Genetic structure of lake and stream populations in a Pyrenean amphibian (Calotriton asper) reveals evolutionary significant units associated with paedomorphosis
Abstract
Differences in environmental conditions such as those between lakes and streams can produce phenotypic variation and ultimately promote evolutionary diversification. Some species of newts and salamanders can occupy these habitats and express alternative phenotypes: metamorphs that lose gills at metamorphosis and paedomorphs that retain them at the adult stage. Whereas this process is facultative in some species, it is obligatory in others, thus suggesting that isolation and environmental pressures may have canalized developmental pathways. In this study, we focused our research on the Pyrenean brook newt, Calotriton asper, which is present in both lakes and streams, but whose fully aquatic paedomorphic individuals are only present in lakes. We aimed to determine the genetic structure and differentiation of two paedomorphic populations, including their surrounding stream and lake metamorphic populations, to test whether populations of paedomorphs can constitute evolutionary significant units. Although gene flow was identified between lakes and nearby stream populations, there was a low percentage of dispersers, and the paedomorphic populations were genetically differentiated from the populations of metamorphs. It is likely that the studied lakes have offered peculiar conditions that have allowed the development of a paedomorphic phenotype. These populations and phenotypes therefore constitute good models to understand local adaptations. As each of these populations of paedomorphs can be considered evolutionary significant units that cannot be replaced by other nearby populations in case of a population crash, conservation actions should be focused directly on them.
1 INTRODUCTION
Polymorphic species can exhibit phenotypic variation as a result of adaptation to different environmental conditions (Fusco & Minelli, 2010; Seehausen, 2015; West-Eberhard, 1989). Therefore, adapted alternative phenotypes are expected to match the resource availability with their functional abilities (Gross, 1991; Schlichting & Pigliucci, 1998; Skulason & Smith, 1995). In this aspect, differences in environmental conditions (e.g., lake and stream habitats) can drive the phenotypic variation among different populations that can promote adaptation and ultimately even evolutionary diversification (Caspers et al., 2014; Ferchaud & Hansen, 2016; Goedbloed et al., 2017; Schluter, 2000). Indeed, these environments have important differences, such as water flow rates, that can result in different behavioral responses (Kaya & Jeanes, 1995) and morphologies (Izen, Stuart, Jiang, & Bolnick, 2016). Conversely, the terrestrial lands surrounding freshwater habitats can represent natural barriers to dispersal of aquatic organisms, which can affect divergence patterns (Isselin-Nondedeu et al., 2017; Weber, Bradburd, Stuart, Stutz, & Bolnick, 2017). Whereas streams and rivers allow aquatic dispersal through the riverine networks, high-altitude lakes do not unless they are connected by lotic waters. Therefore, gene flow between pond or lake and stream populations can play an important role in modulating these divergences (Hendrix, Schmidt, Schaub, Krause, & Steinfartz, 2017; Weber et al., 2017). Therefore, despite the existence of overland dispersal in stream species (Campbell Grant, Nichols, Lowe, & Fagan, 2010), more isolation and differentiation are expected in lakes than in streams, which is particularly crucial for exclusively aquatic species.
Some species of newts and salamanders can skip their terrestrial stage through a process called paedomorphosis, which involves the retention of adult traits such as gills in the larvae (Denoël, Joly, & Whiteman, 2005; Gould, 1977; Laudet, 2011). Paedomorphs are favored in permanent and predator-free environments (Denoël & Ficetola, 2014), whereas metamorphs can take advantage of productive temporary waters and by dispersal and survival in favorable terrestrial landscapes when water quality is deteriorated (Denoël, Dalleur, Langrand, Besnard, & Cayuela, 2018; Denoël, Whiteman, & Wissinger, 2007; Mathiron, Lena, Baouch, & Denoël, 2017). Moreover, the persistence of paedomorphs and metamorphs in the same habitats is favored by resource partitioning (Lejeune, Sturaro, Lepoint, & Denoël, 2018).
Research on unpredictable habitats, such as ponds, shows that paedomorphosis can be facultative (i.e., a polyphenism) in some species (Denoël & Ficetola, 2014; Semlitsch, 1987). Several arguments support this hypothesis. First, sexual behavior experiments show that both morphs are compatible; that is, the two phenotypes can largely intercross (Denoël, Poncin, & Ruwet, 2001; Whiteman & Semlitsch, 2005). Second, after the removal of the perturbation, metamorphic colonizers can lay eggs that can turn into paedomorphs (Denoël & Winandy, 2015). Third, detrimental conditions can induce individuals to opt for paedomorphosis or metamorphosis (Mathiron et al., 2017). Ultimately, the analysis of gene flows between alternative phenotypes confirms that paedomorphs and metamorphs can form a unique population in a shared aquatic habitat (Oromi, Michaux, & Denoël, 2016). However, the fixation of paedomorphosis (i.e., obligatory paedomorphosis) in some species and families suggests that strong environmental conditions have influenced one of the alternative phenotypes and that an ecological and sexual isolation has occurred between the distinct phenotypes (Bonett, Steffen, Lambert, Wiens, & Chippindale, 2014). The experimental selection of paedomorphosis in each generation can promote one of the two phenotypes (Semlitsch & Wilbur, 1989), which is likely to have favored a quantitative trait locus (QTL) of metamorphic failure (Page, Boley, Smith, Putta, & Voss, 2010; Voss & Shaffer, 1997). Population variation in the experimental response to environmental cues has been highlighted in the research (Semlitsch, Harris, & Wilbur, 1990; West-Eberhard, 1989). Moreover, genetic evidence has been found of different levels of isolation or specialization among populations (Bonett, Phillips, Ledbetter, Martin, & Lehman, 2018; Percino-Daniel, Recuero, Vázquez-Domínguez, Zamudio, & Parra-Olea, 2016) and of a different genetic basis of paedomorphosis across species (Keinath, Voss, Tsonis, & Smith, 2017). Environmental constraints, such as land aridity, are considered a selective factor promoting the prolonged life in water and paedomorphosis of some Mexican salamanders of the family Ambystomatidae (Voss & Shaffer, 1997) and some plethodontid salamanders from Texas (Bonett et al., 2014).
In the case of the Pyrenean brook newt, Calotriton asper (Dugès, 1852), an endemic newt (Salamandridae) widely distributed across the Pyrenean mountains (Sillero et al., 2014), metamorphic individuals are found in both streams and lakes, whereas paedomorphs have only been described in three high-altitude lakes (Campeny, Montori, & Llorente, 1986; Miró, Sabás, & Ventura, 2018; Oromi, Amat, Sanuy, & Carranza, 2014). One of these populations is already extinct. Although the retention of functional gills in adults is the most typical and unequivocal evidence of facultative paedomorphosis in pond-breeding newts, this difference with metamorphs is less obvious in the lakes with the presence of C. asper paedomorphs. Indeed, paedomorphs simply present gill remnants as a result of a late or incomplete metamorphosis, possibly without functional implication (Oromi et al., 2014). However, this case fits the idea of constraints and isolation in high-altitude lakes (see the best of a bad lot hypothesis in Whiteman et al., 2012). Moreover, the morphology of newts in lakes is characterized by smooth skin with a divergent morphology and a less developed sexual dimorphism than the typical stream-dwelling populations of the Pyrenean newts (described in Oromi et al., 2014). Therefore, the stream and lake populations of C. asper offer the opportunity to determine whether lake populations, particularly those where paedomorphic traits are expressed, are more isolated and present a different genetic structure than stream populations. Thus by studying these peculiar populations, we aim to determine whether populations with paedomorphosis represent evolutionary significant units in need of specific conservation measures.
2 MATERIALS AND METHODS
2.1 Population sampling
Pyrenean brook newts were collected from 14 different localities in the Pyrenees (n = 426 individuals). We limited the sampling to adults to decrease the probability of having siblings. The capture design consisted of eight and six populations in the Western and Central Pyrenees, respectively. Each area included a lake where paedomorphosis was found (i.e., Ibón de Acherito—IAC in the Western and Ibón de Perramó—IPE in the Central Pyrenees), surrounded by nearby stream or lake populations (Figure 1; Table 1). All lakes were of glacial origin and located at high elevations (above 1,850 m). The stream populations were also at relatively high altitude from 1,200 to 1,800 m. All newts were directly returned to the place of capture after morphological examination and tissue sampling (small clips that do not affect the body condition or survival of newts: Arntzen, Smithson, & Oldham, 1999) for DNA analysis. Such manipulations followed ethical standards (e.g., ethical committee of the University of Liege, protocol 1,613). All samples were preserved individually in ethanol.
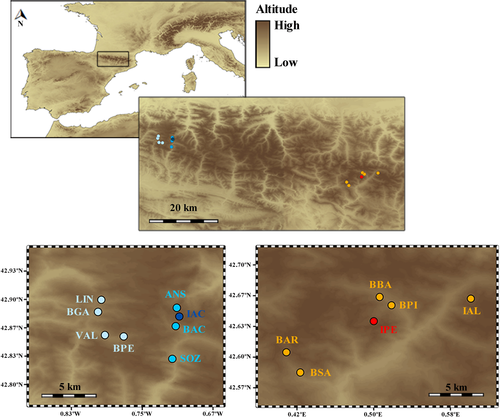
| Population | Code | Zone | Latitude (°) | Longitude (°) | Altitude | Habitat | Process |
|---|---|---|---|---|---|---|---|
| Barranco Gamuetta | BGA | W | 42.89 | −0.80 | 1,324 m | S | M |
| Linza | LIN | W | 42.90 | −0.80 | 1,374 m | S | M |
| Valdagras | VAL | W | 42.86 | −0.79 | 1,266 m | S | M |
| Barranco de Petraficha | BPE | W | 42.86 | −0.77 | 1,427 m | S | M |
| Selva de Oza | SOZ | W | 42.83 | −0.71 | 1,181 m | S | M |
| Barranco de Acherito | BAC | W | 42.87 | −0.71 | 1,385 m | S | M |
| Ansabère | ANS | W | 42.89 | −0.71 | 1,787 m | S | M |
| Ibón de Acherito | IAC | W | 42.88 | −0.71 | 1,882 m | L | P |
| Barbarisa | BAR | C | 42.61 | 0.41 | 2,333 m | L | M |
| Barranco de Sabaril | BSA | C | 42.58 | 0.42 | 1,684 m | S | M |
| Ibón de Perramó | IPE | C | 42.64 | 0.50 | 2,406 m | L | P |
| Barranco de Batisielles | BBA | C | 42.67 | 0.51 | 1,815 m | S | M |
| Barranco del Pino | BPI | C | 42.66 | 0.52 | 1,604 m | S | M |
| Ibón de Alba | IAL | C | 42.66 | 0.61 | 2,301 m | L | M |
- W: Western Pyrenees; C: Central Pyrenees; S: stream; L: lake; M: obligate metamorphosis; P: facultative paedomorphosis.
2.2 Genotyping and data analysis
DNA was extracted using Qiagen DNeasy Blood and Tissue Kit. Genetic analyses were based on 19 microsatellite loci (Ca1, Ca3, Ca5, Ca7, Ca8, Ca16, Ca20, Ca21, Ca22, Ca24, Ca25, Ca29, Ca30, Ca32, Ca35, Ca38, Us2, Us3, and Us7) as previously published in Drechsler et al. (2013). PCR conditions and genotyping followed the methodology provided in Drechsler et al. (2013).
Potential genotyping errors, such as the presence of null alleles, were analyzed using MICRO-CHECKER version 2.2.3 (Van Oosterhout, Hutchinson, Wills, & Shipley, 2004). The presence and frequency of null alleles were additionally examined using FreeNA (Chapuis & Estoup, 2007) following the expectation–maximization (EM) algorithm. The presence of null alleles may result in an overestimation of population differentiation. Thus, the same program was used to compute the FST statistic with and without the excluding null alleles (ENA) correction method. The bootstrap 95% confidence intervals (CIs) for the global FST values were calculated using 50,000 replicates over loci. Allele and genotype frequencies, number of alleles per locus (NA), expected (HE) and observed (HO) heterozygosity, and allelic richness (AR) were obtained for each population using FSTAT version 2.9.3.2 (Goudet, 1995). Genotype frequencies were tested for conformity to the Hardy–Weinberg equilibrium by GENEPOP version 4.253 using Markov chain permutations (1,000 dememorizations, 100 batches, and 1,000 iterations per batch) according to the algorithm of Guo and Thompson (1992). Significance values for multiple tests were adjusted by applying a sequential Bonferroni correction (Rice, 1989). This package was also used to evaluate the marker-to-marker genotypic disequilibrium by adjusting for the Bonferroni correction. The overall and pairwise FST values were calculated to analyze the differentiation among populations in allele frequencies, and FIS was determined to estimate the fixation index using FSTAT version 2.9.3.2 (Goudet, 1995).
The Mantel test (Mantel, 1967) was used to evaluate the isolation by distance (IBD) hypothesis by analyzing the relationship between geographical and genetic distances. Genetic distances were standardized as FST/(1 − FST), and geographical distances were log-transformed (log10) to linearize the relationship between geographical distances and FST values (Rousset, 1997). The significance of the matrix correlation coefficients was estimated in Isolation by Distance Web Service (IBDWS) with 10,000 permutations (Jensen, Bohonak, & Kelley, 2005). IBD was analyzed separately in each cluster.
Population structure was analyzed by STRUCTURE version 2.2 (Pritchard, Stephens, & Donnelly, 2000) to determine the number of possible genetic clusters (K) in our data set using the admixture and correlated allele frequency model. We tested from K = 1 to K = 15 for all the populations, and to K = 9 and K = 7 when analyzing the Western and Central Pyrenees data sets, respectively. A total of 10 independent runs were made with one million Markov chain Monte Carlo (MCMC) iterations, discarding the first 100,000 MCMC steps as a burn-in phase. To avoid a bias as a result of the sensitivity of STRUCTURE due to the sample size of each distinct population, the same simulation has been run with a maximum of 20 samples per population (Vörös et al., 2017). STRUCTURE HARVESTER version 0.6.94 was used to determine the ΔK using the Evanno method (Earl & vonHoldt, 2012). Evanno, Regnaut, and Goudet (2005) suggested that the most probable K is the one obtained from the modal value of the rate of change of the LnP(D) value between successive runs (ΔK). Structure plots were created with Clumpak (Kopelman, Mayzel, Jakobsson, Rosenberg, & Mayrose, 2015), which compares all runs at each value of K to identify the optimal clustering scenarios (Kopelman et al., 2015). Moreover, analyses of molecular variance (AMOVA) were performed in ARLEQUIN 3.5.1.2 (Excoffier & Lischer, 2010) by grouping the sampling localities as indicated by STRUCTURE.
To obtain the spatial genetic structure of the populations within each zone (Central and Western Pyrenees), the number and composition of panmictic groups as well as the spatial boundaries among them were estimated using a Bayesian model computed with GENELAND version 2.0.0 (Guillot, Mortier, & Estoup, 2005) in R (RCoreTeam, 2013). The software implements a Markov chain Monte Carlo (MCMC) procedure to determine the best clustering of samples based on genetic and geographical information. The geographical information is taken into account at the Bayesian prior level, so that clusters corresponding to spatially structured groups are considered to be more likely than clusters that are randomly distributed in space. Five million MCMC iterations sampled each 1,000 steps with a 50,000 burn-in period, and the same maximum number of clusters as in STRUCTURE was run to estimate the model parameters and posterior probabilities of group membership (p).
Furthermore, a discriminant analysis of principal components (DAPCs: Jombart, Devillard, & Balloux, 2010) using the R package “adegenet” (Jombart, 2008) was performed to corroborate the clustering suggested by STRUCTURE. The minimum number of principal components required to account for at least 90% of the variation was selected. We then explored the best value of K from one to 15 for the global data set, or 9 and 8, respectively, for the Western and Central Pyrenees and computed individual membership probabilities to inferred clusters. The find.clusters function was used to detect the number of clusters in the data set. It uses K-means clustering which decomposes the total variance of a variable into between-group and within-group components. We used the Bayesian information criterion (BIC) to identify meaningful Ks that summarized our data. The best number of clusters has the lowest associated BIC.
We also employed a landscape genetic-based causal modeling approach (Cushman, McKelvey, Hayden, & Schwartz, 2006; Cushman, Wasserman, Landguth, & Shirk, 2013) to test for mountain barrier effects while accounting for altitude and slope (resistance surfaces) and geographical (Euclidean) distances on observed genetic distances among populations (pairwise FST). We first obtained the elevation raster from a digital elevation model of the Pyrenees at 90-m resolution (The Consultative Group for International Agriculture Research's—Consortium for Spatial Information (CGIA-CSI), available at http://srtm.csi.cgiar.org). We then constructed the resistance surfaces assuming a linear relationship between elevation and resistance (resistance = elevation). The optimal altitude range for C. asper is at elevations from 750 to 1,500 m (Montori & Llorente, 2014). This coincides with the maximum genetic diversity found for this species from 1,000 to 1,500 m altitude (Valbuena-Ureña et al., 2018). From 1,500 m upwards, the genetic diversity indices decrease, meaning that altitude may have some resistance to the connectivity of their populations. Most of the present study area is over 1,000 m, so there are few areas below this altitude that could represent some resistance. Starting from 1,000 or 1,500 m, the resistance increases with altitude. Moreover, Montori, Llorente, and Richter-Boix (2008) suggested that at high altitudes this species becomes more aquatic and, thus, less likely to disperse (more resistance). Therefore, these resistance surfaces consisted of a linear effect starting at four different minimum altitude thresholds (0, 1,000, 1,500, and 2,000 m a.s.l.), thus resulting in four different resistance surfaces. The resistance matrix for the slope effect was constructed by assuming a linear relationship between slope and resistance (resistance = slope). Then, the least cost paths between all population pairs in each cluster with the four elevation-based resistance models and the slope-resistance model were calculated. We used ArcGIS 10.6 (ESRI, Redlands, CA, USA) for the ecological resistance distance matrix for each model and the XLSTAT 2018.3 package ( http://www.xlstat.com/en/products/xlstat-plspm) to assess the relative support for each model based on partial Mantel tests.
The GENECLASS2 version 2.0 program (Piry et al., 2004) was used to estimate the potential recent migrants (first generation migrants) among populations using the Bayesian method of Rannala and Mountain (1997). We implemented Monte Carlo resampling using 10,000 simulated individuals and a threshold of 0.01 to calculate the assignment probabilities. This test determines the probability of individuals coming from a reference locality or of being a resident (Paetkau, Slade, Burden, & Estoup, 2004). This program computes the probability of the multilocus genotype of each individual to be encountered in a given population. The assignment criterion values of the simulated individuals are then computed, stored, and sorted, so that the probability of an observed multilocus genotype can be estimated as the rank of its corresponding criterion value within the distribution of simulated criterion values. In addition, the effective population size (Ne) for each population and genetic clusters derived from STRUCTURE was calculated using the COLONY version 2.0.4.4 program (Jones & Wang, 2010) because it considers possible genotyping errors and the presence of null alleles. COLONY uses a maximum likelihood method to conduct sibship assignment analyses to estimate Ne under the assumption of random mating. COLONY was run using the maximum likelihood approach for a dioecious/diploid species, with medium length runs and random mating, assuming polygamy for both males and females (as is the case for most salamanders) with no sibship prior. We did not use the option “update allelic frequencies,” and other parameters were used as a default.
3 RESULTS
3.1 Population genetic diversity
The presence of null alleles, verified by the MICRO-CHECKER software, was detected in eight loci in different populations (data not shown). A maximum of two null alleles per population was detected. The highest percentage of null alleles was found for locus Ca16 in the population Barranco de Batisielles (BBA; 40%). In total, null allele frequency estimates ranged from 0.05% to 9.6% with 2.7% on average across all loci. Global FST values calculated with and without correcting for null alleles had overlapping 95% confidence intervals (FST not using ENA = 0.2284 and FST using ENA = 0.2238 with the respective 95% CI [0.1672–0.3055] and [0.1625–0.3012]), which means that the impact of null alleles can be neglected. Classical measures of population differentiation are only slightly biased with a null allele frequency ranging from 5% to 8% on average across loci. Given that the average percentage of null allele frequency across loci (2.7%) is lower than 5%, and FST did not vary after excluding null alleles, all loci were kept for further statistical analyses. The 19 microsatellite loci were polymorphic, and the number of alleles per locus (NA) ranged from 1 (Locus Ca38: BAR, BSA, IPE, and IAC populations) to 11 (Locus Ca16: IAC population). The genetic diversity indices of the sampled C. asper populations are shown in Table 2. Deviation of the Hardy–Weinberg equilibrium was observed in five tests (Ca3 in BSA, Ca7 in BPE, Ca16 in BBA, Ca19 in BAR, and Ca29 in IAL) caused by heterozygote deficiency, which could be explained by non-amplifying alleles. We found no evidence of a significant deviation from linkage disequilibrium after applying the Bonferroni correction.
| POP | n | N A | P A | AR | H O | H E | F IS |
|---|---|---|---|---|---|---|---|
| BGA | 50 | 7.000 | 1 | 4.093 | 0.609 | 0.633 | 0.049 |
| LIN | 39 | 6.632 | 3 | 4.102 | 0.606 | 0.621 | 0.037 |
| VAL | 39 | 6.000 | 1 | 3.937 | 0.641 | 0.619 | −0.022 |
| BPE | 21 | 6.263 | 3 | 4.333 | 0.644 | 0.650 | 0.034 |
| SOZ | 21 | 5.789 | 1 | 4.188 | 0.642 | 0.656 | 0.047 |
| BAC | 6 | 4.158 | 0 | 4.158 | 0.675 | 0.615 | −0.007 |
| ANS | 20 | 4.368 | 1 | 3.419 | 0.579 | 0.573 | 0.016 |
| IACa | 44 | 6.316 | 4 | 3.497 | 0.595 | 0.598 | 0.017 |
| BAR | 12 | 4.684 | 4 | 3.876 | 0.605 | 0.609 | 0.049 |
| BSA | 20 | 5.316 | 3 | 3.810 | 0.597 | 0.601 | 0.032 |
| IPEa | 69 | 4.053 | 2 | 2.821 | 0.448 | 0.457 | 0.028 |
| BBA | 6 | 3.737 | 1 | 3.737 | 0.553 | 0.564 | 0.111 |
| BPI | 15 | 4.421 | 1 | 3.508 | 0.544 | 0.569 | 0.078 |
| IAL | 64 | 4.684 | 4 | 3.347 | 0.495 | 0.505 | 0.028 |
- POP: population code (see Table 1 for a full description); n: number of sampled individuals; NA: mean number of alleles per locus; PA: number of private alleles; AR: allelic richness; HO: observed heterozygosity; HE: expected heterozygosity; FIS: fixation index; values in bold showed P < 0.05. aPopulations with paedomorphosis.
3.2 Genetic structure and landscape genetic analyses
The differences among the populations estimated by FST (Supporting Information Table S1) ranged from −0.0007 (BGA and BPE) to 0.362 (ANS and IPE).
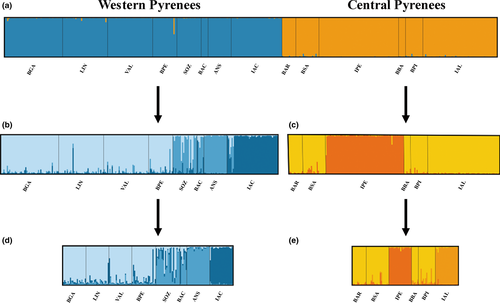
The results of STRUCTURE, which considers the Evanno method, identified the most probable clustering value of K = 2, corresponding to the geographical grouping into two populations, namely, Western and Central Pyrenees (Figure 2a). The separate analysis of the western zone showed three genetic clusters (K = 3; Figure 2b): the westernmost populations grouped into cluster W1 (BGA, LIN, VAL, and BPE) and the remaining populations grouped into W2 (SOZ, BAC, and ANS) while keeping the IAC population (a lake with paedomorphs) assigned to a distinct cluster (W3). We found the same pattern by using a simulation of a maximum of 20 individuals per population: K = 3, with the same grouping of populations (Figure 2d). In the central zone, the most probable K value was 2: the former C1 grouping all central lake- and stream-dwelling populations (BAR, BSA, BBA, BPI, and IAL), with the exception of the lake IPE (a lake with paedomorphs) that was clearly separated into C2 (Figure 2c). Although the approach using the Evanno method cannot support K = 1, the pairwise distances between the populations (Supporting Information Table S1) showed that the most divergent population was the paedomorphic population (IPE), thus supporting K = 2. For the limited data set within the central zone (i.e., with a maximum of 20 individuals per population), all populations were grouped into the same clusters (IPE separated from the other central populations), except from IAL (Ibon de Alba, a lake without paedomorphs) that, in this case, was also clustered separately (K = 3; Figure 2e). In accordance with the above structure, the global AMOVA results showed that the variation between the regions (Western and Central Pyrenees) was responsible for 24% of the variation (Table 3). This variation did not exceed 10% when the two main clusters (Western and Central) were analyzed separately, and most of the variation was within the population. The best K values in the DAPC analyses were 7 for the global data set (decrease of BIC from 2 to 7, after which BIC increased, suggesting that seven clusters should be retained), four for the western populations, and three for the Central ones (Supporting Information Figure S1). Population clustering was in agreement with the likelihood-based method in STRUCTURE: both sectors (Central and Western Pyrenees) grouped separately; when analyzing the structure within Central and Western Pyrenees, IPE clustered separately from the remaining central populations, and IAC clustered in a single group with no admixture from the remaining western populations (Supporting Information Figure S2). IAL clustered in a separate group with DAPC as it had in STRUCTURE with the limited data set. Comparing the different clustering solutions using the BIC from K = 2 to 7 for the global data set, the resulting clustering always separated first in both sectors (Western and Central Pyrenees). The paedomorphic populations were clearly differentiated and were both along the first DA axis, which is the one that explains most of the variability.
| Source of variation | SS | VC | %Var | F i | p |
|---|---|---|---|---|---|
| Zones (Central and Western Pyrenees) | |||||
| Among groups | 858.406 | 1.909 | 23.96 | 0.313 | <0.00001 |
| Among populations within groups | 458.463 | 0.581 | 7.30 | 0.096 | <0.00001 |
| Within populations | 4590.326 | 5.477 | 68.74 | 0.239 | <0.00001 |
| Cluster W (Western) | |||||
| Among groups | 1720.440 | 0.565 | 8.60 | 0.01 | <0.00001 |
| Among populations within groups | 56.772 | 0.099 | 1.51 | 0.016 | <0.00001 |
| Within populations | 2786.569 | 5.904 | 89.89 | 0.086 | <0.00001 |
| Cluster C (Central) | |||||
| Among groups | 124.944 | 0.307 | 5.29 | 0.15 | <0.00001 |
| Among populations within groups | 104.702 | 0.566 | 9.75 | 0.102 | <0.00001 |
| Within populations | 1803.757 | 4.928 | 84.95 | 0.053 | <0.00001 |
- SS: sum of squares; VC: variance components; %Va:= percentage of variation, Fi: fixation index.
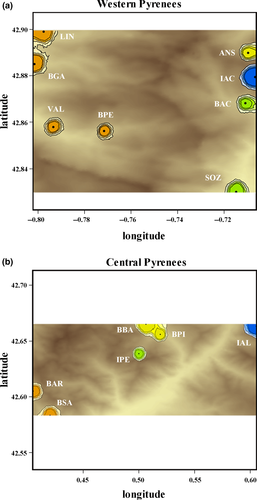
The landscape genetic analyses performed in GENELAND, which takes the spatial position of the individuals into account, provided slightly different distributions of the estimated number of populations with a mode of K = 4 for each zone (Western and Central Pyrenees) (Supporting Information Figure S3). The grouping of populations was similar to that obtained with STRUCTURE for the western zone, with IAC (lake with paedomorphs) clearly separate in a single cluster. In the Central Pyrenees, IPE (lake with paedomorphs) still clustered separately, while the remaining populations were separated into three clusters (Figure 3; Supporting Information Figure S3). IAL also clustered separately.
The causal modeling approach revealed a mountain barrier effect to gene flow for the western cluster, as genetic distances showed highly significant correlations with the altitude and slope (Supporting Information Table S2). However, partial Mantel tests did not suggest any potential effect of an elevation barrier on the genetic structure for the central cluster, as we found no significant correlation between genetic distances and any of the tested variables (Supporting Information Table S2).
3.3 Detection of migrants and effective population size
Assignment tests implemented in GENECLASS2 identified only 11 potential migrants, assuming that the reference population comes from a unique gene pool (Table 4). Indeed, most of the individuals were assigned to their origin population with low average assignment probabilities.
| POP | Cluster | n | PM | Highest AProb |
|---|---|---|---|---|
| BGA | W.1 | 50 | 2 (4%) | 0.37 |
| LIN | W.1 | 39 | 0 (0%) | 0.37 |
| VAL | W.1 | 39 | 1 (2.6%) | 0.46 |
| BPE | W.1 | 21 | 1 (4.8%) | 0.36 |
| SOZ | W.2 | 21 | 1 (4.8%) | 0.31 |
| BAC | W.2 | 6 | 0 (0%) | 0.30 |
| ANS | W.2 | 20 | 0 (0%) | 0.36 |
| IACa | W.3 | 44 | 2 (4.5%) | 0.41 |
| BAR | C.1 | 12 | 1 (8.3%) | 0.27 |
| BSA | C.1 | 20 | 0 (0%) | 0.28 |
| IPEa | C.2 | 69 | 0 (0%) | 0.47 |
| BBA | C.1 | 6 | 0 (0%) | 0.14 |
| BPI | C.1 | 15 | 2 (13.3%) | 0.26 |
| IAL | C.1 | 64 | 1 (1.6%) | 0.45 |
- POP: origin and assigned population; PM: estimated number of potential migrants (and percentage within the population); AProb: average probability of assignment; aPopulations with paedomorphic individuals. See Table 1 for population codes.
The effective population size ranged from 35 to 69 when considering localities separately and from 51 to 408 when considering clusters defined by STRUCTURE (Table 5). The three populations sampled with fewer than 15 individuals were excluded from the analysis because they showed incongruent infinite values.
4 DISCUSSION
Variation in habitat use may have profound evolutionary consequences, influencing gene flow and selection (Garcia-Dorado, 1986; Jones & Probert, 1980; de Meeûs, Michalakis, Renaud, & Olivieri, 1993; Ravigné, Olivieri, & Dieckmann, 2004). In the present study, we show that populations expressing paedomorphosis have a different genetic structure than the other populations inhabited only by metamorphs. This finding exemplifies the differentiation and specialization occurring in some “isolated” lakes in comparison with streams. Therefore, this result has implications for our understanding of both the evolution of amphibians and paedomorphosis across lotic and lentic habitats and of conservation biology, as targeted protection of lakes is needed to maintain the genetic structure of their populations and possibly the potential for developmental and morphological variation.
4.1 Differentiation in lacustrine populations with and without paedomorphosis
Considering all lake populations (i.e., with and without paedomorphosis), genetic differentiation with respect to nearby stream populations was relatively low. However, as the number of migrants found in this study was low, the percentage of dispersers, which guaranteed the connectivity among populations of C. asper, was likely small. Notice that the low probabilities of assignment populations could suggest an effect of unsampled areas in the assignation tests. Therefore, these low probabilities can be due to the gene flow situation among these populations. However, in Calotriton, there are not so many intermediate populations in mountainous areas. The low number of migrants is in line with results of capture–mark–recapture data, which suggest that this species mainly moves short distances, that is, less than 50 m (Montori et al., 2008). Similarly, previous genetic studies found strong differentiation by distance in this species (Milá, Carranza, Guillaume, & Clobert, 2010). However, more work is needed to determine how this species disperses in natural populations. Indeed, movements may have upstream or downstream biases in stream salamanders (Lowe, Mcpeek, Likens, & Cosentino, 2008) and pond versus stream habitat specialization may also be associated with different dispersal strategies in salamanders (Hendrix et al., 2017). Particularly, the recent postglacial colonization of isolated lakes suggests better dispersal capabilities than previously thought (Carranza & Amat, 2005).
As identified by STRUCTURE, the most differentiated genetic clusters within the central and western zones of the Pyrenees corresponded specifically to lakes with paedomorphs (IAC and IPE). Their differentiation was confirmed by DAPC and GENELAND analysis. The distinction of the two populations with paedomorphs using these three approaches highly suggests the robustness of this result. With DAPC and GENELAND, as well as with STRUCTURE analysis, on the limited data set, a lake without paedomorphosis (IAL) was also identified as being well-differentiated from the other populations. No relief structure seemed to have an effect on gene flow in the central zone, but causal modeling results in the western cluster suggest a potential effect of elevation on genetic distances, implying that topography may to some extent restrict across-slope gene flow. This gene flow barrier may result in a certain isolation of those populations located at higher altitudes. However, it has to be noted that ANS and IAC seem easily connected both in terms of ecological cost distance and Euclidian distance but are genetically the most isolated. Moreover, IAC is in fact geographically located among connected populations (e.g., ANS, BAC, and SOZ). This pattern may suggest specific dispersal routes between these localities and/or local adaptations in IAC (see hereafter).
The low number of migrants from nearby populations along with the specific ecological traits of these lakes could have played an important role in the particular development, morphology, and genetics of these populations, which express paedomorphosis. Paedomorphic individuals have adaptations for life in the aquatic environment, not the terrestrial one. It may also be hypothesized that all the individuals in IAC display specific adaptations. For instance, in that lake, newts had a smooth skin, more typical of the larval aquatic stage (Oromi et al., 2014). Moreover, most animals were thin in IAC. Consequently, their sexual differentiation was not obvious: They had small cloacal development despite their adult stage (Oromi et al., 2014; M. Denoël, pers. obs.), and their general aspect suggested constraints from the aquatic environments. Low temperatures can limit the development (Sprules, 1974), and oligotrophy provides less food than other aquatic habitats where metamorphs can acquire high-energy input and good body condition (Denoël, Lena, & Joly, 2007; Denoël, Whiteman, et al., 2007). A low growth condition fits well with the best of a bad lot hypothesis proposed by Whiteman (1994), as supported by long-term surveys in paedomorphic salamanders (Whiteman et al., 2012). Alpine newts from some oligotrophic Alpine lakes also had a lean appearance and less developed gills in some years, suggesting that poor local conditions could particularly affect newt populations in such environments (Denoël, Lena, et al., 2007). Research on Mexican ambystomatids also showed that, in constraining lakes (where there are high salt levels), only salt-adapted populations could maintain, therefore explaining the absence of invasion from nearby populations of metamorphs (Percino-Daniel et al., 2016). Although it is not a matter of salt in IAC, it can be hypothesized that they are adapted to resist the local conditions. For instance, C. asper lived deeper in the lake (coming to the shoreline at night) than the less numerous syntopic palmate newts, Lissotriton helveticus (M. Denoël, pers. obs.). Future research on the physiology of these populations would give a better insight into this matter. Neutral markers were adequate to highlight gene flows and differentiation, but understanding of the local adaptations would need the application of genomics tools. Finally, an interesting point is that Bd chytrids (Batrachochytrium dendrobatidis) have been found in midwife toads (Alytes obstetricans) in IAC and upstream to ANS (Ansabère Lake), but with an enzootic and an epizootic disease dynamic (chytridiomycosis), respectively (Bates et al., 2018). In IAC, after a severe decline, local adaptations and/or conditions favored resistance to disease, whereas declines were continuous in ANS (Bates et al., 2018). C. asper maintained there, but whether the thinness of the newts may be due more to chytrids than other ecological conditions (coldness and/or lack of food) remains to be determined.
Lacustrine populations with and without paedomorphosis, as well as stream populations of C. asper, had similar low effective population sizes. However, the levels of genetic diversity (AR, HE and HO) were moderate (Valbuena-Ureña et al., 2018 for C. asper; Valbuena-Urena, Soler-Membrives, Steinfartz, Orozco-terWengel, & Carranza, 2017; for C. arnoldi), and no sign of inbreeding was detected. This pattern was previously outlined and discussed by Valbuena-Urena et al. (2017). In terms of maintaining genetic diversity, a small effective population size may not necessarily be a limiting factor. For example, life history strategies may be another factor modulating the levels of genetic diversity of certain amphibians (Fouquet et al., 2015; Paz, Ibáñez, Lips, & Crawford, 2015; Valbuena-Urena et al., 2017). The ability to disperse, survive, and reproduce depends on the reproductive mode, body size, and breeding habitat (Dalongeville, Andrello, Mouillot, Albouy, & Manel, 2016). In addition, different reproductive behaviors or mechanisms can counteract the effects of genetic drift (Allentoft & O'Brien, 2010). Although population sizes are not known, it has to be pointed out that a large number of individuals were found in some lakes and streams. For instance, there were more than 500 adults of C. asper counted at night in IAC (M. Denoël and F. Amat, pers. obs.).
4.2 Evolutionary significant units and the conservation of paedomorphic populations
Despite being a widespread process in all caudate families, populations of paedomorphs are particularly endangered by the alterations of freshwater habitats, especially in lakes where alien species are introduced (Contreras, Martínez-Meyer, Valiente, & Zambrano, 2009; Denoël et al., 2009). Metamorphs are also threatened by such introductions (Miró et al., 2018), but paedomorphic newts cannot disperse on land and are therefore more threatened because of their aquatic traits. The consequences of these introductions are particularly detrimental for endemic paedomorphic species, as shown by the near extinction in the wild of the axolotl, Ambystoma mexicanum (Contreras et al., 2009). In facultative paedomorphic species, the species can survive fish introductions but usually not the paedomorphic phenotype (Denoël, Džukić, & Kalezić, 2005). However, threat removal can enable, in some circumstances, the resilience of paedomorphs in these facultative paedomorphic populations (Denoël & Winandy, 2015). This is mainly the case when paedomorphosis is still expressed in surrounding populations.
The differentiated genetic structure of the populations of C. asper with paedomorphs from the other Pyrenean populations (i.e., those that are obligatorily metamorphic) can be considered evolutionary significant units (see e.g., Crandall, Bininda-Emonds, Mace, & Wayne, 2000; Fraser & Bernatchez, 2001). These populations are much at risk as only three populations are known, with one inhabiting a lake where chytrids have been detected and have caused amphibian decline (Bates et al., 2018) and another one that disappeared following fish introductions (Campeny et al., 1986). These introductions were not anecdotic but occurred in most Pyrenean lakes where they disturbed local communities and led some species to decline even to the point of extirpation (Miró & Ventura, 2014; Miró et al., 2018). Prioritizing efforts for the conservation of isolated lakes (Ventura et al., 2017), particularly the remnants of populations of paedomorphs, is essential because introductions continue to occur at present in the Pyrenees, and fish removal is a tedious and costly task in such habitats. It is unlikely that reintroductions from nearby streams may save these populations in the case of local crashes because of their specific genetic structure and evolution in particular habitats. Therefore, preventing all alien species introductions in such habitats is essential for their preservation. Protecting nearby populations can facilitate the resilience of C. asper in such populations, but not likely their genetic integrity, adaptive potential to the new ecological conditions, resistance to pathogens, and paedomorphosis (Bates et al., 2018; Denoël, Scimè, & Zambelli, 2016).
ACKNOWLEDGEMENTS
We are particularly grateful to Sebastian Steinfartz, who made this study possible and provided great support to the acquisition of microsatellite data. We also wish to thank two anonymous reviewers for their constructive comments on our manuscript and Laurane Winandy for field assistance. This work was supported by the Biodiversity Conservation Plan of ENDESA, S.A. (ENEL Group) for D.S.; Fonds de la Recherche Scientifique—FNRS under grant numbers J.008.13 and J.0112.16 and Fonds Spéciaux de la Recherche (University of Liège) for M.D. (Research Director at F.R.S.–FNRS); and the Marie Curie COFUND Fellowship for N.O. while at the University of Liège. The research and capture permits for newts were issued by Departamento de Agricultura, Ganaderia y Medio Ambiente (Gobierno de Aragon, Spain).




