 | Yes | Portugal/ Mogadouro |
| FG92 | Plecotus | 40.45/ −7.36 | MK817186 | MK817240 | MK817235 | MK817216 | Yes | Portugal/ Trancoso |
| FG93 | Bio3 | 40.93/ −7.69 | MK817166 | MK817248 | MK817219 | MK817216 | Yes | Portugal |
| FG94 | Bio3 | 41.44/ −7.00 | MK817186 | MK817254 | MK817219 | MK817216 | Yes | Portugal |
| FG95 | Plecotus | 40.10/ −8.18 | MK817186 | MK817240 | MK817235 | MK817216 | Yes | Portugal/ Lousã |
| FG96 | Plecotus | 40.88/ −8.25 | MK817166 | MK817248 | n/a | MK817216 | Yes | Portugal/ Arouca |
| FG164 | Ecosativa | 41.53/ −7.51 | MK817186 | MK817240 | MK817235 | MK817216 | Yes | Portugal/ Vila Pouca de Aguiar |
| FG165 | Ecosativa | 41.52/ −7.51 | MK817186 | MK817251 | MK817219 | MK817216 | Yes | Portugal/ Vila Pouca de Aguiar |
| FG166 | Ecosativa | 41.55/ −7.51 | MK817186 | MK817242 | MK817235 | MK817216 | Yes | Portugal/ Vila Pouca de Aguiar |
| FG167 | Ecosativa | 41.45/ −7.75 | MK817166 | MK817248 | MK817219 | MK817216 | Yes | Portugal/ Vila Pouca de Aguiar |
| FG933 | MVZ [MVZ:Mamm:192570] | 27.92/ 101.33 | n/a | n/a | n/a | n/a | Yes | China/ Yunnan |
| FG934 | MVZ [MVZ:Mamm:192571] | 27.92/ 101.33 | MK817187 | n/a | MK817192 | MK817190 | n/a | China/ Yunnan |
| FG935 | MVZ [MVZ:Mamm:192573] | 27.92/ 101.33 | n/a | n/a | n/a | n/a | Yes | China/ Yunnan |
| FG936 | MVZ [MVZ:Mamm:193379] | 27.92/ 101.33 | MK817188 | n/a | MK817191 | MK817189 | n/a | China/ Yunnan |
| FG1052 | NHM-AUB | - | n/a | n/a | n/a | n/a | Yes | Lebanon |
| FG1053 | NHM-AUB | - | n/a | n/a | n/a | n/a | n/a | Lebanon |
| FG1054 | NHM-AUB | - | n/a | n/a | n/a | n/a | n/a | Lebanon |
| FG1551 | Toni Guillén [ROM:MAM:118321] | - | MK817165 | MK817238 | MK817237 | MK817218 | Yes | Laos |
| FG1552 | Toni Guillén | - | MK817165 | MK817239 | MK817236 | MK817217 | Yes | Laos |
| FG1735 | Sébastien Puechmaille | 43.62/ 4.77 | MK817186 | MK817240 | MK817219 | MK817216 | Yes | France/ Saint-Martin-de-Crau |
| FG16 | CIBIO | 41.17/ −7.05 | MK817186 | MK817258 | n/a | n/a | Yes | Portugal/ Torre de Moncorvo |
| FG3144 | Senckenberg [38739] | - | n/a | n/a | n/a | n/a | n/a | Afghanistan/ Kabul |
| FG3145 | Senckenberg [33526] | - | n/a | n/a | n/a | n/a | n/a | France |
| FG3146 | Senckenberg [77805] | - | n/a | n/a | n/a | n/a | n/a | Kyrgyzstan |
| FG3147 | Senckenberg [77806] | - | mini | n/a | n/a | n/a | n/a | Kyrgyzstan |
| FG3148 | Senckenberg [77807] | - | n/a | n/a | n/a | n/a | n/a | Kyrgyzstan |
| FG3149 | Senckenberg [77808] | - | mini | n/a | n/a | n/a | n/a | Kyrgyzstan |
| FG3150 | Senckenberg [91142] | - | mini | n/a | n/a | n/a | n/a | Kyrgyzstan |
| FG3151 | Senckenberg [91143] | - | mini | n/a | n/a | n/a | n/a | Kyrgyzstan |
- a Complete mitochondrion genome available on genbank (Mata V.A et al., (2017). First complete mitochondrial genomes of molossid bats (Chiroptera: Molossidae). Mitochondrial DNA Part B, 2(1), 152–154)
- b This samples were amplified using internal primers that differentiate T. teniotis from T. latouchei. Due to differences in sequence length, the two samples from T. teniotis amplified using these primers were not included in the phylogenetic and evolutionary analysis.
- c Samples identified as belonging to Chaerephon plicatus
- d Samples identified as belonging to T. latouchei




