Variation in morphology and functional performance across distinct evolutionary lineages of the Moorish gecko (Tarentola mauritanica) from the Iberian Peninsula
Abstract
Deciphering the mechanisms that underlie morphological and functional diversity is essential for understanding how organisms adapt to their environment. Interestingly, phenotypic divergence does not necessarily correspond to the geographic and genetic separation between populations. Here, we explored the morphological and functional divergence among populations of two genetically differentiated clades of the Moorish gecko, Tarentola mauritanica. We used linear and geometric morphometrics to quantify morphological variation and investigated how it translates into biting and CLIMBING PERFORMANCE, to better understand the mechanisms potentially underlying population and lineage divergence. We found marked morphological differences between clades, both in body size and head shape. However, much of this differentiation is more strongly related to local variation between populations of the same clade, suggesting that recent ecological events may be more influential than deep evolutionary history in shaping diversity patterns in this group. Despite a lack of association between morphology and functional diversification in the locomotor system of the Moorish gecko, straightforward links are observed between head morphology and biting performance, providing more hints on the possible underlying causes. Indeed, variation in bite force is mostly determined by size variation and sexual dimorphism, and differences between the two clades concern how sexual variation is expressed, reinforcing the idea that both social and ecological factors contribute in shaping differentiation. Interestingly, the individuals from the islets off the coast of Murcia exhibit particular morphological and functional traits, which suggests that the ecological conditions related to insularity may drive the phenotypic differentiation of this population.
1 INTRODUCTION
Determining the degree of correspondence between phenotypic and phylogeographic clustering is a very important instrument for assessing the natural history of distinct populations or lineages and for evaluating how selective pressures have acted on them (Zamudio, Bell, & Mason, 2016). A population is a group of individuals of a species that interbreed and live in the same place at the same time. However, the geographic and temporal separation between different populations is not always accompanied by genetic or phenotypic divergence. Indeed, in nature different populations can differ both genetically and phenotypically (e.g., Murphy et al., 2017), only genetically (e.g., Torres-Carvajal & Mafla-Endara, 2013), only phenotypically (e.g., Herrel et al., 2008), or not be different at either the molecular or the phenotypic level, with implications for taxonomic decisions (Mallet, 1995; Padial et al., 2010). Teasing apart phylogeographic, ecological, and phenotypic differentiation may inform us on the processes that drive population divergence, eventually leading to the emergence of new species.
Indeed, the adaptation of populations to distinct environments is a primary mechanism for ecological speciation (Coyne & Orr, 2004; Rundle & Nosil, 2005; Schluter, 2000). In this process, speciation occurs as a result of divergent selection on the phenotypic traits of individuals exploiting alternative ecological niches (Chase & Leibold, 2003). Remarkably, the phenotypic traits involved in ecological speciation frequently include morphological features involved in crucial ecological functions, such as locomotion and prey capture (Schluter, 2000). In the same context, deciphering the mechanisms that underlie morphological and functional diversity is essential for understanding how animals adapt to their environment. Functional performance is the physical ability of an organism for accomplishing an ecologically relevant task; and morphology is the first visible indicator of selection on functional traits, since it is frequently forged by performance requirements (Irschick, Meyers, Husak, & Le Galliard, 2008).
The basic biological traits of lizards are well studied at the microevolutionary level, which makes them emerging models in ecology and evolution, providing a solid basis for formulating hypotheses to explain phenotypic diversification (Camargo, Sinervo, & Sites, 2010). Locomotor and biting performance are the most commonly studied functional traits in lizards (e.g., Gomes, Carretero, & Kaliontzopoulou, 2016; Herrel, Zaaf, Vanhooydonck, Aerts, & Van Damme, 2000; Kaliontzopoulou, Adams, van der Meijden, Perera, & Carretero, 2012; Kaliontzopoulou, Bandeira, & Carretero, 2013; Vanhooydonck et al., 2014). Both are involved in many crucial ecological and social tasks, and, as such, they hold a large evolutionary potential (Irschick et al., 2008). Running performance is associated with habitat use, escape from predators, prey capture, and territory defense (Herrel et al., 2000). Bite force is used in prey acquisition, territory defense, and mating (Kaliontzopoulou et al., 2012; Verwaijen, Van Damme, & Herrel, 2002). In geckos, the study of functional morphology has focused mainly on the biomechanical particularities of the adhesive system used for locomotion (e.g., Collins, Russell, & Higham, 2015; Higham & Russell, 2010; Johnson & Russell, 2009), while less attention has been given to how performance and morphology vary across distinct intraspecific evolutionary entities, and how they are related to each other. From the few studies available, CLIMBING PERFORMANCE seems to be negatively affected by head size, particularly if surfaces are very steep (Cameron, Wynn, & Wilson, 2013), and is also influenced by limb length and body proportions, at least in some species (e.g., Collins et al., 2015; Higham & Russell, 2010; Johnson & Russell, 2009). On the other hand, bite force in geckoes seems to follow the general biomechanical pattern observed in many lizard groups, with a positive correlation between relative head size and biting capacity (Cameron et al., 2013; Massetti, Gomes, Perera, Rato, & Kaliontzopoulou, 2017).
Here, we explore the morphological and functional divergence of different populations of the Moorish gecko, Tarentola mauritanica (Linnaeus, 1758), present in the Iberian Peninsula. This paraphyletic species complex is a member of the Family Phyllodactylidae (Gamble, Bauer, Greenbaum, & Jackman, 2008), and it is distributed across the Mediterranean regions of North Africa and Southern Europe (Rato et al., 2015; Vogrin, Corti, Pérez Mellado, Baha El Din, & Martínez-Solano, 2017). The species includes six mitochondrial evolutionary clades situated, respectively, in the following: Central and South-western Morocco (Clade I); the Iberian Peninsula (Clade II); Southern Europe and North Africa (Clade III); Central Morocco (Clade IV); Eastern Canary Islands (Clade V, assigned as T. angustimentalis); and Southern Iberia, North-eastern Morocco, and North-western Algeria (Clade VI) (Harris, Batista, Carretero, & Ferrand, 2004; Harris, Batista, Lymberakis, & Carretero, 2004; Harris, Carretero, Corti, & Cascio, 2009; Perera & Harris, 2008; Rato, Carranza, & Harris, 2012). Because T. mauritanica is frequently associated with humanized environments, accidental anthropogenic introductions can sometimes occur (Arnold, Burton, & Ovenden, 2002). This is the case of the European populations from Clade III, whose current geographic distribution results from a recent introduction and successive expansion from North Africa (Harris, Batista, Lymberakis, et al., 2004). Genetic evidence that each of these clades should be considered as a different species is very robust, confirmed through a multilocus Bayesian species delimitation (Rato, Harris, Carranza, Machado, & Perera, 2016). Diversification of the different lineages occurred during the Miocene and Pleistocene, associated with both divergence and conservatism of the macroclimatic niche related to temperature and precipitation (Rato et al., 2015). These results have been corroborated through ecophysiological studies in the two lineages distributed across the Iberian Peninsula (Clades II and III), which exhibit significantly distinct water loss patterns, supporting the hypothesis that this physiological trait is possibly related to the cladogenesis of T. mauritanica (Rato & Carretero, 2015). Furthermore, this same study detected that populations from Clade III exhibited greater variability concerning water loss values evidencing its ecophysiological flexibility, which has been suggested to enhance its invasive success across Europe.
The objective of this study was to examine morphological and functional variation in the two main clades of T. mauritanica from the Iberian Peninsula (Clades II and III), in order to identify the potential contribution of functional morphology in population and lineage divergence. These two evolutionary units diverged around 2.47 Mya, and they represent the most recent cladogenetic event within this species complex (Rato et al., 2012). Our objective is to test whether morphological and functional variation accompany the geographic, physiological, and molecular separation between these two clades, and to obtain a better understanding of the functional relationship between morphology and performance in the Moorish gecko. For this purpose, we use linear and geometric morphometrics (GM) to investigate variation among five populations representing the two lineages and considering body size, relative head dimensions, head shape, and limb proportions. Furthermore, we consider variation in bite force and climbing speed performance, and investigate how they relate to morphological variation. A strong association between morphology and functional performance would point to ecological or social requirements as a strong driver of divergence in phenotypic traits between populations and/or clades. By investigating intraspecific lineages and distinct populations within them, we are able to target two distinct evolutionary time frames, which in turn allows us to investigate the relative contribution local adaptation and evolutionary history in shaping the observed phenotypic variation.
2 MATERIALS AND METHODS
2.1 Specimens examined
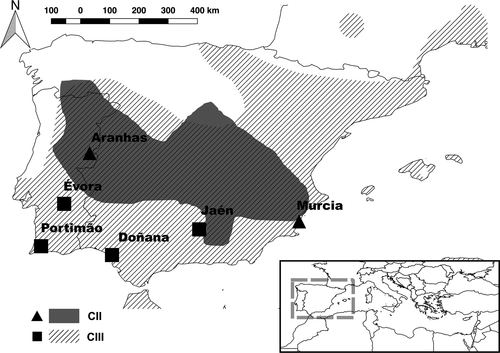
To quantify variation between populations and clades in both morphology and functional performance, we captured a total of 37 males and 46 females of T. mauritanica, all adults. Individuals were caught by noosing (García-Muñoz & Sillero, 2010) or by hand, in five different localities in the Iberian Peninsula covering the two main clades present in Europe (Clade II and Clade III). These include two populations from Clade II (CII), with 12 females and five males from Aranhas (AR), and 10 females and 10 males from Isla Grosa in Murcia (MU); and three populations from Clade III (CIII) with seven females and five males from Évora (EV), eight females and seven males from Jaén (JA), and nine females and 10 males from Portimão (PO) (Figure 1). Individuals were sexed either by inspecting for the presence of hemipenes or by placing a white light against the skin, dorsally to the tail base (Atzori et al., 2007).
2.2 Morphological and functional characters recorded
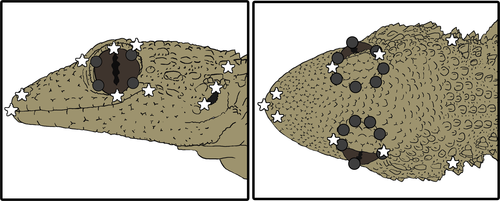
We used linear biometry and GM to describe variation in total and relative body, head and limb size, and head shape, respectively. Biometric traits measured included the following: snout-vent length (SVL), head length (HL), head width (HW), head height (HH), forelimb length (FLL), and hindlimb length (HLL). To quantify variation in head shape, we used tpsDig (Rohlf, 2015) to record the position of 23 dorsal and 14 lateral landmarks and then superimposed landmark coordinates to obtain shape variables, following all data procedures described in Massetti et al., 2017 (Figure 2) and using the geomorph R package (Adams, Collyer, Kaliontzopoulou, & Sherratt, 2016; Adams & Otárola-Castillo, 2013).
To quantify functional performance, we measured maximal bite force and CLIMBING PERFORMANCE using standard protocols, also described in detail in Massetti et al., 2017.
2.3 Data analyses
We used general linear models (GLMs) to explore diversity in functional morphology among populations and clades of T. mauritanica. We used SVL as a response variable and clade (CLA), population (POP), SEX, and all possible interactions as predictors, to test for differences between populations and clades in body size, while taking potential differences between the sexes into account. To investigate variability in relative head and limb dimensions, we used each morphological trait individually as the response variable and SVL, CLA, POP, SEX, and all interaction terms as predictors. Throughout, we considered POP as a factor nested within CLA.
To test for differences in dorsal and lateral head shape between populations and clades, while also considering potential variation between sexes, we fit GLMs using residual permutations as implemented in the function procD.lm of geomorph R- package (Adams & Otárola-Castillo, 2013; Adams et al., 2016), including shape as the response variable and CLA, POP, SEX, and their interaction as predictors. Furthermore, we examined a model with logCS, CLA, POP, and SEX as predictors to take the possible influence of head size into account. Throughout, we also considered all interaction terms.
In order to investigate variation across populations and clades in bite force, we examined a GLM with CLA, POP, SEX, and all interactions as predictors, as well as a GLM considering also SVL to take size variation into account. To test for the presence of variation in bite force across populations and clades independently of the effect of head dimensions, we examined a model with bite force as the response variable; SVL, HH, HW, and HL as covariates; and CLA, POP, and SEX and their interaction as predictors. In addition, we used partial least squares (PLS) analysis to investigate the size-free, multivariate association between bite force and head dimensions, using the plsr function of the pls R package (Mevik & Wehrens, 2007). For this analysis, we first corrected bite force and each head dimension for size effects using the residuals of a linear regression of each of these traits on SVL. Similar to what we did for linear measurements, we also explored the association between bite force and head shape as quantified through GM, using the function two.b.pls of geomorph R package (Adams & Otárola-Castillo, 2013; Adams et al., 2016).
Finally, to investigate the variability of CLIMBING PERFORMANCE between clades and populations, we examined a GLM using CLIMBING PERFORMANCE as the response variable and CLA, POP, SEX, and all interaction terms as predictors. Then, we performed another GLM including also SVL as a predictor to take the effect of size into account. To test for the effect of limb length on CLIMBING PERFORMANCE, we examined two GLMs, one with FLL and another with HLL as predictors.
All statistical analyses were performed using R v. 3.3.1 (R Core Team, 2016).
3 RESULTS
3.1 Linear biometry
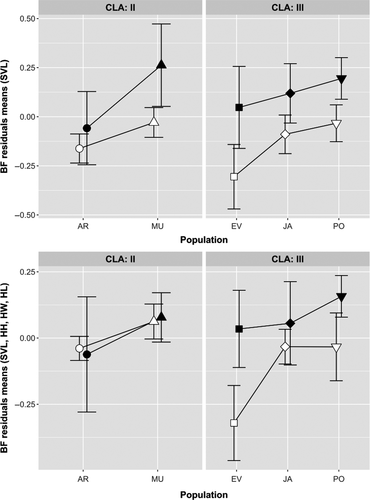
The GLM test performed on SVL indicated the existence of significant effects of both clade and population on body size, and the presence of sexual size dimorphism in the entire dataset, where males were larger than females (Table 1, Figure 3a). Post hoc comparisons showed that populations from clade CII were smaller in body size than those from CIII, where individuals from Isla Grosa (MU) were sharply smaller than those of any other continental population (all p < 0.05). Sexual dimorphism in body size was identifiable in CIII (p < 0.05) but not in CII (p > 0.05). Regarding head dimensions, we only identified significant variation across clades in relative HH, which was smaller in CII compared to CIII, a pattern driven by the individuals from AR population, which had markedly flatter heads than other populations (Figure 3b; pairwise comparisons of AR with all other populations, sexes pooled: all p < 0.05); this feature was particularly marked among females (pairwise comparisons of AR with all other populations, females only: all p < 0.05). For HW, we found significant effects of SEX and the CLA × SEX interaction, an effect that reflected the presence of sexual dimorphism for this trait in populations of CII, and the lack of it in those of CIII (Figure 3c). Post hoc comparisons showed that both HW and HH were sexually dimorphic for CII (p < 0.05 in all cases), but not for CIII (p > 0.05 in all cases). The GLMs performed on limb size indicated a significant effect of population, but not clade, on both fore- and hindlimb length (Table 1). Specifically, individuals from the PO population had shorter limbs than those from other populations (Figure 3d,e), including those belonging to the same clade, with the only exception of AR hindlimb (p < 0.05 in all pairwise comparisons of PO with other populations for HLL and FLL).
| Snout-vent length | |||
|---|---|---|---|
| SS | F | p-value | |
| Body | |||
| CLA | 0.691 | 92.570 | 0.001 |
| SEX | 0.273 | 36.643 | 0.001 |
| POP | 0.493 | 22.025 | 0.001 |
| CLA × SEX | 0.004 | 0.517 | 0.465 |
| POP × SEX | 0.013 | 0.594 | 0.577 |
| Head height | Head width | Head length | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SS | F | p-value | SS | F | p-value | SS | F | p-value | |
| Head | |||||||||
| SVL | 1.274 | 294.255 | 0.001 | 1.875 | 1514.709 | 0.001 | 1.704 | 1551.523 | 0.001 |
| CLA | 0.026 | 5.960 | 0.023 | 0.0E0 | 4.0E-4 | 0.984 | 0.001 | 0.946 | 0.368 |
| SEX | 0.071 | 16.312 | 0.001 | 0.013 | 10.540 | 0.003 | 0.006 | 5.021 | 0.052 |
| SVL × CLA | 0.002 | 0.571 | 0.445 | 0.003 | 2.022 | 0.147 | 8.0E-5 | 0.072 | 0.817 |
| POP | 0.037 | 2.827 | 0.043 | 0.005 | 1.334 | 0.278 | 0.002 | 0.460 | 0.779 |
| SVL × SEX | 3.70E-4 | 0.085 | 0.744 | 1.80E-4 | 0.145 | 0.695 | 0.002 | 2.232 | 0.165 |
| CLA × SEX | 3.40E-4 | 0.078 | 0.783 | 0.006 | 4.760 | 0.017 | 1.0E-5 | 0.010 | 0.921 |
| SVL × POP | 0.025 | 1.895 | 0.109 | 0.002 | 0.454 | 0.702 | 0.011 | 3.417 | 0.031 |
| SVL × CLA × SEX | 0.002 | 0.381 | 0.492 | 0.001 | 0.406 | 0.523 | 0.002 | 1.413 | 0.243 |
| POP × SEX | 0.018 | 1.390 | 0.183 | 0.001 | 0.216 | 0.857 | 0.013 | 3.943 | 0.009 |
| SVL × POP × SEX | 0.005 | 0.421 | 0.665 | 0.007 | 1.754 | 0.111 | 0.006 | 1.962 | 0.086 |
| Forelimb length | Hindlimb length | |||||
|---|---|---|---|---|---|---|
| SS | F | p-value | SS | F | p-value | |
| Limbs | ||||||
| SVL | 1.534 | 382.114 | 0.001 | 1.404 | 529.849 | 0.001 |
| CLA | 0.008 | 1.941 | 0.156 | 0.008 | 3.053 | 0.135 |
| SEX | 1.85E-6 | 4.60E-4 | 0.977 | 0.001 | 0.209 | 0.688 |
| SVL × CLA | 0.002 | 0.517 | 0.479 | 0.004 | 1.567 | 0.296 |
| POP | 0.047 | 3.924 | 0.012 | 0.054 | 6.748 | 0.002 |
| SVL × SEX | 0.004 | 1.038 | 0.297 | 0.010 | 3.694 | 0.057 |
| CLA × SEX | 0.001 | 0.146 | 0.706 | 4.40E-4 | 0.166 | 0.659 |
| SVL × POP | 0.002 | 0.187 | 0.895 | 0.015 | 1.830 | 0.132 |
| SVL × CLA × SEX | 1.79E-5 | 0.004 | 0.949 | 4.0E-4 | 0.151 | 0.668 |
| POP × SEX | 0.021 | 1.754 | 0.161 | 0.006 | 0.723 | 0.482 |
| SVL × POP × SEX | 0.018 | 1.467 | 0.225 | 0.021 | 2.606 | 0.024 |
Note
- Significant effects are marked in bold letter. See Materials and Methods for variable abbreviations.

3.2 Head shape
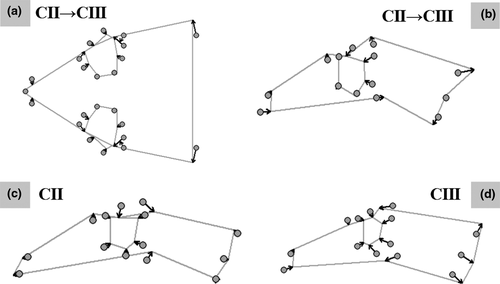
General linear models on projected Procrustes residuals indicated the presence of significant differences between clades on both dorsal and lateral head shape (Table 2), with CII exhibiting relatively larger eyes, and a reduction in the rear part of the head (Figure 4a,b). Also, while both clades showed the same type of sexual dimorphism in dorsal head shape (i.e., CLA×SEX not significant), the pattern of sexual differentiation changes across clades in lateral head shape (Figure 4c,d). Indeed, although males had relatively smaller eyes than females in both clades, males of CII also exhibited flatter heads and slightly longer snouts, while in CIII males exhibited a widening of the posterior part of the head. Following the general pattern observed for linear measurements, logCS of both the dorsal and lateral view of the head was affected by CLA, SEX, and CLA × POP, but the results on head shape variation remained unchanged when taking variation in logCS into account (Table 2).
| Dorsal | Lateral | |||||
|---|---|---|---|---|---|---|
| SS | F | p-value | SS | F | p-value | |
| Shape | ||||||
| CLA | 0.008 | 3.406 | 0.009 | 0.015 | 3.332 | 0.002 |
| SEX | 0.012 | 5.238 | 0.001 | 0.011 | 2.425 | 0.021 |
| CLA × POP | 0.008 | 1.190 | 0.245 | 0.026 | 1.931 | 0.008 |
| CLA × SEX | 0.001 | 0.523 | 0.710 | 0.008 | 1.853 | 0.037 |
| CLA × POP × SEX | 0.004 | 0.618 | 0.779 | 0.013 | 0.936 | 0.417 |
| LogCS | ||||||
| CLA | 0.568 | 57.579 | 0.001 | 0.460 | 42.474 | 0.001 |
| SEX | 0.271 | 27.490 | 0.001 | 0.471 | 43.477 | 0.001 |
| CLA × POP | 0.219 | 7.387 | 0.001 | 0.379 | 11.641 | 0.001 |
| CLA × SEX | 0.004 | 0.453 | 0.482 | 0.012 | 1.147 | 0.269 |
| CLA × POP × SEX | 0.008 | 0.256 | 0.835 | 0.020 | 0.624 | 0.557 |
| Shape | ||||||
| logCS | 0.015 | 6.403 | 0.001 | 0.017 | 4.036 | 0.002 |
| CLA | 0.007 | 3.060 | 0.011 | 0.017 | 4.040 | 0.002 |
| SEX | 0.008 | 3.499 | 0.006 | 0.008 | 1.962 | 0.052 |
| logCS × CLA | 3.52E-4 | 0.155 | 0.994 | 0.006 | 1.477 | 0.148 |
| CLA × POP | 0.008 | 1.110 | 0.288 | 0.021 | 1.611 | 0.025 |
| logCS × SEX | 0.001 | 0.251 | 0.939 | 0.005 | 1.209 | 0.221 |
| CLA × SEX | 0.002 | 0.902 | 0.398 | 0.010 | 2.284 | 0.007 |
| logCS × CLA × POP | 0.007 | 1.054 | 0.283 | 0.012 | 0.953 | 0.337 |
| logCS × CLA × SEX | 0.001 | 0.606 | 0.593 | 0.004 | 0.824 | 0.472 |
| CLA × POP × SEX | 0.008 | 1.206 | 0.121 | 0.014 | 1.097 | 0.131 |
| logCS × CLA × POP × SEX | 0.006 | 0.844 | 0.381 | 0.011 | 0.835 | 0.387 |
Note
- Significant effects are marked in bold letter. See Materials and Methods for variable abbreviations.
3.3 Functional performance
General linear models on performance traits indicated a significant effect of SVL, SEX, and POP on bite force, but the absence of any effect, apart from SVL, on CLIMBING PERFORMANCE (Table 3). Males exhibited higher absolute bite forces than females in all populations (all p < 0.05), but relative bite force was not sexually dimorphic in AR and JA after size correction. In addition, relative bite force was stronger in MU and PO and weaker in EV and AR, whereas JA exhibited intermediate values (Figure 5, all pairwise comparisons with p < 0.05). When taking variation in SVL and in head dimensions into account, we found that the effects of SEX, POP, and CLA×SEX remained significant (Table 3). This last significant interaction term is reflected in the absence of sexual dimorphism in bite force in CII after accounting for the effect of head dimensions and SVL (p > 0.05), while sexual differences remain in CIII, especially in the EV and PO populations (pairwise comparisons between the sexes for EV, PO, and CIII, all p < 0.05) (Figure 5).
| Bite force | Climbing | |||||
|---|---|---|---|---|---|---|
| SS | F | p | SS | F | p | |
| SVL | 18.331 | 440.398 | 0.001 | 1.530 | 22.780 | 0.001 |
| CLA | 0.001 | 0.018 | 0.911 | 0.123 | 1.824 | 0.151 |
| SEX | 1.711 | 41.096 | 0.001 | 0.032 | 0.473 | 0.461 |
| SVL × CLA | 0.078 | 1.866 | 0.174 | 0.002 | 0.031 | 0.870 |
| CLA × POP | 0.392 | 3.139 | 0.027 | 0.206 | 1.024 | 0.301 |
| SVL × SEX | 0.004 | 0.094 | 0.754 | 0.014 | 0.206 | 0.616 |
| CLA × SEX | 0.006 | 0.136 | 0.670 | 0.125 | 1.857 | 0.139 |
| SVL × CLA × POP | 0.052 | 0.420 | 0.684 | 0.007 | 0.037 | 0.992 |
| SVL × CLA × SEX | 0.085 | 2.033 | 0.118 | 0.021 | 0.318 | 0.552 |
| CLA × POP × SEX | 0.052 | 0.419 | 0.646 | 0.071 | 0.352 | 0.727 |
| SVL × CLA × POP × SEX | 0.125 | 1.000 | 0.286 | 0.282 | 1.401 | 0.161 |
| Bite force | |||
|---|---|---|---|
| SS | F | p | |
| SVL | 18.331 | 786.377 | 0.001 |
| HH | 1.327 | 56.936 | 0.001 |
| HW | 0.665 | 28.507 | 0.001 |
| HL | 0.276 | 11.842 | 0.005 |
| CLA | 0.033 | 1.403 | 0.354 |
| SEX | 0.464 | 19.885 | 0.001 |
| CLA × POP | 0.476 | 6.806 | 0.001 |
| CLA × SEX | 0.130 | 5.582 | 0.019 |
| CLA × POP × SEX | 0.130 | 1.861 | 0.105 |
Note.
- Significant effects are marked in bold letter. See Materials and Methods for other variable abbreviations.
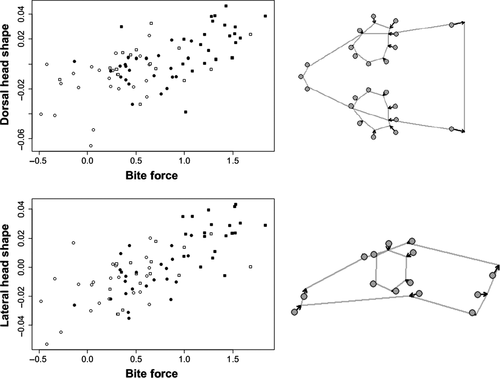
PLS analysis of the multivariate association between bite force and head dimensions, after removing size effects, yielded significant results (r = 0.629, p = 0.001; Figure 6). Examination of the morphology PLS vector indicated that relative HH was the most relevant for determining bite force (r = 0.93), followed by relative HW (r = 0.74), and relative HL (r = 0.63). PLS analysis of the association between bite force and head shape as quantified through GM also showed a significant relationship for both dorsal (r = 0.614, p = 0.001) and lateral (r = 0.725, p = 0.001) head shape, where higher bite forces were associated with an enlargement of the posterior part of the head, the shortening of the snout, and the reduction in relative eye size. This association remained significant for lateral head shape also after accounting for logCS effects (r = 0.545, p = 0.001), while becoming nonsignificant for dorsal head shape (r = 0.298, p = 0.15).
Finally, we found no significant association between limb size and CLIMBING PERFORMANCE (ANOVA; FLL: F = 0.12, p = 0.76; HLL: F = 0.01, p = 0.90).
4 DISCUSSION
Our study on functional performance and morphology among populations from two phylogenetically differentiated clades of T. mauritanica in the Iberian Peninsula, highlighted the presence of variation among populations and between clades, confirming some of the previous observations about the association between these traits in the Moorish gecko (Massetti et al., 2017). While the two phylogenetically differentiated clades were morphologically differentiated, both in body size and head shape, much of this differentiation was driven by variation between populations of the same clade, suggesting that local adaptation and recent ecological events—including insularity—may be more influential than deep evolutionary history in shaping patterns of phenotypic diversity. Straightforward links are observed between head morphology and biting performance, where bite force is mostly determined by size variation and sexual dimorphism, reinforcing the idea that both social and ecological factors contribute in shaping differentiation. In turn, we observed a lack of association between morphology and functional diversification in the locomotor system of the Moorish gecko, supporting the idea that the adhesive system of geckoes is more relevant for locomotion than linear biometric traits. Our findings shed more light on the ecological and evolutionary mechanisms potentially underlying population divergence and cladogenesis in this species complex, adding to our understanding of diversification.
4.1 Sources of morphological variation
Our investigation of morphological variation across clades and populations of the Moorish gecko in the Iberian Peninsula indicated that individuals from the populations of clade CII were smaller in body size than those of CIII (Figure 3), confirming the results obtained in a previous ecophysiological study (Rato & Carretero, 2015). Despite a general tendency for individuals from AR to exhibit smaller body sizes as compared to those from populations of CIII, the ones most influential in driving the smaller body size of CII were the individuals from Isla Grosa (MU), which were remarkably smaller than those of any other continental population, possibly pointing to insularity as a driving factor. Island miniaturization is a common evolutionary pattern, with numerous examples among reptiles, including—for instance—the Sphaerodactylus geckos from the West Indies (Hedges & Thomas, 2001), the Leptotyphlops snakes from the Lesser Antilles (Blair Hedges, 2008), or the famous Brookesia chameleons from Madagascar (Glaw, Köhler, Townsend, & Vences, 2012), to mention just a few. The most common effect of miniaturization on morphology is reduction and structural simplification, ranging from general underdevelopment to the complete loss of organs (see references in Hanken & Wake, 1993). Many hypotheses have been put forward to explain the causality of island dwarfism, some suggesting that it is due to a relaxation in predation (Boekschoten & Sondaar, 1966; Sondaar, 1977), others pointing to it as a way of coping with resource shortage on small islands (Roth 1992; Burness, Diamond, & Flannery, 2001), and yet others proposing that it increases fitness, as it entails shorter gestation periods and generation times (e.g., Raia & Meiri, 2006). Regarding the species complex considered here, genetic evidence indicates that the populations from the Murcian islets form a distinct evolutionary cluster within the Iberian clade of T. mauritanica (Rato et al., 2016), suggesting a lack of gene flow with mainland populations for a considerable part of their recent evolutionary history. As such, further studies would be necessary to assess whether this is a case of insular dwarfism and elucidate the precise ecological mechanisms that drove the marked divergence in body size of this population.
Similar to what was observed for body size, local differentiation in specific populations is also an important determinant of variation in the relative size of different body parts. For instance, individuals from Clade II exhibited flatter heads than those from Clade III (Figure 3). Yet, we found again that Clade II shows extensive variability, with the AR population exhibiting a relatively flatter head compared to MU, or with any other population considered here. Head height is related to microhabitat and refuge use in other lizard species (Bieke Vanhooydonck & Van Damme, 1999; Zaaf, Van Damme, Herrel, & Aerts, 2001; Zaaf & Van Damme, 2001), and the pattern observed here could be a consequence of more restrictions on this trait in the AR population. Indeed, this particular population inhabits an urban environment, where geckoes are more exposed to urban predators and a reduced diversity of refugia is available (i.e., cats, Koenig, Shine, & Shea, 2002; Gillies & Clout, 2003), although this may not necessarily be the case in other urban populations. Alternatively, the variation observed for this trait within Clade II could be due to the smaller body size of the individuals from MU. Hypothetically, being smaller may have permitted the individuals from MU to evolve a relatively higher head without compromising their ability to use small crevices as refugia. Also, because the habitat of Isla Grosa consists mainly of small bushes and rock clusters (CR, pers. obs.), there are no real steep surfaces where a larger head could have a negative effect on CLIMBING PERFORMANCE (Cameron et al., 2013). Unfortunately, our dataset only included two populations from Clade II, hampering inferences related to the direction of change among populations in this, or any other, trait within this phylogenetic group. As such, a study including more populations from Clade II would be necessary to determine what ecological conditions, related or not to insularity, triggered the observed morphological changes.
However, local variation was also observed within Clade III, suggesting that insularity is not the only factor causing population differentiation within phylogenetic clades. This is the case, for instance, of fore- and hindlimb length, which was relatively shorter in individuals from PO compared to those of any other population (Figure 3d,e). Again, variation in relative limb length is frequently associated with habitat use in lizards in general (Garland & Losos, 1994; Losos, 1990; Sinervo & Losos, 1991) and in geckoes in particular (Zaaf & Van Damme, 2001), and it could explain the variation observed here, as the PO population inhabits a natural environment, characterized by large boulders with steep surfaces, while the other two studied populations of Clade III (EV and JA) were sampled in city walls. Of course, confirming this hypothesis would require a more detailed study of microhabitat choice in these, and possibly additional, populations.
Despite extensive morphological variation across populations within phylogenetic clades, we also found some evidence of morphological differentiation between clades. Unexpectedly, however, this differentiation seems to be more strongly associated with variation in the degree and direction of sexual dimorphism in the two clades, rather than to raw morphological differences. This was the case, for instance, for the presence of identifiable sexual dimorphism in SVL in CIII but not in CII (Figure 3a). The opposite was the case for HW; while males of both populations of Clade II had visibly wider heads than corresponding females, none of the populations of Clade III were dimorphic for this trait (Figure 3c). This pattern appears also for relative HH, but in this case, it appears primarily affected from the variability of the individuals from MU. This is in contrast with what was found in a previous study on populations of Clade III from the southernmost extreme of the distribution range of this group in Spain (Massetti et al., 2017), but it is in accordance with a study performed on Italian populations, which belong to the same evolutionary lineage (Zuffi, Sacchi, Pupin, & Cencetti, 2011), suggesting once again that extensive geographic variation exists in T. mauritanica. First, this pattern could be due to variation in dorsal shape in the analyzed populations not measurable with linear biometry. Indeed, this possibility is supported by the presence of significant sexual dimorphism in dorsal head shape, without differences in the degree of sexual dimorphism across populations or clades (Table 2). However, when considering lateral head shape, the same pattern emerges, where a significant CLA×SEX interaction again suggests differences between clades in sexual dimorphism. Considering that such variation in sexual dimorphism is repeatedly observed in head relative dimensions and shape, and given the importance of the head for social interactions in lizards (Herrel, Spithoven, Van Damme, & De Vree, 1999; Husak, Lappin, Fox, & Lemos-Espinal, 2006; Husak, Lappin, & Van Den Bussche, 2009; Lappin & Husak, 2005), it is possible that the two Iberian phylogenetic clades of the Moorish gecko may also be differentiated in terms of territorial behavior and mating systems. Such an idea would need to be further explored and formally tested through behavioral observations, but it seems to also be supported by our results on functional performance (see below).
4.2 Functional diversification
Combined with the morphological patterns discussed above, our investigation of functional variation across populations and clades of T. mauritanica in the Iberian Peninsula provides more hints to the processes that may drive phenotypic differentiation within this gecko species complex. Interestingly, the results obtained on functional performance were more clear-cut than those related to morphological traits. This is not necessarily surprising, as morphology is under the influence of several different evolutionary pressures, including functional optimization, whereas functional performance may be more directly related to ecological and social tasks and resulting selective processes (Irschick et al., 2008). In the case of the variation observed for limb length, and the marked differentiation of the PO population, morphological patterns do not correspond to differences between the considered groups (i.e., sexes, populations, and clades) (Table 3), supporting the idea that, whatever the ecological factors driving local geographic variation, these are probably not related to habitat use. This same pattern is also reflected in the lack of association between climbing speed and limb length (see Results). First, the reason for this lack of the typical morphology–function association may be that the morphological variability observed aims to enhance other aspects of locomotor performance not quantified here (e.g., acceleration; Scales & Butler, 2015). Most probably, though, it reflects the particularities of the locomotor system of geckoes and the much higher importance of the adhesive pads in determining the locomotor capacity of these organisms. Accordingly, previous studies suggest that as soon as limb size accomplishes the needs of the adhesive system, any change of it is hardly relevant for geckoes (Zaaf & Van Damme, 2001).
Contrasting this lack of association between linear biometry and functional diversification in the locomotor system of the Moorish gecko, straightforward links are observed between head morphology and biting performance, providing more hints on the possible underlying causes. Following the typical pattern for lizards, we found that biting performance is related to head size and relative head dimensions, where HH was the most relevant in determining bite force, followed by HW and HL (see Results). In agreement, both lateral and dorsal head shape are related to bite force, where the posterior part of the head accommodating the adductor muscles (Herrel, Aerts, Fret, & De Vree, 1999; Herrel, Spithoven, et al., 1999) was particularly relevant in enhancing bite performance and lateral head shape was significant also once the effect of size was removed (Figure 6). Relative HH is often directly associated with bite force in several lizard species (Herrel, De Grauw, & Lemos-Espinal, 2001; Herrel, Aerts, et al., 1999; Herrel, Spithoven, et al., 1999; Herrel, De Grauw, et al., 2001; Herrel, Van Damme, et al., 2001; Kaliontzopoulou, Carretero, & Llorente, 2008; Van Damme, De Vree, & Herrel, 1995), including T. mauritanica (Massetti et al., 2017). The probable reason is that usually higher heads provide more space for the mandible adductor muscles and give them the possibility to insert more perpendicularly to the lower jaw, increasing the force on the quadrato-articular joint (Haas, 1973; Herrel, De Grauw, et al., 2001; Herrel, Van Damme, et al., 2001). Head width tends instead to be representative of the volume of the abductor muscles since wider heads can accommodate more jaw adductor musculature without affecting the amount of space available for other organs (Brock, 1938; Herrel, De Grauw, et al., 2001; Herrel, Van Damme, et al., 2001). Both these head dimensions varied extensively across our sample, with traceable consequences for functional differentiation across sexes, populations, and clades.
Regarding sexual differentiation, the functional morphology of the biting system followed the typical lizard pattern in the two Iberian clades of T. mauritanica, where males exhibited higher biting capacities, relative to their body size, as compared to females (Figure 5). Both male–male antagonism and mate acquisition are known to drive sexual selection underlying this phenotypic pattern (Herrel, Aerts, et al., 1999; Herrel, Spithoven, et al., 1999; Husak et al., 2006, 2009; Lappin & Husak, 2005). What is more interesting, however, is how the morphological distinctiveness between phylogenetically differentiated clades of the Moorish gecko is reflected in sexual dimorphism in functional performance. Indeed, we found that, after taking differences between the sexes in body size and head dimensions into account, bite-force sexual dimorphism is maintained in Clade III, but not in Clade II (Figure 5). From a functional morphology perspective, this fact possibly suggests that sexual dimorphism in bite force is determined in different ways in the two studied clades. For Clade II, exaggerated head proportions seem to be exclusively responsible for sexual differentiation in bite performance. By contrast, in Clade III functional sexual differences seem to be related to other head features not measured in this study or linked to head properties impossible to detect from external morphology (i.e., skeletal, muscular, and physiological modifications). Put another way, sexual dimorphism in biting performance is maintained stable (relative to body size) across phylogenetically differentiated lineages of T. mauritanica, as indicated by a nonsignificant CLA×SEX interaction term (Table 3). This means that functional sexual dimorphism is maintained despite of variation in morphological sexual dimorphism, in what could be viewed as a many-to-one mapping (Wainwright, Alfaro, Bolnick, & Hulsey, 2005) of sexual differentiation of function on form.
Another interesting pattern is the visible functional differentiation of the insular population from Isla Grosa (MU). Indeed, we found that, despite their smaller size, the male individuals from MU present the highest heads and, together with the males from PO, the stronger bite forces (Figure 5). This reinforces the idea that the ecological conditions related to insularity may drive the phenotypic differentiation of this population, as higher biting performance would allow individuals to increase their chances of capturing and immobilizing prey (Herrel, Van Damme, et al., 2001; Verwaijen et al., 2002). If further supported by data on prey availability and/or size, this could represent an important adaptation to an isolated and low on resources environment as these Murcian islets. By contrast, we found no evidence that populations of Clade III exhibit higher biting capacities, neither in absolute terms nor relative to their body size and head dimensions. As such, increased antagonistic capacity for either resource exploitation or direct interference is probably not particularly relevant for the colonizing success that this lineage has exhibited across large part of Europe. Rather, ecophysiological flexibility seems as a more feasible mechanism through which individuals of Clade III have managed to invade new environments and establish populations throughout the Mediterranean region (Rato & Carretero, 2015).
Put together, our results enhance our understanding of the patterns of phenotypic differentiation within and among the two phylogenetic clades of the Moorish gecko present in the Iberian Peninsula, and they provide hints to the ecological and evolutionary processes that drive such differentiation. In general, individuals of the Iberian clade seem to be smaller, with relatively larger eyes and a more conical head compared to those of the European one. However, much of this differentiation is rather more strongly related to local variation between populations of the same clade, suggesting that recent ecological events may be more influential than deep evolutionary history in shaping diversity patterns in this group. Nevertheless, to determine accurately which ecological factors drove the observed patterns, a wider sampling of populations would be required. At the moment, our results show little correspondence of divergence in both morphological and performance traits with the previously obtained phylogenetic distinctiveness between Iberian clades of T. mauritanica (Harris, Batista, Carretero, et al., 2004; Harris, Batista, Lymberakis, et al., 2004; Perera & Harris, 2008; Rato et al., 2012, 2016).
ACKNOWLEDGEMENTS
The authors would like to acknowledge all the people that helped capturing the animals in the field, namely to Miguel Carretero, Marta Silva, Diana Marguč, Olga Sawościanik, and Marcos Ferrández. CR was supported by a postdoctoral grant (SFRH/BPD/92343/2013), VG by a doctoral grant (SFRH/BD/93237/2013), and AK by an IF contract (IF/00641/2014/CP1256/CT0008), all from Fundação para a Ciência e a Tecnologia (FCT, Portugal). Capture of the animals was performed under the following licences; 201500066531 from the Consejería de Agricultura y Agua, Murcia Region (Spain); 201599900067073 from the Consejería de Medio Ambiente y Ordenación del Território, Andalucia (Spain); and 258/2015/CAPT from the Instituto da Conservação da Natureza e das Florestas (Portugal).




