Molecular phylogeny of neodalyellid flatworms (Rhabdocoela), including three new species from British Columbia
Abstract
Rhabdocoel flatworms are abundant members of marine meiofaunal communities worldwide, contributing to a reservoir of biodiversity that thrives in sediments and on macroalgae. Yet, they are relatively understudied due to bias in meiofaunal collection techniques, time-intensive identification and a lack of taxonomic expertise. Here, sampling of intertidal habitats in British Columbia, Canada, was undertaken to enhance our understanding of rhabdocoel diversity in this region. Six species of neodalyellid rhabdocoel were discovered and characterized with DNA sequence and morphological data: Pogaina paranygulgus, Anoplodium hymanae, Baicalellia pusilla n. comb., B. solaris n. sp., B. daftpunka n. sp. and Tamanawas kalipis n. g., n. sp. The latter three species are new to science. A phylogenetic analysis of neodalyellid rhabdocoels, inferred from 18S and 28S ribosomal RNA gene sequences of 39 taxa, provided further insight into their interrelationships, trait evolution and systematics, including the recognition of a new genus, Tamanawas, and the suppression of Canetellia and Coronopharynx.
1 INTRODUCTION
Meiofaunal animals are useful bioindicators of marine ecosystem health (Kennedy & Jacoby, 1999; Zeppilli et al., 2015). Yet, studying impacts on meiofaunal biodiversity requires delineation of present biodiversity to establish a baseline. Although meiofaunal communities are traditionally considered to be dominated by nematodes, flatworms can also be extremely abundant, and their biomass in sandy habitats has been found to either equal or exceed that of nematodes (Martens & Schockaert, 1986). More recent surveys based on environmental DNA sequence (eDNA) data have shown that flatworms can rank as the second- or third-richest meiofaunal lineage in marine sediments (Fonseca et al., 2010, 2017). Their low richness and abundance rankings in some studies likely reflect the fact that delicate flatworms are often damaged and become unrecognizable by traditional sampling techniques (e.g. fixation and subsequent sieving of sediments) and must be either alive or well-preserved to be identified. Species identification is often laborious and based on complex reproductive structures; thus, a lack of taxonomic expertise has also contributed to sampling bias against flatworms.
The Rhabdocoela is a species-rich group of microturbellarian flatworms characterized by their small size (typically 0.5–1.5 mm long), the presence of a bulbous pharynx and a simple, sac-like gut (Ehrenberg, 1831). Most rhabdocoels either inhabit sediments or occur epiphytically in marine and freshwater environments. However, some taxa are known from limnoterrestrial environments or have become ecto- or endosymbiotic in other aquatic invertebrates (Jennings, 1997; Van Steenkiste, Davison, & Artois, 2010). While morphology of the reproductive organs, and sclerotized structures of the male copulatory organ in particular (e.g. stylet), usually provides robust characters for species identification, diagnosis and classification of taxa above species and genus level have proved more difficult. Rhabdocoela was formerly subdivided into the Dalyellioida, Typhloplanoida and Kalyptorhynchia based on pharynx morphology and the presence of a proboscis. Yet, molecular phylogenetic studies based on 18S and 28S ribosomal RNA (rRNA) gene sequences found the former two taxa to be paraphyletic, and they were merged to form the Dalytyphloplanida, consisting of freshwater Limnotyphloplanida, and marine Thalassotyphloplanida and Neodalyellida (Van Steenkiste et al., 2013; Willems et al., 2006). The latter clade is composed of mostly marine “Dalyellioida”, the promesostomid Einarella argillophyla and Solenopharyngidae. About half of neodalyellid species are free-living, while the other half are endosymbiotic representatives of the Graffillinae, Pterasticolidae and Umagillidae.
Around 1,700 described species of rhabdocoels are known worldwide, 180 of which belong to the Neodalyellida. Only 81 species of rhabdocoels, 18 of which are neodalyellids, are described from the Northeast Pacific Ocean (Tyler, Schilling, Hooge, & Bush, 2006–2018). To advance our understanding of rhabdocoel diversity in this region, we undertook sampling of various intertidal habitats in British Columbia, Canada. Species diversity and phylogeny of thalassotyphloplanids are reported in Van Steenkiste and Leander (2017) and Van Steenkiste, Herbert, and Leander (2018). In this study, we focus on neodalyellid diversity and phylogeny. Tamanawas kalipis n. g., n. sp., Baicalellia daftpunka n. sp. and Baicalellia solaris n. sp. are new to science and are described and discussed here for the first time. We also report on Anoplodium hymanae, Pogaina paranygulgus and Baicalellia pusilla n. comb. (formerly Coronopharynx pusillus) and provide further details on their morphology. Furthermore, we include nuclear ribosomal sequences for nine new taxa and provide an updated molecular phylogenetic analysis of the Neodalyellida to infer patterns of character evolution.
2 MATERIALS AND METHODS
2.1 Collection of organisms
During 2015–2016, a total of 32 individuals, representing five free-living neodalyellid species, were collected for morphological and molecular phylogenetic analysis, from beaches, rocky intertidals and estuarine mudflats in British Columbia, Canada. Rhabdocoel specimens were isolated from sand and algae with the MgCl2 decantation method, while the oxygen depletion method was used to extract animals from mud and finer sediments (Schockaert, 1996). Ten individuals representing two species of the Umagillidae were collected from subtidal sea cucumber Parastichopus californicus; three sea cucumbers were dissected to inspect the coelom cavity and intestine for the presence of umagillids.
2.2 Microscopy
Specimens were studied alive with DIC light microscopy and later mounted whole using Amman's lactophenol (100 g phenol, 100 ml lactic acid, 200 ml glycerine, 100 ml water) to preserve the stylet. Specimens intended for histological sectioning were fixed in marine Bouin's solution (RICCA Chemical Company, Arlington, TX, salinity adjusted with Instant Ocean Sea Salt), embedded in paraffin, serially sectioned (5 μm sections) and stained with Gill II haematoxylin, using Eosin Y as a counterstain (both Leica Biosystems, Wetzlar, Germany).
Live specimens, whole mounts and histological sections were photographed using a Zeiss Axioplan 2 microscope equipped with a Zeiss Axiocam 503 colour camera. Schematic diagrams were drawn using Adobe Illustrator (Adobe Systems Incorporated, San Jose, CA). Measurements were taken from preserved and live specimens along the longitudinal axis of the animal or its stylet (i.e. axial) using Fiji v. 1.47 (Wayne Rasband, National Institutes of Health). Holotypes were deposited in the Swedish Museum of Natural History (SMNH, Stockholm, Sweden), and other specimens remain in the Beaty Biodiversity Museum (BBM, University of British Columbia, Vancouver, Canada).
2.3 DNA extraction, amplification and sequencing
DNA was extracted from single specimens of nine species, amplified and sequenced according to the protocol used by Van Steenkiste and Leander (2017), with the exception that nearly complete 18S rRNA and partial 28S rRNA amplicons (henceforth referred to as 18S and 28S) were 1,633–1791 bp and 1,293–1,328 bp, respectively. The sequencing and PCR amplification primers and thermocycling conditions used are listed in Supporting Information Table S1. Full sequences were assembled in Geneious v.9.1.5 (Biomatters) and deposited in GenBank under the accession numbers listed in Table 1.
| Taxa | Locality | Coordinates | 18S accession # | 28S accession # |
|---|---|---|---|---|
| Outgroup | ||||
| Einarella argillophyla Luther, 1948 | Gullmaren, Sweden | 58°17′21′′N; 11°30′23′′E | AY775757 | NA |
| Trisaccopharynx westbladi Karling, 1940 | Kristineberg, Sweden | NA | AY775774 | NA |
| Solenopharyngidae sp. | Costa Paradiso, Sardinia, Italy | 41°03′09′′N; 8°56′16′′E | KC529519 | KC529640 |
| Austradenopharynx sp. | Poonindie, South Australia, Australia | 34°35′31′′S; 135°54′12′′E | KC529521 | KC529642 |
| Adenopharynx mitrabursalis Ehlers, 1972 | Sylt, Germany | 55°02′06′′N; 08°24′29′′E | KC529520 | KC529641 |
| Ingroup | ||||
| Tamanawas kalipis n. sp. | Victoria, BC | 48°24′12′′N, 123°21′03′′W | MH337259 | MH337262 |
| Seritia elegans Westblad, 1953 | Korsfjorden, Bergen, Norway | 60°11′00′′N; 05°12′15′′E | KC529517 | KC529638 |
| Wahlia microstylifera Westblad, 1930 | Korsfjorden, Bergen, Norway | 60°11′00′′N; 05°12′15′′E | KC529518 | KC529639 |
| Umagillidae sp. | Bamfield, BC | 48°51′18′′N, 125°09′45′′W | MH337260 | MH337261 |
| Anoplodium hymanae | Bamfield, BC | 48°51′18′′N, 125°09′45′′W | MH327508 | NA |
| Anoplodium stichopi Bock, 1925 | NA | NA | AF167424 | NA |
| Vejdovskya pellucida Schultze, 1851 | Hallands Väderö, Skåne, Sweden | 56°25′57′′N; 12°34′18′′E | KC529512 | NA |
| Vejdovskya ignava Ax, 1951 | Hanko, Finland | 59°49′21′′N; 22°58′21′′E | KC529513 | KC529635 |
| Provortex balticus 1 (Schultze, 1851) Graff, 1882 | Sandhammaren, Skåne, Sweden | 55°23′07′′N; 14°11′57′′E | KC529511 | NA |
| Provortex tubiferus Luther, 1948 | Bohuslän, Sweden | NA | AJ312269 | NA |
| Provortex balticus 2 Schultze, 18511851 | NA | NA | AJ312268 | AJ315648 |
| Provortex karlingi Ax, 1951 | Hallands Väderö, Skåne, Sweden | 56°26′00′′N; 12°34′26′′E | KC529510 | NA |
| Eldenia reducta Ax, 2008 | Zwin, the Netherlands | 51°21′56′′N; 03°22′18′′E | KC529502 | KC529627 |
| Bresslauilla relicta Reisinger, 1929 | Hanko, Finland | 59°49′50′′N; 23°09′33′′E | KC529515 | KC529636 |
| Neodalyellida sp. 1 | Donaña NP, Andalusia, Spain | 36°52′16′′N; 06°25′45′′W | KC529524 | KC529644 |
| Neodalyellida sp. 2 | Doha, Qatar | 25°19′09′′N; 51°32′16′′E | KC529525 | KC529645 |
| Balgetia semicirculifera Karling, 1962 | Sylt, Germany | 55°01′34′′N; 08°25′52′′E | KC529503 | KC529628 |
| Dalyellioida sp. | Costa Paradiso, Sardinia, Italy | 41°03′09′′N; 8°56′16′′E | KC529523 | KC529643 |
| Pogaina sp. 1 | iSimangaliso, KwaZulu-Natal, South Africa | 28°23′44′′S; 32°25′28′′E | KC529507 | KC529632 |
| Pogaina sp. 2 | Poonindie, South Australia, Australia | 34°35′31′′S; 135°54′15′′E | KC529508 | KC529633 |
| Pogaina sp. 3 | Kaneohe bay, Oahu, Hawai'i, USA | 21°30′47′′N; 157°50′09′′W | KC529506 | DK28S65 |
| Pogaina paranygulgus Karling, 19861986 | Surrey, BC | 49°05′09′′N; 122°51′39′′W | MH337254 | MH337258 |
| Pterastericola australis Cannon, 1986 | Australia | NA | AJ012518 | AY157161 |
| Pterastericola psilastericola Jespersen & Luetzen, 1972 | Skagerrak, Sweden | 58°46′39′′N; 10°41′55′′E | KC529516 | KC529637 |
| Baicalellia beauchampi (Ax, 1956) comb. nov. | Tjäreskäret, Västerbotten, Sweden | 63°28′2′′N; 19°46′3′′E | KC529504 | KC529629 |
| Baicalellia brevituba Luther, 1918 | Zwin, the Netherlands | 51°21′56′′N; 03°22′18′′E | KC529505 | KC529630 |
| Baicalellia canadensis Ax & Armonies, 1987 | Sandy Neck Estuary, Massachusetts, USA | 41°44′01.0′′N; 70°22′49.8′′W | KC869833 | KC869886 |
| Baicalellia solaris n. sp. | Victoria, BC | 48°24′12′′N; 123°21′03′′W | MH337256 | MH337255 |
| Baicalellia daftpunka n. sp. | Victoria, BC | 48°24′12′′N; 123°21′03′′W | MH337253 | MH337257 |
| Baicalellia pusilla (Luther, 1962) n. comb. | Nanaimo, BC | 49°11′43′′N; 123°57′32′′W | MH337252 | NA |
| Graffilla buccinicola Jameson, 1897 | NA | NA | AJ012521 | AJ313232 |
| Pseudograffilla arenicola Meixner, 1938 | Törö, Stockholms Län, Sweden | 58°48′42′′N; 17°47′42′′E | KC529514 | NA |
| Dalyellioida “houdini” sp. | Darwin, Australia | 12°26′06′′S; 130°49′58′′E | KC529522 | NA |
| Provorticidae sp. | Waimea Valley, Oahu, Hawaii, USA | 21°38′06′′N; 158°03′14′′W | KC529509 | KC529634 |
- Taxa in bold are new sequences for this study.
2.4 Molecular phylogenetic analyses
New sequences were aligned with existing neodalyellid 18S and 28S sequences downloaded from GenBank (Table 1) using the structural Q-INSI algorithm in MAFFT (Katoh & Toh, 2008). This resulted in a total of 39 18S and 28 28S sequences, including outgroup taxa. The latter were selected based on current knowledge of neodalyellid relationships (Van Steenkiste et al., 2013).
The 5′ and 3′ ends of the alignments were trimmed in Geneious v9.1.7 (www.geneious.com; Kearse et al., 2012). Ambiguous positions were selected with Aliscore v2.2 (Misof & Katharina, 2009) and removed with Alicut v2.3 (Kueck, 2009). The final 18S (1792 bp) and 28S (1184) alignments were concatenated, and best-fit partitioning schemes and models of molecular evolution were recovered in PartitionFinder v.1.1.0 using a greedy search with PhyML and the Bayesian information criteria (BIC) (Lanfear, Calcott, Ho, & Guindon, 2012). This resulted in two partitions corresponding with the 18S and 28S sequences and the GTR + GAMMA + I model for both partitions. Phylogenetic analyses using maximum likelihood and Bayesian inference were performed in RAxML v8.2.9 (Stamatakis, 2014) and MrBayes v3.2.6 (Ronquist & Huelsenbeck, 2003), respectively, using XSEDE in the CIPRES Science Gateway v3.3 (https://www.phylo.org/) and the same settings as in Van Steenkiste and Leander (2017).
For the 50% majority-rule consensus tree obtained, nodes with both bootstrap supports <70% and posterior probabilities <95% were collapsed using TreeGraph 2 (Stöver & Müller, 2010). The final concatenated tree was edited, and bootstrap supports and posterior probabilities were labelled using Adobe Illustrator.
3 RESULTS
3.1 Taxonomic summary
| Neodalyellida Willems et al., 2006 |
| Provorticidae Beklemishev, 1927 |
| Provorticinae Luther, 1962 |
| Tamanawas kalipis n. g., n. sp. |
| Neokirgellinae Oswald, Makarkin, Tyler, & Rogozin, 2010 |
| Pogaina paranygulgus Karling, 1986 |
| Baicalellia solaris n. sp. |
| Baicalellia daftpunka n. sp. |
| Baicalellia pusilla (Luther, 1962) n. comb. |
| Umagillidae Wahl, 1910 |
| Anoplodium hymanae Shinn, 1983 |
3.2 Tamanawas n. g
3.2.1 Diagnosis
Provorticinae with eyes; doliiform pharynx situated anteriorly; ovovitellaria; compact paired testes in first body half; long, muscular copulatory organ with coiled, tubiform male copulatory stylet.
Type species: Tamanawas kalipis n. sp.
Other species: Tamanawas halileimonia (Ax, 1960) n. comb., Tamanawas helictos (Ax, 1956) n. comb. (see Section 3.3.7 on T. kalipis n.g., n. sp.).
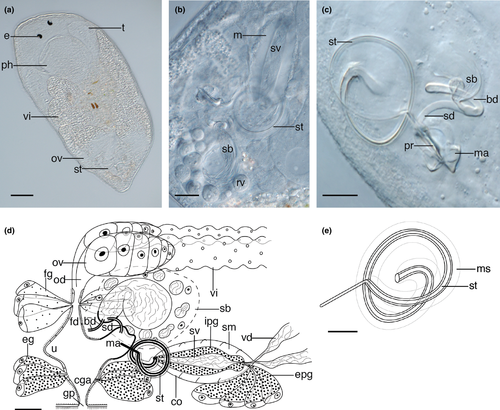
3.3 Tamanawas kalipis n. g., n. sp. (Figure 1)
3.3.1 Locality
Macroalgae in rocky lower intertidal at Clover Point, Victoria, British Columbia, Canada (48°24′12′′N; 123°21′03′′W; September 2, 2016): type locality.
3.3.2 Material
Observations on two live animals. Histological sections of one animal and two whole mounts; one whole mount is the designated holotype (SMNH, Type-9044), the other whole mount and serial sections are paratypes (BBM, MI4099-MI4100).
3.3.3 Etymology
The genus name refers to the animal's elusive nature, the species epithet to its occurrence in the intertidal. In Chinook jargon of the Pacific Northwest: Tamanawas: spirit/sorcerer, kalipi: rough tide.
3.3.4 DNA sequences
18S (GenBank accession MH337259); 28S (GenBank accession MH337262).
3.3.5 Diagnosis
Species of Tamanawas with a narrow, tubiform, 137–139 μm-long stylet forming one and a half coils with a diameter of 30–33 μm, ending in a straight protrusion of 20–22 μm; efferent and afferent system consisting of female duct and sclerotized spermatic duct, respectively; syncytial seminal bursa with blind sclerotized duct; eyes with large lenses; short protrusions around pharynx opening.
3.3.6 Description
Animals about 0.3–0.6 mm long with rounded anterior end and slightly tapering caudal end. Body is transparent with diatoms visible in the gut. Large doliiform pharynx (about one fifth of total body length) situated in the first third of the body, directly posterior to a pair of eyes with large 6 μm-long lenses (Figure 1a). Pharynx provided with circular and longitudinal muscles and has very short, hair-like protrusions around the wide proximal opening.
Short, paired testes (Figure 1a) lie in the anterior body third, at each side of the pharynx. Long vasa deferentia leave the testes posteriorly and connect to the proximal end of the copulatory organ in the last third of the body. Paired, elongate vitellaria (Figure 1a) run dorsolaterally, beginning parallel to the middle of the pharynx. They merge with the paired ovaries at the posterior end of the body, thus forming ovovitellaria.
Long, sausage-shaped copulatory bulb (Figure 1b,d) surrounded by one thick spiral muscle layer. Extracapsular prostate glands enter the copulatory bulb proximally. Within the bulb, the intracapsular seminal vesicle is narrow and lies medial to the prostatic gland necks. Distal to the copulatory bulb, the ejaculatory duct and prostate glands enter the sclerotized stylet. The stylet (Figure 1b–e) is a narrow tube of relatively consistent diameter which forms a spiral shape consisting of one and a half coils of length 137–139 μm (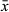 = 138 μm; n = 2), with widest point diameter of 31–33 μm (
= 138 μm; n = 2), with widest point diameter of 31–33 μm (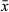 = 32; n = 2). Coils encased within a muscular tube or sleeve. Stylet terminates in a straight protrusion which projects at about 90° to the coil, slightly thinner in diameter than the coil itself and measuring 20–22 μm (
= 32; n = 2). Coils encased within a muscular tube or sleeve. Stylet terminates in a straight protrusion which projects at about 90° to the coil, slightly thinner in diameter than the coil itself and measuring 20–22 μm (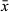 = 21 μm; n = 2). The protrusion enters the male atrium (Figure 1c,d), which is strongly sclerotized and forms folds which may be mistaken in whole mounts for projections of the stylet (Figure 1c). Male atrium connects to the common genital atrium (Figure 1d), which is surrounded by eosinophilic glands and contains glandular secretions.
= 21 μm; n = 2). The protrusion enters the male atrium (Figure 1c,d), which is strongly sclerotized and forms folds which may be mistaken in whole mounts for projections of the stylet (Figure 1c). Male atrium connects to the common genital atrium (Figure 1d), which is surrounded by eosinophilic glands and contains glandular secretions.
Ovaries connected to the common genital atrium through an efferent and afferent system (Figure 1d). The latter consists of a syncytial seminal bursa positioned between the ovaries. The syncytial tissue appears in close contact with the ovaries. Its large central sperm vesicle is folded over on itself (Figure 1b,c) and surrounded by several smaller vesicles (Figure 1b,d). It is slightly sclerotized at the end where it connects to a curved, strongly sclerotized spermatic duct which runs to the distal part of the male atrium. Another blind, sclerotized duct extends laterally from the central vesicle (Figure 1c,d; see Section 4). In the efferent system, female glands enter the female duct where the oviducts merge (Figure 1d). A protrusion (Figure 1d) of the common genital atrium or distal end of the female canal might function as a uterus. The epithelia of the common and female genital atria appear to be syncytial.
3.3.7 Remarks
Tamanawas kalipis n. g., n. sp. shares many morphological similarities with members of the provorticine genus Vejdovskya. These features include a doliiform pharynx, compact, paired testes lying close to the pharynx in the first body half, ovovitellaria, a long, muscular, male copulatory organ, and an elongate, tubiform copulatory stylet. A seminal bursa is reported for most species of Vejdovskya, often large in size as found in T. kalipis n. g., n. sp. (Ax, 1951, 1954, 1956, 1997; Karling, 1957; Luther, 1962).
The genus Vejdovskya has been diagnosed by a combination of characters, some of which are unclear or not present in some species. For instance, the position and size of the seminal vesicle and prostate glands in the copulatory bulb varies among species and two long tactile sensory cilia in the front end are not mentioned in the descriptions of Vejdovskya parapellucida (Ax, 1997) and Vejdovskya simrisiensis (Karling, 1957). Ax (2008) subdivided the genus into two groups. The first group consists of Vejdovskya ignava Ax, 1951; V. simrisiensis Karling, 1957; V. parapellucida Ax, 1997; V. pellucida Schultze, 1851 and Vejdovskya mesostyla Ax, 1954; all of which lack eyes, have ovovitellaria in the second body half, and an elongate, nonspiralling stylet. The second group consists of Vejdovskya halileimonia and Vejdovskya helictos, which differ from the other species in this genus by the presence of eyes and a spiral stylet (Ax, 2008). As such, the latter two species seem closely related to T. kalipis n. g., n. sp.
Vejdovskya halileimonia has three to four large stylet coils (Ax, 1960) which are similar in appearance to the one and a half coils observed in T. kalipis n. g., n. sp. The presence of a “muscular sleeve or cuff” which surrounds and follows the curves of the stylet, is also shared by T. kalipis n. g., n. sp. While V. halileimonia also has elongate vitellaria, it diverges from T. kalipis by having a connection between them, just distal to the pharynx, which is significantly smaller in size compared with the large pharynx in T. kalipis n. g., n. sp. Although a seminal bursa was not observed in live specimens of V. halileimonia, its presence cannot be excluded. Vejdovskya helictos has a large pharynx relative to body size and a stylet formed by six weak spiral coils (Ax, 1956). In addition, V. helictos also has a small, sclerotized, curved tube, which Ax (1956) predicts connects to the bursa and is likely homologous with the sclerotized spermatic duct we report in T. kalipis n. g., n. sp. Attached to the sclerotized tube and close to the stylet, Ax (1956) also describes a weakly-sclerotized ring which he predicts is part of a bursa in V. helictos. Perhaps this is part of the sclerotized wall of the male genital atrium as in T. kalipis n. g., n. sp.
Because of the unique morphological similarities (eyes, spiral stylet, spermatic duct) between V. helictos, V. halileimonia and T. kalipis n. g., n. sp. and the distinct phylogenetic position of T. kalipis n. sp. (see Figure 6 and Section 4), we propose the recognition of a new genus, Tamanawas n. g., and transfer V. halileimonia and V. helictos to this genus. Further morphological details have to be filled in for T. halileimonia n. comb. and T. helictos n. comb. to confirm similarities and homologies in the male and female genital system. Nonetheless, these three species differ in the number and extent of stylet coiling and the sclerotization of the male genital atrium and spermatic duct (not observed in T. halileimonia n. comb.).
3.4 Pogaina Marcus, 1954; Pogaina paranygulgus Karling, 1986 (Figure 2)
3.4.1 New localities
Estuarine mudflats in two locations: (a) Mud Bay Park, Surrey, BC (49°05′09′′N; 122°51′39′′W; October 28, 2015); (b) Ladysmith Inlet, Nanaimo, BC (49°01′28′′N; 123°50′59′′W; March 21, 2016).
3.4.2 Known distribution
Northeast Pacific Ocean: California (Karling, 1986).
3.4.3 Material
Observations on several live animals. Eleven whole-mounted specimens (BBM, MI4101–4111).
3.4.4 DNA sequences
18S (GenBank accession MH337254); 28S (GenBank accession MH337258).
3.4.5 Remarks
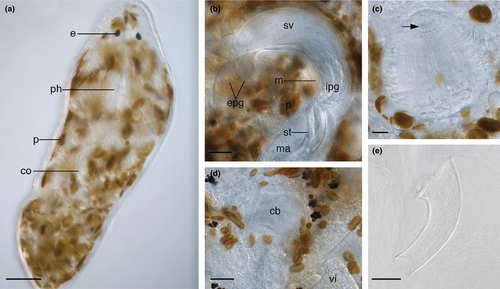
Animals transparent and about 0.4–0.7 mm long. Internal anatomy in accordance with the description of Karling (1986). Small oviform pharynx situated directly posterior to a pair of lenticular eyes (Figure 2a). Around 14 small, tooth-like protrusions around the proximal pharynx opening (Figure 2c). Protrusions more numerous than “about 12” described by Karling (1986). Paired testes relatively short and situated laterally, directly posterior to the pharynx. Paired ovaries distinct from the paired vitellaria and situated laterally, posterior to copulatory organ. Brown algal photobionts present throughout the parenchyma. Some are black and probably degraded (Figure 2d).
Copulatory bulb (Figure 2b) situated posterior to the pharynx in the midsection of the animal, taking the shape of a curved cylinder. The wall is formed by a thick layer of internal circular and external longitudinal muscles. Within the bulb, the seminal vesicle is proximal to the prostate glands. The extracapsular portion of the prostate glands are large and enter the copulatory bulb proximally on the convex side. Prostate gland necks merge with the copulatory duct inside the copulatory stylet. The stylet (Figure 2b,e) is a 74–104 μm-long curved funnel (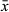 = 85 μm; n = 7) with a 25–38 μm-wide proximal pore (
= 85 μm; n = 7) with a 25–38 μm-wide proximal pore (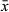 = 30 μm; n = 7) and narrow 6–16 μm-wide distal pore (
= 30 μm; n = 7) and narrow 6–16 μm-wide distal pore (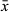 = 11 μm; n = 7). The large range is likely due to stylet compression during mounting; in live animals, the proximal and distal pores measured around 29 and 6 μm, respectively. Male atrium (Figure 2b) fits loosely around the stylet. Copulatory bursa (Figure 2d) surrounded by a weakly-sclerotized basal membrane and a layer of circular muscle.
= 11 μm; n = 7). The large range is likely due to stylet compression during mounting; in live animals, the proximal and distal pores measured around 29 and 6 μm, respectively. Male atrium (Figure 2b) fits loosely around the stylet. Copulatory bursa (Figure 2d) surrounded by a weakly-sclerotized basal membrane and a layer of circular muscle.
Previously described from Californian mudflats and tide pools (Karling, 1986), this is the first report of P. paranygulgus in British Columbia.
3.5 Baicalellia Nasonov, 1930
3.5.1 New diagnosis
Provorticidae with eyes; doliiform pharynx situated anteriorly, sometimes with protrusions around the opening; elongate vitellaria connected to posterior ovaries; paired testes sometimes connected by anterior transverse commissure; tubiform stylet; copulatory and seminal bursae; gonopore in posterior body third.
3.5.2 Remarks
The genera Coronopharynx and Canetellia are suppressed and their representatives transferred to the genus Baicalellia based on the results of our phylogenetic analysis (see Section 4 below). As such, the diagnoses of Nasonov (1930) and Luther (1962) had to be amended and a new diagnosis is provided.
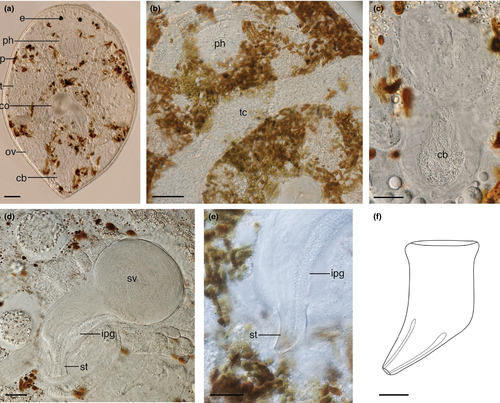
3.6 Baicalellia solaris n. sp. (Figure 3)
3.6.1 Localities
Macroalgae in rocky lower intertidal at three locations: (a) Clover Point, Victoria, British Columbia, Canada (48°24′12′′N; 123°21′03′′W; March 3, 2016): type locality; (b) West Beach boulders, Calvert Island, British Columbia, Canada (51°39′07′′N; 128°08′33′′W; April 9, 2016).
3.6.2 Material
Observations on several live animals and serial sections of one specimen (BBM, MI4118). Seven whole mounts, one of which designated as the holotype (SMNH, Type-9045), the others paratypes (BBM, MI4112–4117).
3.6.3 Etymology
The species name refers to its symbiotic relationship with photosynthetic microalgae. Solaris (Lat.): of the sun.
3.6.4 DNA sequences
18S (GenBank accession MH337256); 28S (GenBank accession MH337255).
3.6.5 Diagnosis
Species of Baicalellia with a tubiform, 22–35 μm-long stylet consisting of a 13–20 μm-wide straight tube which tapers distally at 65° into an asymmetrical funnel with flexible distal opening; photobionts in the parenchyma; short pointed protrusions around the pharynx opening; anastomosing testes posterior to pharynx.
3.6.6 Description
Animals about 0.5–0.6 mm long with rounded anterior end and slightly tapering caudal end (Figure 3a). The body is transparent but appears spotted due to the presence of brown algal photobionts in the parenchyma. A companion paper will discuss the nature of these photobionts. Relatively small doliiform pharynx in the first third of the body directly posterior to the large lenticular eyes. Pharynx provided with circular and longitudinal muscles, and very small pointed protrusions around the proximal opening (arrow in Figure 3b).
Internal organization similar to other species of the genus Baicalellia Nasonov, 1930 (see Ax, 1995; Luther, 1962; Nasonov, 1930, 1932). Paired testes (Figure 3a) at each side reach from just posterior to the pharynx to the posterior third. They are connected by an anterior transverse commissure (Figure 3b), located just posterior to the pharynx. Vasa deferentia leave the testes posteriorly and can become widened to form extracapsular seminal vesicles. Paired vitellaria (Figure 3a) run dorsolaterally and produce vitellocytes that surround maturing egg cells in the paired ovaries at the posterior end of the body, thus forming ovovitellaria.
Copulatory bulb (Figure 3d) of the male copulatory organ consists of two halves. Its proximal half contains a very large intracapsular seminal vesicle receiving the vasa deferentia. About halfway down the bulb, just distal from the intracapsular vesicle, extracapsular prostate glands enter the copulatory bulb. The distal half of the copulatory bulb contains the ejaculatory duct and gland necks filled with prostate secretion. This half of the bulb is surrounded by two spiral muscle layers. Distal to the copulatory bulb, the ejaculatory duct and prostate secretion enter the weakly-sclerotized stylet (Figure 3d–f). The latter is an asymmetrical tube with no projections. From the base to the distal apex it measures 22–35 μm ( = 28 μm; n = 7). The proximal half is a 13–20 μm-wide (
= 28 μm; n = 7). The proximal half is a 13–20 μm-wide (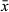 = 15 μm; n = 7) straight tube with a thickened proximal rim. The distal half abruptly tapers into an asymmetrical funnel at an angle of about 65° to a narrow distal opening of 3–4 μm. The distal opening is extremely flexible and widens to the same width as the proximal part of the stylet (Figure 3d) to facilitate evacuation of sperm and prostate secretion into the male atrium (Figure 3e). The latter fits tightly around the stylet and opens into a large common genital atrium.
= 15 μm; n = 7) straight tube with a thickened proximal rim. The distal half abruptly tapers into an asymmetrical funnel at an angle of about 65° to a narrow distal opening of 3–4 μm. The distal opening is extremely flexible and widens to the same width as the proximal part of the stylet (Figure 3d) to facilitate evacuation of sperm and prostate secretion into the male atrium (Figure 3e). The latter fits tightly around the stylet and opens into a large common genital atrium.
A large copulatory bursa (Figure 3c) surrounded by thick circular muscles protrudes from the dorsal wall of the common genital atrium. Through a sphincter, the common genital atrium enters a short female canal. The latter connects to the ovovitelloducts and to a muscular syncytial seminal bursa with a thickened basal membrane. Sperm are visible in the seminal bursa. A uterus is absent.
3.7 Baicalellia daftpunka n. sp. (Figure 4)
3.7.1 Localities
Macroalgae in rocky lower intertidal, Clover Point, Victoria, British Columbia, Canada (48°24′12′′N; 123°21′03′′W; March 3, 2016): type locality.
3.7.2 Material
Observations on several live animals. Three whole mounts, one of which designated as the holotype (SMNH, Type-9046), the others paratypes (BBM MI4119–4120).
3.7.3 Etymology
The species name refers to the helmet-shaped male copulatory stylet. Daftpunka: Daft Punk, an electronic music duo who wear helmets to conceal their identities while performing publicly.
3.7.4 DNA sequences
18S (GenBank accession MH337253); 28S (GenBank accession MH337257).
3.7.5 Diagnosis
Species of Baicalellia with 61–75 μm-long, upside-down helmet-shaped tubiform stylet with irregular rounded window and relatively wide distal opening; large pharynx with long tentacle-like protrusions; anastomosing testes in posterior body half.
3.7.6 Description
Animals 0.5–0.8 mm long, transparent, and with rounded anterior end and slightly tapering caudal end. Directly posterior to the lenticular eyes is the large doliiform pharynx (Figure 4b), which has around 26 long, tentacle-like protrusions around the proximal opening and is provided with circular and longitudinal muscles. Diatoms are visible in the gut.
Organization of ovovitellaria, vasa deferentia, prostate glands and intracapsular seminal vesicle (Figure 4c,d) as in B. solaris n. sp. Anastomosing paired testes in posterior half, lateral to the copulatory organ. The bulb of the latter is surrounded by two spiral muscle layers. The stylet (Figure 4e,f) is a 61–75 μm-long (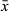 = 69 μm; n = 2), asymmetrical, upside-down helmet-shaped sclerotized tube. Distally the tube curves to a relatively wide opening, next to which there is an irregular rounded window. Sclerotization is stronger on one side of this window creating the impression of a pincer-like extension.
= 69 μm; n = 2), asymmetrical, upside-down helmet-shaped sclerotized tube. Distally the tube curves to a relatively wide opening, next to which there is an irregular rounded window. Sclerotization is stronger on one side of this window creating the impression of a pincer-like extension.
A seminal bursa (Figure 4d) with vacuolar epithelium is present, but its connection to the rest of the female system could not be observed. A large copulatory bursa (Figure 4d) is present at the distal end of the animal.
3.8 Baicalellia pusilla (Luther, 1962) n. comb. syn. Coronopharynx pusillus Luther, 1962 (Figure 5)
3.8.1 New locality
Coarse sand and shell hash in lower intertidal, Departure Bay, Nanaimo, BC (49°11′43′′N; 123°57′32′′W; April 12, 2015).
3.8.2 Known distribution
Northeast Pacific Ocean: Oregon (Karling, 1986), Gulf of Alaska (Ax & Armonies, 1990); Baltic Sea (Fenchel & Jansson, 1966; Luther, 1962, 1963); North Sea (Armonies, 1986; Tulp, 1974); White Sea (Kotikova & Joffe, 1988).
3.8.3 Material
Live animal observations and one whole-mounted specimen (BBM, MI4121). Histological sections of a specimen under the name of Vejdovskya pusilla, collected in 1947 in Tvärminne, Finland, and belonging to the collections of the Finnish Museum of Natural History (HLA.75939, http://id.luomus.fi/HLA.75939), correspond with B. pusilla n. comb. and are most likely part of the original material used by Luther (1962) to describe C. pusillus. Yet, Luther (1962) does not designate a holotype. Therefore, these sections are designated as the lectotype of B. pusilla n. comb.
3.8.4 DNA sequences
18S (GenBank accession MH337252).
3.8.5 Remarks
Animals transparent and about 0.8 mm long, falling within the 0.5–1 mm length range of previously described Pacific specimens. Food visible in the gut (Figure 5a). Large doliiform pharynx (Figure 5b) posterior to lenticular eyes with the conspicuous crown of about 26 long, pointed tentacles around the proximal opening. The tentacles appear to have small bristles on them (described by Luther, 1962 as “flagellated brushes”), although this was not reported in the other Pacific forms.
Genital organization consistent with the description of Luther (1962). Long, paired vitellaria reach from the pharynx to the paired ovaries, with which they form ovovitellaria. This is consistent with the Alaskan (Ax & Armonies, 1990) and Baltic (Luther, 1962) specimens but differs from the Oregon morphotype (Karling, 1986), which was reported to have distinct ovaries and vitellaria.
Paired testes not connected and located in posterior body third. Copulatory organ (Figure 5c) located between the testes and surrounded by spiral muscles. Prostate glands enter the seminal vesicle in the middle of the copulatory organ and long, thin gland necks are visible inside the stylet. The stylet (Figure 5c) is a cylindrical, 37 μm-long, slightly tapering tube. It has a proximal, curved spur on one side, measuring 34 μm. This is longer than the spur on the Alaskan (22–23 μm) and Oregonian (17 μm) forms (Ax & Armonies, 1990; Karling, 1986). Pacific populations previously described from estuarine habitats on the Oregon (Karling, 1986) and Alaskan (Ax & Armonies, 1990) coasts.
3.9 Distinctions between B. solaris n. sp., B. daftpunka n. sp. and B. pusilla n. comb
The general organization of B. solaris n. sp. and B. daftpunka n. sp. strongly resembles members of Baicalellia Nasanov, 1930, supporting their phylogenetic position based on DNA sequence data (see Figure 6 and Section 4). As is typical of Baicalellia, the vitellaria are elongate and caudally join paired ovaries to form ovovitellaria, the gonopore is located in the posterior body third, paired testes are connected by a transverse commissure, stylets are tubiform and copulatory and seminal bursae are present. Baicalellia solaris n. sp. differs from other species in this genus because of the morphology of its stylet and the presence of algal photobionts in its parenchyma. Baicalellia groenlandica has the most similar stylet to B. solaris, described as a slightly-curved tube, although it differs by curving continuously from base to apex rather than only in the distal half, and the possession of a small, tooth-like distal protrusion (Ax, 1995). In the past, only Pogaina was known to harbour symbiotic microalgae among marine rhabdocoels. As such, this is the first time photobionts are reported in another marine rhabdocoel genus. Baicalellia daftpunka is also unique because of its stylet morphology. The helmet-shaped stylet of B. daftpunka is unlike those previously described for species of Baicalellia. The stronger sclerotization on one side of its rounded, distal window gives the impression of a pincer-like extension, such that the stylet may appear superficially similar to the stylet of Baicalellia anchoragenesis (Ax & Armonies, 1990). Based on the results of the phylogenetic analysis (Figure 6) and morphological similarities to the genus Baicalellia, Canetellia beauchampi, Canetellia nana and C. pusillus are transferred to Baicalellia (see Section 4).
The genus Baicalellia was erected by Nasonov (1930) to accommodate seven freshwater “dalyellioid” species from Lake Baikal. Subsequent additions of species to this genus are mostly euryhaline species from freshwater and brackish water environments around the world. The only truly marine species is Baicalellia forcipifera (Van Steenkiste, Volonterio, Schockaert, & Artois, 2008). As such, B. solaris n. sp. and B. daftpunka n. sp., both found in the rocky marine intertidal, confirm that representatives of this genus can also be exclusively marine. The addition of the latter two species, Baicalellia beauchampi n. comb., Baicalellia nana n. comb. and B. pusilla n. comb (all brackish water species) brings the total number of Baicalellia species to 23.
3.10 Anoplodium Schneider, 1858; Anoplodium hymanae Shinn, 1983
3.10.1 New localities
Endosymbiotic in the coelom of holothurian Parastichopus californicus collected at 15–20 m depth between Wizard Island and Helby Island, Bamfield, BC (48°51′18′′N; 125°09′45′′W; June 3, 2015; August 31, 2015).
3.10.2 Known distribution
Parastichopus californicus from Cowlitz Bay and Colin's Cove, Washington, USA (Shinn, 1983).
3.10.3 Material
Observations on several live animals and serial sections of one specimen (BBM, MI4123). One whole-mounted specimen (BBM, MI4122).
3.10.4 DNA sequences
18S (GenBank accession MH327508).
3.10.5 Remarks
Anatomy consistent with the description by Shinn (1983). Differs from other Anoplodium species in having a pseudostratified epidermis consisting of large ciliated cells and numerous, smaller basal cells, anterior male accessory glands and a single, median ovary, which it shares only with Anoplodium mediale (Ozaki, 1932). This is the first time this species has been found in British Columbia.
3.11 Molecular phylogenetic relationships
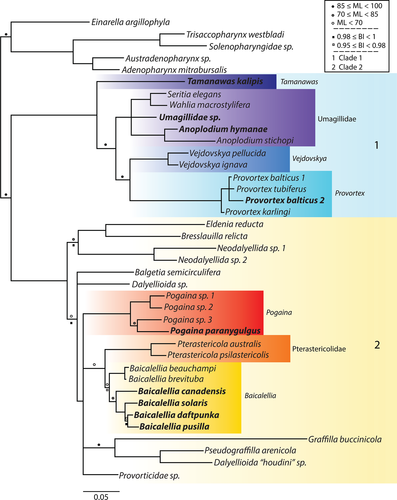
Our phylogenetic analyses are based on the concatenated dataset including 39 taxa and 3,077 bp. This consists of partial 18S rRNA (1,792 bp) and 28S rRNA sequences (1,184 bp) for 39 and 28 neodalyellid species, respectively. ML and Bayesian tree topologies were congruent and are summarized in Figure 6.
The outgroup includes four members of the monophyletic Solenopharyngidae, and the promesostomid E. argillophyla. The ingroup consists of two sister clades that are consistent with Van Steenkiste et al. (2013), but some relationships within them are updated: (a) Clade 1 includes the provorticine genera Provortex and Vejdovskya (Provorticidae), and Umagillidae; the polytomy of these taxa is resolved here. Umagillidae and Provorticinae are sister taxa, and T. kalipis n. g., n. sp. falls basal to them; (b) Clade 2 includes the provorticid Eldenia reducta and several members of Neokirgellinae (Provorticidae), Pterastericolidae, and Graffillidae. Baicalellia sensu Nasonov is paraphyletic because of the position of B. beauchampi n. comb. (formerly C. beauchampi) and B. pusilla n. comb. (formerly C. pusillus). These species are now assigned to Baicalellia. The sister group relationship of Baicalellia and Pterastericola recovered by Van Steenkiste et al. (2013) is supported, but the branching of Pogaina as basal to these taxa is not well supported in the analysis. Rather, the Baicalellia-Pterastericola group falls within a polytomy containing Pogaina, Provorticidae sp. and the group of graffillids Pseudograffilla arenicola, Graffilla buccinicola, and Dalyellioida “houdini” sp. We find support for a basal clade with Eldenia reducta, Bresslauilla relicta and Neodalyellida spp.
4 DISCUSSION
4.1 Clade 1: Tamanawas, Provorticinae and Umagillidae
The inclusion of T. kalipis n. g., n. sp. and two umagillids (A. hymanae and undescribed Umagillidae sp.) resolves the polytomy of the Provorticinae–Umagillidae clade recovered in Van Steenkiste et al. (2013). The basal position of T. kalipis n. g., n. sp. to all umagillids and Vejdovskya + Provortex suggests that a relatively long tubular stylet and the presence of an afferent and efferent system in the atrial organs are most likely plesiomorphic traits. Umagillids typically have a double connection between the common atrium and the female system. This consists of a female duct (efferent) as in other neodalyellids, but also a vagina leading to a seminal bursa (afferent) that connects to the ovovitellarian junction, often via a seminal receptacle. Perhaps the spermatic duct of T. kalipis n. sp. is homologous with the umagillid vagina. In addition, sclerotization is present in parts of the umagillid bursa and/or associated ducts, as seen in T. kalipis n. g., n. sp., although absent from the Collastominae (Cannon, 1982). A bursal valve, defined as a common plug at which two sclerotic ducts (leading from the vagina and to the seminal receptacle, respectively) enter the bursa (Cannon, 1987), is present in many members of the Umagillinae and was proposed by Cannon (1982) as the ancestral condition. This may be homologous with the point at which the spermatic duct and a second sclerotized duct enter a sclerotized part of the bursa in T. kalipis n. g., n. sp., although here the second duct appears blind rather than connecting to a seminal receptacle.
For several species of Provortex, including P. balticus, P. pallidus, P. tubiferus, P. karlingi and P. psammophilus (Ax, 1951; Luther, 1948; Meixner, 1938; Schultze, 1851), a double connection is also reported. Here, the afferent system consists of a non-sclerotized spermatic duct connected to a bursa. Although not reported in Vejdovskya and other species of Provortex, a double connection may also be present. As such, the double connection to the female system may be a synapomorphy that distinguishes Clade 1 from Clade 2. The presence of an afferent and efferent system is not uncommon among other dalytyphloplanids and can have various degrees of complexity including a bursa, sclerotized structures, sphincters and insemination ducts (e.g. Trigonostomidae; see (Van Steenkiste & Leander, 2017). This complex system is most likely involved in mediating the transport of sperm to the ovaries during sexual reproduction. As such, it is possible that sexual selection resulted in the evolution of these important diagnostic traits in the atrial system of neodalyellids and other rhabdocoels.
Umagillidae, Provortex and V. ignava all have a uterus, albeit generally more established in form than in T. kalipis n. g., n. sp. (Ax, 2008; Cannon, 1982). Some umagillids (e.g. species of Anoplodium, Collastoma monorchis and Ozametra arbora; Cannon, 1982) have lost the stylet in the male genital system. In addition, eyes are lost in all umagillids and all current species of Vejdovskya. While the loss of eyes can easily be understood in endosymbiotic taxa such as umagillids and pterastericolids, it is not clear why some free-living neodalyellid taxa such as Vejdovskya, Einarella and some solenopharyngids are eyeless.
4.2 Clade 2: Pogaina, Pterasticolidae, Baicalellia and relatives
The addition of B. solaris n. sp., B. daftpunka n. sp., B. pusilla n. comb. (formerly C. pusillus) and Baicalellia canadensis to the data set of Van Steenkiste et al. (2013) provides insights into the interrelationships within Baicalellia. B. pusilla n. comb. and B. beauchampi n. comb. (formerly C. beauchampi) fall within Baicalellia. This supports the observation by Ax (2008) that there is no adequate morphological justification for the genus Canetellia.
Both B. pusilla n. comb. and B. beauchampi n. comb. share Baicalellia-like characters (paired, elongate ovovitellaria, paired testes, long, muscular copulatory organ, seminal and copulatory bursae and posterior gonopore), with the exception that they lack a transverse commissure between the testes. Baicalellia canadensis (Ax & Armonies, 1987) also lacks this connection. As such, the transverse commissure cannot be considered as a diagnostic character for the genus. Unconnected testes may represent multiple independent secondary losses of the anastomosing commissure, although it is possible that this character shows plasticity during the animal's development. This seems to be the case in the rhabdocoel Lurus evelinae, whose paired testes coalesce in some specimens and not in others (Van Steenkiste et al., 2008).
Baicalellia pusilla n. comb. is the sister taxon to B. daftpunka n. sp., with which it shares long, tentacle-like protrusions around the pharynx opening. Such tentacles, which may have a sensory function, are also shared by Baicalellia albicauda, Baicalellia nigrofasciata, Baicalellia baicali, Baicalellia posieti, Baicalellia evelinae and B. beauchampi n. comb. (Ax, 1956; Marcus, 1946; Nasonov, 1930), but are not reported in the remaining 15 Baicalellia species. Very short, tooth-like protrusions surround the opening of the B. solaris n. sp. pharynx (similar to those seen in some Pogaina species). Long, tentacle-like protrusions are present in several other neokirgellines (e.g. Kalyla, Daelja, Neokirgella, Selimia; Beklemischev, 1927; Marcus, 1951; Ax, 1959), but none of these taxa have been included in a phylogenetic analysis so far. As such, assessing homologous characters in neodalyellid pharynx morphology remains problematic and more detailed morphological studies are needed to support future phylogenetic studies.
Based on the phylogenetic position of B. pusilla n. comb. and B. beauchampi n. comb. and their morphological resemblance to species in Baicalellia, Canetellia and the monotypic Coronopharynx are suppressed. The second described Canetellia species, C. nana, is not included in this phylogenetic analysis, but its limited description suggests it differs from B. beauchampi n. comb. only in its stylet morphology and vasa deferentia that leave the testes proximally rather than distally (Ax, 2008). For that reason, it is also included within Baicalellia.
The endosymbiotic Pterasticolidae is confirmed as the sister group to Baicalellia, but differs from species of Baicalellia in having unpaired gonads, a small, bean-shaped copulatory organ usually in the anterior half of the body, an ootype and sometimes a pseudovagina. The single testis in Pterastericolidae lends support to the hypothesis that Baicalellia-like anastomosing testes could have been the plesiomorphic condition in the common ancestor of these taxa. Pterastericolids also have several adaptations to an endosymbiotic lifestyle: adhesive organs, no eyes and loss of cilia during their lifetime (Cannon, 1987).
The polytomy of Pogaina, the Baicalellia + Pterasticola sister group, Provorticidae sp. and the graffillid group P. arenicola + G. buccinicola + Dalellioida “Houdini” sp. limits the discussion about the evolution of traits in these taxa. Pogaina and Baicalellia have many shared characters, and the results of our phylogenetic analysis complicate a clear diagnosis for these genera based on morphological characters. The presence of photobionts (reported as “zooxanthellae”) in the parenchyma was the main diagnostic character to identify Pogaina (Ax, 1970). Yet, zooxanthellae are reported in B. evelinae and now photobionts have also been found in B. solaris n. sp., which may provide a consistent source of energy in coastal waters with changing seasonal availability of microalgae. As such, the two main characters that allowed Pogaina to be distinguished from Baicalellia (separate, nonanastomosing testes and zooxanthellae) are no longer valid. It is possible that some species of Pogaina that were solely described on stylet morphology and the presence of photobionts belong to Baicalellia.
Representatives of Baicalellia and Pogaina share the presence of a copulatory and seminal bursa. Two bursae are not reported for all species, but this likely reflects problems with identification and/or their absence in sexually immature specimens (i.e. Baicalellia rectis, Pogaina japonica; Ax, 2008). Here “copulatory bursa” and “seminal bursa” are used sensu Van Steenkiste et al. (2018) in an attempt to standardize the terminology. The copulatory bursa is a blind, usually muscular sack protruding from the common genital atrium, that likely serves to store a mate's sperm. This organ has also been referred to as a “bursa copulatrix” (Nasonov, 1930). The seminal bursa is a sack connected to the female system that may serve to resorb redundant sperm. It is often syncytial, contains spermatic vesicles and has also been referred to as “phagocytic organ,” “seminal receptacle” (Nasonov, 1930) or “syncytial tissue” (Ax & Armonies, 1990). Use of the term “seminal receptacle” is reserved for any additional sperm storage structures. For now, we refrain from providing new morphology-based diagnoses for the taxa Pogaina and Baicalellia sensu novo until a very thorough systematic revision, including both morphological and DNA sequence data, of more representatives can be carried out.
5 CONCLUSIONS
Our sampling campaign of neodalyellids in British Columbia has contributed to knowledge of rhabdocoel species diversity in the Northeast Pacific Ocean, providing descriptions of three novel species and updates to three previously described species. New 18S and 28S sequences from the six described species and three additional, undescribed species allowed us to improve inferences about the phylogenetic relationships within the Neodalyellida. These results have led to the following conclusions: (a) confirmation of the paraphyly of the Provorticidae and Neokirgellinae; (b) a double connection to the female reproductive system may be a synapomorphy for Clade 1 consisting of T. kalipis n. sp., Umagillidae and Provorticinae; (c) monophyly of Provorticinae (Vejdovskya and Provortex); (d) the basal position of T. kalipis n. sp. in Clade 1 necessitates the recognition of a new genus Tamanawas n. g., into which T. halileimonia n. comb. and T. helictos n. comb. are transferred based on shared characters; (e) Coronopharynx, Canetellia and Baicalellia cannot be differentiated based on morphological and DNA sequence data, resulting in suppression of the former two genera in favour of Baicalellia; (f) Baicalellia and Pogaina lack distinguishing diagnostic characters at the morphological level but are clearly differentiated in the molecular phylogenetic analysis.
As is evidenced by the polytomies in the analysis, many neodalyellid relationships remain uncertain. Increased sampling efforts will not only improve knowledge of the diversity and importance of rhabdocoels in meiofaunal communities, but also provide data to improve molecular phylogenetic inferences and to identify diagnostic morphological characters at the genus level and above. Increased sampling of this group should also shed light onto the prevalence and evolution of photobionts.
ACKNOWLEDGEMENTS
We are extremely grateful to María Herranz, Greg Gavelis, Emma Gavelis and Jane Yangel for their assistance in the field, and to the Hakai Research Institute and the Bamfield Marine Sciences Centre for supporting sampling campaigns. We also thank Dr. Maarten Vanhove and Katja Lylund (Finnish Museum of Natural History) for providing us with high-quality micrographs of the serial sections of “Vejdovskya pusilla.” This work was supported by the Tula Foundation through the Centre for Microbial Diversity and Evolution (CMDE) and the National Science and Engineering Research Council of Canada (NSERC 2014-05258).




