Insights into chromosomal evolution of Cicadomorpha using fluorochrome staining and mapping 18S rRNA and H3 histone genes
Abstract
The infraorder Cicadomorpha (Hemiptera) is a cosmopolitan species-rich lineage of phytophagous insects. They have holocentric chromosomes and vary greatly in diploid number across families, with X0 as the predominant sex male mechanism. Here, we advance the understanding of chromosome mapping of repetitive elements of four families of cicadomorphan insects, the spittlebugs (Cercopidae), leafhoppers (Cicadellidae), and treehoppers (Aetalionidae and Membracidae). Sampled individuals from 19 species show considerable variation in diploid number, which may have originated from fusions between autosomes or between autosomes and the ancient X. The distribution of CMA3+ blocks, primarily observed in low numbers in autosomal regions, was a conserved trait. Likewise, fluorescence in situ hybridization (FISH) mapping revealed mainly one locus per haploid genome for the 18S rRNA gene and for H3, each of which is located on distinct chromosomes. Despite the extensive variation in the number of autosomes and sex systems, the number of loci of ribosomal and H3 genes remained stable and may reflect the ancestral genome organizations in these groups. These results shed light on the chromosomal-level organization in Cicadomorpha and provide new insights into the evolutionary history of karyotypes and repetitive elements in this diverse insect lineage.
1 INTRODUCTION
The hemipteran infraorder Cicadomorpha is a diverse lineage of phytophagous insects, including nearly 30,000 described species found in a wide range of tropical and temperate environments around the globe (Cryan, 2005; Dietrich, 2002). Cicadomorphans are currently classified in four superfamilies: Cicadoidea (cicadas), Cercopoidea (froghoppers and spittlebugs), Membracoidea (leafhoppers and treehoppers), and Myerslopioidea (ground-dwelling leafhoppers), which together with planthoppers (infraorder Fulgoromorpha) constitute the suborder Auchenorrhyncha (Cryan, 2005; Cryan & Urban, 2012). These sap-feeding insects are characterized by their specialized piercing–sucking mouthparts, which can cause damage to crops and pasture, and facilitate the transmission of plant pathogens (Dietrich, 2002, 2005; Peck & Thompson, 2008).
The most distinctive cytogenetic feature of auchenorrhynchan insects is their holocentric chromosomes, a trait shared with all other Hemiptera (Kuznetsova & Aguin-Pombo, 2015). Despite their outstanding diversity and economic significance, the karyotypes of auchenorrhynchans are still poorly characterized with regard to chromosome banding and mapping of repetitive DNAs (Kuznetsova & Aguin-Pombo, 2015). Their diploid number ranges from 2n = 8 to 2n = 38 in females, with XX♀/X0♂ as the predominant sex chromosome system (Boring, 1907; Halkka, 1964; Kuznetsova & Aguin-Pombo, 2015). Earlier contributions have reported extensive variation in the amount and location of C-heterochromatin in auchenorrhynchan chromosomes (Noda & Tatewaki, 1990; Perepelov, Bugrov, & Maryańska-Nadachowska, 2002; Kuznetsova, Maryańska-Nadachowska, & Nokkala, 2003; Kuznetsova, Maryańska-Nadachowska, & Nokkala, 2009; Maryańska-Nadachowska, Kuznetsova, Lachowska, & Drosopoulos, 2012; Kuznetsova & Aguin-Pombo, 2015). Species-level differences include variation in base pair richness, as detected by fluorochrome banding, which indicates that GC-rich regions are more prevalent than AT-rich chromosomal blocks (Anjos, Rocha, Paladini, Mariguela, & Cabral-de-Mello, 2016; Kuznetsova & Aguin-Pombo, 2015; Kuznetsova, Maryańska-Nadachowska, Anokhin, & Aguin-Pombo, 2015; Kuznetsova et al., 2009; Maryańska-Nadachowska, Kuznetsova, & Abdul-Nour, 2008). Chromosomal analysis based on the mapping of DNA sequences by fluorescence in situ hybridization (FISH) has not been extensively addressed in this group. The chromosomal location of the 18S rRNA gene (ribosomal RNA; 18S) and U1 snRNA gene (small nuclear RNA: U1) and the presence of telomeric repeats (TTAGG)n at chromosome ends have been described, but the chromosomal distribution of additional repetitive elements is still poorly known (Anjos, Paladini, Mariguela, & Cabral-De-Mello, 2018; Anjos et al., 2016; Golub, Kuznetsova, & Rakitov, 2014; Kuznetsova, Grozeva, Hartung, & Anokhin, 2015; Maryańska-Nadachowska, Anokhin, Gnezdilov, & Kuznetsova, 2016; Maryańska-Nadachowska, Kuznetsova, & Karamysheva, 2013).
Repetitive sequences are suitable markers to improve our understanding of genome organization and chromosomal evolution in species with holocentric chromosomes (Bardella, Fernandes, & Cabral-de-Mello, 2016; Nguyen & Carabajal Paladino, 2016; Pita et al., 2013). In order to shed light on the dynamics of karyotypes and repetitive DNAs in Auchenorrhyncha, we analyzed chromosomes from 19 species belonging to four families (Aetalionidae, Cercopidae, Cicadellidae, and Membracidae) using a combination of traditional staining techniques and FISH for 18S and histone H3 probes. We discuss the data in a comparative framework to offer predictive hypotheses about the processes leading to the observed variation.
2 MATERIAL AND METHODS
We studied 19 cicadomorphan species in the following families (number of sampled species in parentheses): Aetalionidae (1), Cercopidae (10), Cicadellidae (2), and Membracidae (6). Exemplars were collected in the municipality of Rio Claro, São Paulo State, Southeastern Brazil (four males and three females per species; Table 1). Exemplars were stored in 100% ethanol for DNA extraction and species identification (whole body except part of the abdomen). Chromosome analysis was performed on testes from four anesthetized males per species, which were immersed in distilled water for 5 min, and subsequently fixed in modified Carnoy's solution (3:1, absolute ethanol: glacial acetic acid). The testis follicles were macerated in a drop of 50% acetic acid on microscope slides, which were then dried at 40–45°C on a heating block. Chromosomes were stained with 5% Giemsa, and fluorochrome staining (CMA3/DA/DAPI) was performed according to the procedure outlined in Schweizer, Mendelak, White, and Contreras (1983).
| Family Species | Karyotype (2n) | CMA3+ banding | FISH | |
|---|---|---|---|---|
| 18S | H3 | |||
| Cercopoidea | ||||
| Cercopidae | ||||
| Deois flavopicta (Stål, 1854) | 2n = 19,X0#1 | 2tA | 2iA | 2iA |
| Deois mourei Cavichioli & Sakakibara, 1993 | 2n = 19,X0#1 | 2tA | 2iA | 2iA |
| Deois schach (Fabricius, 1787) | 2n = 19,X0#1 | 2tA | 2iA | 2iA |
| Mahanarva fimbriolata (Stål, 1854) | 2n = 19,X0#2 | 2iA#2 | 2iA | 2iA |
| Mahanarva liturata (Le Peletier & Serville, 1825) | 2n = 19,X0#2 | 2tA,2iA#2 | 2iA | 2iA |
| Mahanarva quadripunctata (Walker, 1858) | 2n = 19,X0#2 | 2iA#2 | 2iA | 2iA |
| Mahanarva spectabilis (Distant, 1909) | 2n = 19,X0#2 | 2iA#2 | 2iA | 2iA |
| Mahanarva tristis (Fabricius, 1803) | 2n = 19,X0#2 | 7tA,1tX#2 | 2iA | 2iA |
| Mahanarva vittata (Walker, 1851) | 2n = 19,X0#2 | 2iA,1tX#2 | 1tX | 2tA |
| Notozulia entreriana (Berg, 1879) | 2n = 15,X0#1 | 2iA | 2tA | 2tA |
| Membracoidea | ||||
| Aetalionidae | ||||
| Aetalion reticulatum (Linneaeus, 1758) | 2n = 21,X0#3 | 2tA | 2tA | 4tA |
| Cicadellidae | ||||
| Ferrariana trivittata* (Signoret, 1854) | 2n = 19,X0 | 2tA | 2tA | 2iA |
| Oncometopia facialis* (Signoret, 1854) | 2n = 17,X0 | 2tA | 2tA | 2iA |
| Membracidae | ||||
| Amblyophallus exaltatus* (Fabricius, 1803) | 2n = 20,neo-XY | 1tX | 2tXY | 2tA |
| Bolbonota melaena* (Germar, 1835) | 2n = 21,X0 | Absent | 2iA | 2iA |
| Enchenopa sp.* | 2n = 19,X0 | Absent | 2tA | 2tA |
| Horiola picta* (Coquebert, 1801) | 2n = 21,X0 | 2tA | 2tA | 2tA |
| Membracis foliatafasciata* (DeGeer, 1773) | 2n = 13,X0 | Absent | 4iA,8tA,1tX | 2tA |
| Neotynelia pubescens* (Fabricius, 1803) | 2n = 21,X0 | 2tA | 2tA | 2tA |
Genomic DNA was obtained from one representative of each family (Aetalion reticulatum, Mahanarva quadripunctata, Membracis foliatafasciata, and Oncometopia facialis) using the phenol–chloroform procedure as proposed by Sambrook and Russell (2001). The 18S and H3 sequences were amplified through polymerase chain reaction (PCR) using primers available in the literature (Cabral-de-Mello, Moura, & Martins, 2010; Cabrero, López-León, Teruel, & Camacho, 2009). The 18S probe was labeled with biotin-14-dATP through nick translation (Invitrogen, San Diego, CA, USA), while the H3 probe was labeled through PCR with digoxigenin-11-dUTP (Roche, Mannheim, Germany). Two-color FISH assays were performed for each probe according to the protocol presented in Pinkel, Straume, and Gray (1986) with modifications specified in Cabral-de-Mello et al. (2010). Probes labeled with biotin-14-dATP and digoxigenin-11-dUTP were detected using streptavidin-Alexa Fluor 488 (Invitrogen) and anti-digoxigenin-Rhodamine (Roche), respectively. All preparations were counterstained with 4,6-diamidino-2-phenylindole (DAPI) and mounted in Vectashield (Vector, Burlingame, CA, USA). Chromosome banding and FISH signals were observed with the aid of an Olympus BX61 epifluorescence microscope equipped with appropriate filters. Photographs were acquired using a DP70 cooled digital camera, which were then combined into multifocal images and manually edited in Adobe Photoshop CS2.
3 RESULTS
3.1 Karyotypes
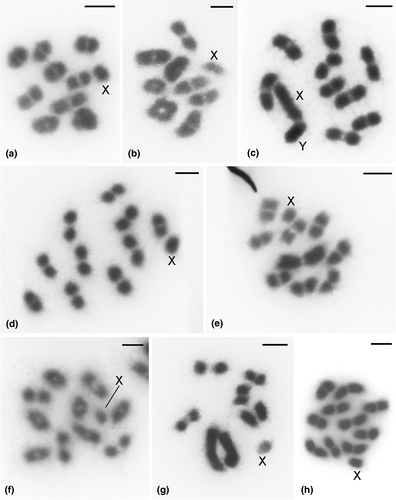
Herein, the chromosomes of eight species of Auchenorrhyncha are studied for the first time (Figure 1, Table 1). The diploid number of examined species ranged from 2n = 13 (Membracis foliatafasciata) to 2n = 21 (Neotynelia pubescens, Bolbonota melaena, and Horiola picta). Chromosome size varied slightly in most species (Figure 1), but in Enchenopa sp. (Figure 1e), M. foliatafasciata (Figure 1g), and N. entreriana (Figure 2d), large autosomal pairs were noticed, and in A. exaltatus, the sex chromosomes were large (Figure 1c). The two leafhopper species (Cicadellidae) differed in their diploid number: 2n = 19 in Ferrariana trivittata (Figure 1a) and 2n = 17 in Oncometopia facialis (Figure 1b). Out of the six species of Membracidae sampled, three have 2n = 21, Bolbonota melaena (Figure 1d), Horiola picta (Figure 1f), and Neotynelia pubescens (Figure 1h). The other three treehopper species have 2n = 20 (Amblyophallus exaltatus, Figure 1c), 2n = 19 (Enchenopa sp., Figure 1e), and 2n = 13 (Membracis foliatafasciata, Figure 1g). All species of Cercopidae (except Notozulia entreriana; 2n = 15) have 2n = 19, in agreement with previous studies (Anjos et al., 2016; Anjos et al., 2018). Aetalion reticulatum (Aetalionidae) has 2n = 21, as described by Kuznetsova and Kirillova (1993). X0 was the predominant sex system (Table 1, Figure 1). Amblyophallus exaltatus was an exception, as a neo-XY sex system was discovered in this species (Figure 1c).
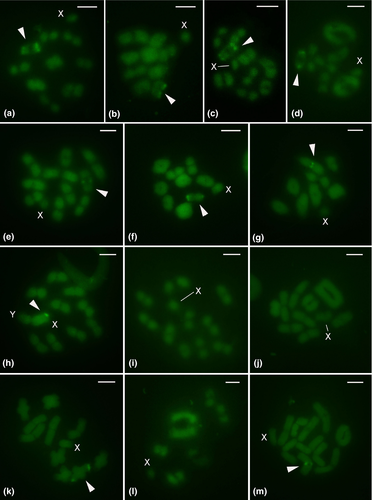
3.2 Fluorochrome staining
Fluorochrome assays revealed homogeneous DAPI staining in all species (results not shown). Most species (nine out of thirteen) showed one CMA3+ block terminally located in one autosomal pair (Table 1; Figures 2a–g,k,m). In Amblyophallus exaltatus, a terminal CMA3+ block was observed on the neo-X chromosome (Table 1; Figure 2h). No CMA3+ blocks were visible in the remaining species (Bolbonota malaena, Enchenopa sp., and Membracis foliatafasciata; Table 1; Figures 2i,j,l). All Mahanarva species exhibited the same CMA3+ banding pattern as described in Anjos et al. (2016) (Table 1).
3.3 FISH mapping of 18S rDNA and H3
Two-color FISH assays of most species revealed one autosomal locus of 18S and one autosomal locus of H3 per haploid genome, located on a distinct autosomal pair (Table 1, Figure 3a–h,j; Figure 4b,c,e–g,i). Exceptions regarding the position of 18S clusters include Mahanarva vittata (Figure 3i) and Amblyophallus exaltatus (Figure 4d), with clusters located on sex chromosomes, and Membracis foliatafasciata (Figure 4h), with multiple loci (a total of 13 in diploid genome) found on autosomes and in the X chromosome. Aetalion reticulatum was the sole exception for the H3 histone gene, as this species had clusters on two pairs of autosomes (Figure 4a).
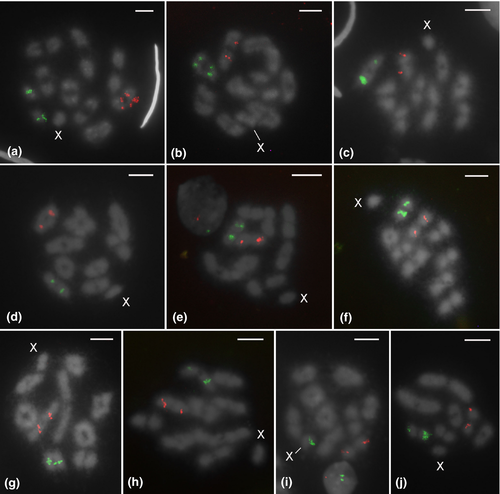
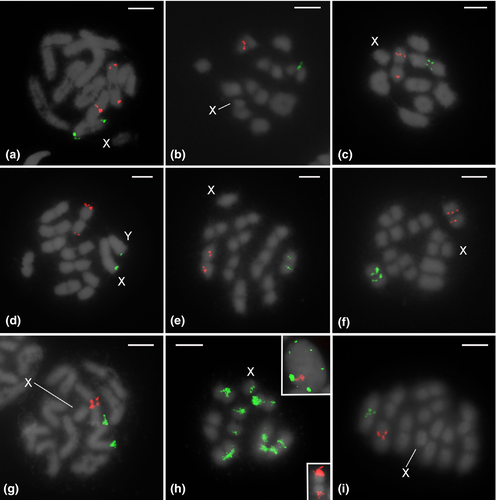
18S and H3 gene clusters were found on interstitial or terminal chromosome regions, depending on the species (Table 1, Figures 3 and 4). Interstitial 18S clusters were detected in all species of the genus Deois (Figures 3a–c), five species of Mahanarva (Figure 3d–h), and Bolbonota melaena (Figure 4e). Terminal sites were observed in the sex chromosomes of M. vittata (Figure 3i) and Amblyophallus exaltatus (Figure 4d), and in one autosomal pair of Notozulia entreriana (Figure 3j), Aetalion reticulatum (Figure 4a), Ferrariana trivittata (Figure 4b), Oncometopia fascialis (Figure 4c), Enchenopa sp. (Figure 4f), Horiola picta (Figure 4g), and Neotynelia pubescens (Figure 4i). 18S clusters were located terminally or interstitially in Membracis foliatafasciata, depending on the chromosome (Figure 4h). Interstitial H3 clusters were present in nearly all cercopids (Figures 3a–h), except Mahanarva vittata (Figure 3i) and Notozulia entreriana (Figure 3j), in which clusters were located at terminal chromosome regions. The two sampled species of Cicadellidae (Figures 4b,c) and one Membracidae (Bolbonota melaena; Figure 4e) also showed interstitial clusters. Terminal clusters were observed in five species of Membracidae (Figure 4d–f–i) and Aetalion reticulatum (Aetalionidae; Figure 4a).
4 DISCUSSION
Our results expand the available evidence concerning karyotypic variability in cicadomorphans and demonstrate that the number and distribution of repetitive elements (including A + G-rich DNAs, 18S, and H3) are highly conserved and presumably evolutionarily stable in these insects. According to Kuznetsova and Aguin-Pombo (2015; and references therein), a putative ancestral chromosome number for Cercopoidea and Membracoidea ranges between 2n = 26–28 and 2n = 22, respectively. However, several species studied here have fewer chromosomes compared with the putative ancestral values. Reduction in chromosome number can be caused by fusions over the course of repeated chromosome diversification events. Large chromosomes and low chromosome numbers in distantly related cicadomorphans (e.g., Membracis foliatafasciata (2n = 13) and Notozulia entreriana (2n = 15)) reinforce our hypothesis that independent chromosome fusions occurred in the group. For instance, the neo-XY sex system (e.g., Amblyophallus exaltatus) could have originated from a fusion between the original X and an autosome. Despite the extraordinary diversity in diploid number—which ranges from 2n = 13 to 2n = 22 in sampled males—most cicadomorphans share the same sex determination system (X0), a feature widely observed in other auchenorrhynchan insects (Kuznetsova & Aguin-Pombo, 2015). This suggests that autosomes are more likely to be involved in chromosomal rearrangements than the sex chromosomes.
Despite the remarkable variation in chromosome number, CMA3+ blocks, when present, were detected in a single autosomal pair in most species, except in three Mahanarva (Anjos et al., 2016) and A. exaltatus. Low specificity in base pair richness appears to be common in Auchenorrhyncha, as most species lack DAPI/CMA3+ blocks and have fewer A + T-rich or G + C-rich regions (Maryańska-Nadachowska et al., 2012). The majority of sampled species have few autosomal CMA3+ blocks, which is also the case of some spittlebugs in the genera Philaenus and Mahanarva (Anjos et al., 2016; Maryańska-Nadachowska et al., 2012). Some rare exceptions include M. tristis, whose chromosomes bear multiple CMA3+ blocks (Anjos et al., 2016), and two planthopper species in the family Issidae, Agalmatium bilobum and Hysteropterum albaceticum, which carry several A + T-rich blocks spread over their chromosomes (Kuznetsova et al., 2009). The presence of CMA3+ blocks on autosomes, which can be terminal or interstitial, is the most common condition in Cicadomorpha. Alternate locations of G + C-rich regions can result from a variety of factors, which include (i) chromosomal rearrangements, such as fusions that lead to the neo-XY sex determination system (presumably in Amblyophallus exaltatus, and previously reported for Philaneus italosignum (Maryańska-Nadachowska et al., 2012); (ii) locus multiplication and subsequent spread of G + C-rich repetitive DNAs in species carrying multiple CMA3+ blocks (Anjos et al., 2016). Base pair richness was previously observed in other hemipteran insects, such as aphids (Bizzaro, Barbolini, & Mandrioli, 1999; Criniti et al., 2005; Mandrioli, Azzoni, Lombardo, & Manicardi, 2011; Marco, Cassanelli, Mazzoni, Bizzaro, & Manicardi, 2009) and heteropterans (Bardella, da Rosa, & Vanzela, 2014; Bardella, Grazia, Fernandes, & Vanzela, 2014).
Furthermore, our results demonstrate that the single autosomal 18S locus is a remarkably conserved trait in Cicadomorpha, although its specific position varies, that is, interstitial or terminal. This feature has also been reported for species in other auchenorrhynchan families, such as Aphrophoridae (Kuznetsova, Grozeva, et al., 2015; Kuznetsova, Maryańska-Nadachowska, et al., 2015; Maryańska-Nadachowska et al., 2013) and Myerslopiidae, the sister group to Membracoidea (Dietrich et al., 2017; Golub et al., 2014). This condition may be ancestral to Cicadomorpha, considering that a similar arrangement of these 18S loci has been observed in planthoppers, that is, Fulgoroidea: Issidae (Maryańska-Nadachowska et al., 2016), a sister lineage to Cicadomorpha (Cryan & Urban, 2012).
Alternate organizational patterns, such as multiple 18S loci dispersed along different chromosomes or located on sex chromosomes, are also observed in Cicadomorpha, albeit rarely. Multiple 18S loci, as observed herein for Membracis foliatafasciata, have also been reported other insects with holocentric chromosomes, like moths and butterflies (Nguyen, Sahara, Yoshido, & Marec, 2010). In Hemiptera, multiple rDNA loci were observed in Triatoma infestans (Panzera et al., 2014), although in lower number in comparison with M. foliatafasciata. This species represents an exceptional case of high 18S loci in Hemiptera. Dispersed 18S loci could result from genomic amplification followed by spread, as previously suggested for repetitive sequences in insect chromosomes (Anjos et al., 2015; Cabral-de-Mello, Cabrero, López-León, & Camacho, 2011; Cabral-de-Mello, Moura, Melo, & Martins, 2011; Cabrero & Camacho, 2008). The occurrence of 18S on sex chromosomes in Mahanarva tristis and Amblyophallus exaltatus suggests putative transposition and large chromosome rearrangements, respectively, which are involved in the relocation of 18S clusters. Sex chromosomes bearing 18S genes resulting from chromosome fusions were also reported in several Philaenus species (Maryańska-Nadachowska et al., 2013).
This is the first time that histone genes have been mapped in species of Cicadomorpha. Similar to the 18S loci, H3 loci were conserved in number and autosome location, but they also varied in terms of their chromosome position (interstitial or terminal). This variation likely resulted from chromosome inversions or transpositions. The consistent placement of H3 loci in autosomes in distinct families suggests that this is a common feature in cicadormorphan insects. Further investigation is warranted in related lineages, such as planthoppers, to confirm whether this represents the ancestral state in Cicadomorpha. The chromosome distribution of H3 loci is identical to that in other insects with holocentric chromosomes, such as true bugs in the families Coreidae and Pentatomidae (Bardella et al., 2016), aphids (Mandrioli & Manicardi, 2013), and tortricid moths (Síchová, Nguyen, Dalíková, & Marec, 2013), as well as in a few insects with monocentric chromosomes (Cabral-de-Mello, Cabrero, et al., 2011; Cabral-de-Mello, Moura, et al., 2011; Cabrero et al., 2009; Teruel, Cabrero, Perfectti, & Camacho, 2010). In acridid grasshoppers, the distribution of these loci may be influenced by strong purifying selection at the chromosome level (Cabrero et al., 2009), a process that could also explain the data reported here.
Our findings provide further evidence to support chromosome number diversity in cicadomorphan insects (Kuznetsova & Aguin-Pombo, 2015). On the other hand, several other chromosome traits were found to be more conserved, such as the presence and location of CMA3+ blocks, as well as H3 and 18S loci. In cicadomorphans, other stable features include the pool of repetitive DNAs (C0t-DNA) and the U1 snRNA loci in spittlebugs (Anjos et al., 2016, 2018). Although these repeated sequences are conserved in number and location, assessing chromosome homology in these groups is a challenging prospect due to the similar size and appearance of holocentric chromosomes. While our data expand the still poorly known chromosome organization in Cicadomorpha, further studies will provide new insights into the chromosome evolution of this fascinating group.
ACKNOWLEDGEMENTS
The authors are grateful to three anonymous reviewers for their valuable suggestions. Special thanks to Dr. Keith Bayless for proofreading an earlier version of this manuscript and to Camilo Flórez Valencia, Giovane Proença, and Iago Bueno for providing images of live insects for the graphical abstract. This study was partially supported by the São Paulo Research Foundation (Fundação de Amparo a Pesquisa do Estado de São Paulo—FAPESP 2014/11763-8) and the Coordination for the Improvement of Higher Education Personnel (Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior—CAPES). AA is supported by FAPESP (2014/06226-3), and OE is supported by CAPES and FAPESP (2012/21398-0; 2014/01793-7). DCCM is the recipient of a career development fellowship from the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq 304758/2014-0).




