Phylogeography of Plectostylus Beck, 1837 (Gastropoda: Stylommatophora: Orthalicoidea): Origin and isolation of the Fray Jorge forest relicts in northern Chile
Abstract
Among the questions surrounding the biogeographical history of the Chilean biota, none has gathered more interest than the origin of the Fray Jorge (FJ) forest relict and its biota. Inserted in a semi-desert area, this forest enclave exists due to the existence of a very particular microclimate in this region. The age of the disjunction and the historical relationship between the FJ biota with the remaining components of South America are explained by two distinct, competing hypotheses: the first suggests that it would have become isolated during the climatic changes of the Paleogene/Neogene, while the second suggests that the isolation is a product of Quaternary glaciations. To discriminate between these competing hypotheses, we used DNA sequence phylogeny methods and molecular genetic dating to the study of a genus of land snails (Plectostylus) that occurs in the FJ relict and throughout Chile. The phylogeny shows a clear distinction between forest and arid clades, and each of these clades is formed by many geographically circumscribed populations. The FJ fragment snails form a clade that is sister to all other forest clades. The separation between the Fray Jorge clade and the other forest clades dates back to the Paleogene/Neogene. Our data suggest that the FJ forest is a relict from the forests that occupied that landscape during the Paleogene/Neogene and retreated due to the aridification of the region. We also observe that the current taxonomy of the Plectostylus genus must be re-evaluated.
1 INTRODUCTION
Among the questions surrounding the biogeographical history of the Chilean biota, one of the most intriguing ones is the origin of the Fray Jorge (FJ) forest relict (30°40′S; 71°35′W; >400 m above sea level—a.s.l.). This is the northernmost forest formation in Chile, a remnant of Valdivian temperate rainforest located over 1,000 km from where this biome is distributed (Villagrán et al., 2004). Inserted in a semi-desert area, in a matrix of xerophile vegetation, this forest enclave exists due to the existence of a very particular microclimate in this region, where there is condensation of fog on the top of the mountains close to the coast. Its isolation associated with a remarkable floristic similarity to the austral forests of Chile has intrigued many scientists for the last 100 years.
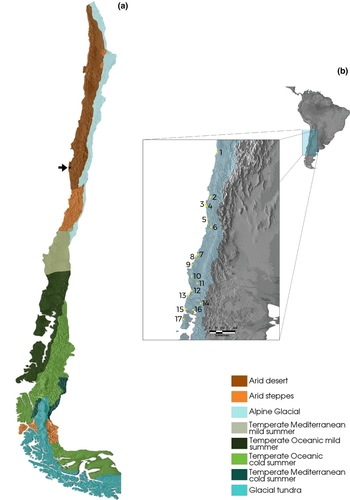
The Chilean climate is heavily influenced by air and sea currents, latitude and altitude. The Andes work as a wall to air circulation, provoking rains in some areas while blocking the humidity from reaching other areas. When considering the large number of variables that influence the climate in this area (high altitudes influencing air mass movement, sea currents), the Andes create not only a complex scenario with many different climates, but also extreme environmental conditions. The different climates that occur in Chile can be summarized in three main groups: arid, temperate and glacial (Figure 1a). As can be seen in Figure 1, FJ is located in an area of desert climate; nevertheless, it presents a vegetation conformation typical of temperate regions of southern Chile, with whom this relict shares a high floristic similarity. Additionally, FJ shares floristic similarities with very distant biotas such as Australasia, South Africa and other South American temperate forests (Troncoso, Villagrán, & Muñoz, 1980; Villagrán & Hinojosa, 1997; Villagrán et al., 2004). These unique attributes made this biome to be classified as a relict, a reminiscent of ancestral forests that covered a large portion of the South American continent in the past under very distinct climatic conditions (Villagrán et al., 2004). This area is currently recognized by UNESCO as a biosphere reserve.
The dominant species in this forest is Aextoxicon punctatum Ruiz and Pavón (Aextoxicaceae), commonly known as olivillo. The distribution of this particular species, as well as many other vascular plants that are found in FJ, is associated with forests that are very distant from FJ. The nearest individuals are more than 800 km away, usually in the austral forests of Chile. The FJ flora has been divided into three groups, according to their phylogenetic relationships and floristic similarities: South American, Australasian and endemic (Villagrán et al., 2004).
The age of the disjunction and the historical relationship between the enigmatic FJ biota with the remaining components of South America have been explained by two distinct, competing hypotheses. Philippi (1884) and later Schmithüsen (1956) suggested that this relict would have become isolated during the Paleogene/Neogene, between 65.5–2.58 million years ago (Mya) as a reminiscence of the warm and humid forests that occupied that region. On the other hand, a number of different authors (Looser, 1935; Muñoz & Pisano, 1947; Skottsberg, 1948; Wolfhügel, 1949; Troncoso et al., 1980) have suggested this relict would have become isolated during the Quaternary (2.59 Mya to the present day), owing its origin to the forest contractions and expansions caused by the climactic fluctuations associated with glacial and interglacial cycles. These two hypotheses are at the core of the debate over the origin of the FJ forest (Villagrán et al., 2004).
The hypothesis proposed by Philippi (1884) and Schmithüsen (1956) is supported by the fossil record of the Paleogene/Neogene from South America and Antarctica (e.g., Romero, 1978, 1986; Hinojosa & Villagrán, 1997). It is also supported by current knowledge on the vegetation of the Paleogene (65.5–23.03 Mya) that indicate a succession of three floras: Neotropical (65–55 Mya), followed by a mixed forest during the Eocene (55–40 Mya) and finally the formation of Antarctic forest (from the Eocene to the Oligocene, 40–23 Mya). This evidence shows the arid area where FJ is inserted was covered with forests during the Paleogene/Neogene and not the Pleistocene. In this context, the discontinuity of the Chilean forests with the remaining South American forests, including FJ, would be a consequence of the fragmentation of a continuous forest that extended along the subtropical region of South America during the Miocene. This fragmentation would be a consequence of the increasing aridity caused in part by the Andes blocking the rainclouds coming from the East during its uplifting in the end of the Plio-Pleistocene. This uplifting and its consequences are likely associated with the expansion of xeric formations forming what is now known as the dry diagonal of South America (a succession of dry formations in South America that runs from Northeast Brazil until The Atacama desert in Chile; Villagrán & Hinojosa, 1997). Alternatively, Villagrán et al. (2004) state that FJ would be a relict of the expansions and retractions of southern Chilean vegetation during the glacial–interglacial cycles of the Quaternary. According to this last hypothesis, the age of the relicts of A. punctatum Ruiz & Pav. from the North of Chile can be assigned to both the late Pleistocene (Looser, 1935; Skottsberg, 1948), and the early to middle Pleistocene (Muñoz & Pisano, 1947; Wolfhügel, 1949; Troncoso et al., 1980).
Although geological data suggest that the Talinay Heights (where the FJ forest is) were established in the late Calabrian (1.8–0.781 m.a.; Troncoso et al., 1980), there is a large body of evidence that is incompatible with a recent origin for this biome. The FJ relicts have no sclerophyll species, which suggests that this forest has been in Talinay since before the expansion of the coastal wet forests in central Chile. Additionally, this hypothesis implies a gradient of floristic similarity from North to South correlated with geographical distance, but the observed pattern suggests a separation of the FJ fragment from an older and widely distributed forest in the Chilean coast (Villagrán & Hinojosa, 1997).
Most of the studies that discuss the origin of the FJ forest are based on botanical data (Muñoz & Pisano, 1947; Pérez & Villagrán, 1985; Hinojosa & Villagrán, 1997). Until the present day, none of these hypotheses have been tested in a phylogeographic framework using Metazoans. Animals that present restricted distributions and low vagility can be a valuable source of information in biogeographical studies (Solem, 1981). Among the animals that present the previous characteristics, we can point land snails, as most species in this group have a very restricted distribution. Additionally, many gastropods have very specific habitat requirements that result in insular, fragmented distributions (Kerney, Cameron, & Jungbluth, 1983; Pfenninger, Posada, & Magnin, 2003), associated with low active dispersal potential (Cowie, 1984; Pfenninger, Bahl, & Streit, 1996; Baur & Baur, 1993). These attributes suggest that land snails are very good models for biogeographical studies (Solem, 1981; Wade, Mordan, & Clarke, 2001).
The genus Plectostylus Beck, 1837 is completely restricted to the Chilean territory and adjacent Andean regions. The group's distribution extends approximately from latitudes 20°S to 42°S. They are found in the Atacama desert, the driest place in the world, more than 1,000 km north of the FJ forest, and at the same time deep into the temperate humid forests of central and southern Chile reaching the Island of Chiloé, 1,200 km south of FJ (Figure 1b for reference). There are currently 12 valid species for this genus with wide, unclear distributions and no phylogenetic hypothesis (Valdovinos & Stuardo, 1988). We believe that a phylogeographic study using Plectostylus mollusks will yield substantial information for the current hypotheses that seek to explain the origin of the FJ forest.
One of the best ways to date events of separation between species or populations is to use molecular estimations of divergence (i.e., a molecular clock). A recent study described the molecular genetic phylogeny for the superfamily Orthalicoidea (Breure & Romero, 2012) in which Plectostylus is inserted. Through the use of multiple DNA sequence markers and fossil calibrations, this study suggested a time of separation for all the important nodes within this clade. The divergence between Plectostylus and the closely related Australian genus Bothriembryon Pilsbry, 1894 was estimated at 31.4 Mya. We believe it is possible to utilize the age calibration described by Breure and Romero (2012) under a DNA marker-based phylogeographic framework to establish the age of the isolation of the FJ forest relict snails with its closest living relative, giving a rough estimate of the age of the isolation event and add another piece of evidence to support one of the competing hypotheses for the origin of this relictual forest. We also believe that a phylogeographic study would allow us to reconstruct a piece of the history of the FJ relict and the group studied, by inferring the phylogenetic history between the animals distributed all across the Chilean territory.
Given the factors explained above, this work has the following objectives: To (a) perform a comprehensive phylogeographic study in the genus Plectostylus using mitochondrial and nuclear DNA markers, allowing for the identification of the lineages that form this complex and establish the evolutionary relationships between those; (b) estimate the time of divergence between the Fray Jorge Plectostylus population and the animals present in the central and southern Chilean forests, and using this information to discriminate between the two competing hypotheses that try to explain the origin of the isolation of this relict.
2 MATERIAL AND METHODS
2.1 Sampling
The animals used in this study were captured in two distinct collecting trips: the first between December 2009 and February 2010 and the second between December 2010 and January 2011. The animals were collected manually. The localities and sample sizes are described in Table 1 and Figure 1b. The complete list of animals collected with voucher names is available in Supporting information Table S1. The localities where the animals were captured range from temperate forest to semi-desert landscapes, in a transect that spans over 2,000 km (Figure 1). The habitats visited range from arid soils to treetops, gradually from northern (where the animals concentrate at the top of the coastal mountains, where the humidity is higher) to southern Chile (Valdivian forests, with over 2,500-mm annual precipitation). The captured animals were preserved in 100% ethanol and transported to the University of São Paulo (USP), where the protocols for DNA extraction and sequencing were implemented. The specimens are deposited in the Malacological Collection of the USP Zoology Museum.
| No | Locality/federal unit | Lat | Long | n | Environment |
|---|---|---|---|---|---|
| 1 | Paposo/Antofagasta (Taltal) | −25.03 | −70.45 | 25 | Arid desert |
| 2 | Las Rojas/Coquimbo | −29.92 | −71.17 | 25 | Arid desert |
| 3 | Fray Jorge Nat. Park (forest)/Coquimbo | −30.66 | −71.68 | 11 | Temperate forest |
| 4 | Fray Jorge Nat. Park (sclerophyll)/Coquimbo | −30.84 | −71.62 | 6 | Arid desert |
| 5 | Cerro Santa Inés/Valparaíso | −32.16 | −71.49 | 30 | Arid desert |
| 6 | La Campana Nat. Park/Valparaíso | −32.98 | −71.12 | 10 | Temperate forest |
| 7 | Los Ruiles Nat. Road/Maule | −35.83 | −72.51 | 2 | Temperate forest |
| 8 | Los Queules Nat. Road/Maule | −35.98 | −72.71 | 2 | Temperate forest |
| 9 | Hualpén/Bio Bio | −36.80 | −73.17 | 40 | Temperate forest |
| 10 | Contulmo Nat. Monument/Araucanía | −38.02 | −73.16 | 25 | Temperate forest |
| 11 | Cerro Ñielol Nat. Monument/Araucanía | −38.73 | −72.59 | 23 | Temperate forest |
| 12 | Mehuín/Los Rios | −39.42 | −73.21 | 5 | Temperate forest |
| 13 | Valdivia/Los Rios | −39.96 | −73.58 | 16 | Temperate forest |
| 14 | Puyehue Nat. Park/Los Lagos | −40.78 | −72.22 | 3 | Temperate forest |
| 15 | Los Muermos/Los Lagos | −41.36 | −73.81 | 15 | Temperate forest |
| 16 | Lahuén Ñadi Nat. Monument/Los Lagos | −41.43 | −73.03 | 3 | Temperate forest |
| 17 | Chiloé Nat. Park/Los Lagos | −42.37 | −74.06 | 33 | Temperate forest |
2.2 DNA extraction amplification and sequencing
Genomic DNA was extracted using the protocol for mollusks developed by Sokolov (2000). The DNA obtained in this step was used for amplification of the markers used in this study. Two DNA markers were selected for this study. The first was the mitochondrial gene Cytochrome Oxidase I (COI). This marker is one of the most commonly used in phylogenetic studies (Hurst & Jiggins, 2005). We targeted a region of approximately 700 base pairs (bp) inside the gene, using the universal primers developed by Folmer, Black, Hoeh, Lutz, and Vrijenhoek (1994). The second marker selected was Internal transcribed Spacer 2 (ITS2) in the nuclear ribosomal DNA cluster. This fragment has been used in many large-scale phylogenetic studies, including over 200 species of land snails in more than 150 genera (Wade & Mordan, 2000; Wade et al., 2001; Wade, Mordan, & Naggs, 2006). This marker was considered highly variable (Baldwin, 1992; Schultz, Maisel, Gerlach, Müller, & Wolf, 2005) and was also used in the more complete phylogeny published to date for the superfamily Orthalicoidea (Breure & Romero, 2012). We used the primers developed specifically for mollusks by Wade and Mordan (2000). These primers amplify the whole ITS2, as well as approximately 70 bp of the 5.8s gene in the 3′ region and over 300 bp of the 28s gene in the 5′ end.
Sequencing reactions were carried out using BigDye Mix Terminator kit (Applied Biosystems, Foster City, USA) and an automatic sequencer (ABI Prism 310 Genetic Analyzer; Applied Biosystems), using the same primers described above. Sequences were assembled and edited using the program Consed/PhredPhrap (Ewing & Green, 1998; Ewing, Hillier, Wendl, & Green, 1998; Gordon, Abajian, & Green, 1998; Gordon, Desmarais, & Green, 2001). Then, the sequences were aligned using Clustal W (Thompson, Higgins, & Gibson, 1994) as implemented in the BioEdit software (Hall, 1999). The COI sequences were translated for verification of stop codons that could indicate a pseudo gene.
2.3 Molecular phylogenetic analyses and time divergence estimates
Each dataset (mtDNA and nDNA) was analyzed separately and as a concatenated file. The software Sequence Matrix (Vaidya, Lohman, & Meier, 2011) was used to concatenate the sequences. For each dataset, two different methods of phylogenetic inference were used. The maximum likelihood (ML) analysis used the model of substitution selected by the program jModelTest 0.1.1 (Posada, 2008) using the corrected Akaike information criterion (AICc; Hurvich & Tsai, 1989) for each dataset. The models and specific partitions used in this analysis are described in Supporting information Table S2. The search for the best maximum likelihood tree was carried over using the software GARLI v0.95 (Zwickl, 2006). For each dataset, 1,000 replicates were run and bootstrap support was estimated using 1,000 replicates. Additionally, we evaluated the ML trees using the [SH]-aLRT test (Anisimova, Gil, Dufayard, Dessimoz, & Gascuel, 2011) as implemented in the PhyML software (Guindon et al., 2010). For every analysis, we used Bothriembryon dux (Pfeiffer, 1861) as the outgroup, due to the availability of the data for this closely related genus (Breure, Groenenberg, & Schilthuizen, 2010).
Time divergence estimates were calculated using BEAST v.2.4.7 (Bouckaert et al., 2014) using a relaxed clock log normal-distribution prior for rate variation and a Yule model for tree branching pattern. We calibrated the node splitting Plectostylus and the outgroup Bothriembryon with a normal with a mean of 31.4 Mya and a sigma parameter of 4 to describe the 95% confidence interval between 20.1 and 45.6 Mya, which is the divergence time estimation inferred by Breure and Romero (2012). We ran a Markov chain Monte Carlo (MCMC) chains for 200 million generations and assessed that the chain had proper mixing and convergence with Tracer v.1.6.0 (Rambaut, Suchard, Xie, & Drummond, 2014) and RWTY v.1.0.1 (Warren, Geneva, & Lanfear, 2017), confirming minimum effective sample size (ESS) of over 200 for every parameter. We discarded the first 20% of the chain run as burn in with LogCombiner v.2.4.2 and summarized a maximum clade credibility (MCC) tree keeping the median heights with TreeAnnotator v.2.4.2.
3 RESULTS
From the 184 specimens collected, 105 were genotyped for COI, while 141 were genotyped for ITS2; of these, 86 have sequences for both markers. For COI, a total of 609 bp were sequenced and 64 unique haplotypes were described. A total of 267 nucleotide sites were variable. For the ITS2 gene, a sequence of 719 bp was obtained and 16 haplotypes were described. A total of 69 nucleotide sites were variable. The sequences are available under access numbers MG738368–MG738472 and MG731243–MG731383. Supporting information Table S1 provides all individuals sequences with their respective sequence IDs. Due to the low variability found in the nuclear marker, we decided to use it only in the concatenated analysis. The concatenated data file used to generate the phylogenetic trees is available on TreeBASE.
3.1 Phylogenetic analysis
The ML tree can be seen in Figure 2 for the concatenated dataset. The differences between the concatenated tree and the mtDNA tree for ML are minimal and regard the arid clade. The concatenated topology presents much better bootstrap values and SH-aLRT support. Because the concatenated dataset shows identical topologies for ML and much better bootstrap value support, we only ran the concatenated dataset for Bayesian inference (BI). The results for BI were identical to the ML analysis and are presented here. We will use the concatenated tree for all the discussion.
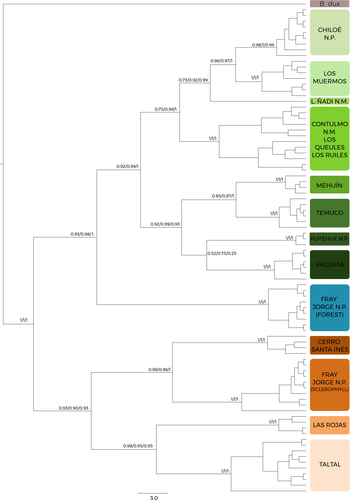
As shown in Figure 2, the Plectostylus species complex is divided into two main clades that correspond not only to two distinct geographical areas but also to very distinct climates. The first one is formed by populations that occur in arid and semiarid areas of northern Chile. The second is formed by populations that inhabit the forest areas of central and southern Chile, and also by the animas from the FJ relict, in the semiarid region. The northern group is composed by four populations: Taltal, Las Rojas, Parque Nacional Fray Jorge xerophyte and Cerro Santa Inés. The structure in this clade has a strong geographical component: Taltal and Las Rojas form a northern clade, while Parque Nacional Fray Jorge xerophyte and Cerro Santa Inés form a southern clade. This group is sister to the forested areas clade that includes FJ (Figure 2). The clades described here do not bear any resemblance to the species described by Valdovinos and Stuardo (1988).
3.2 Time divergence estimates
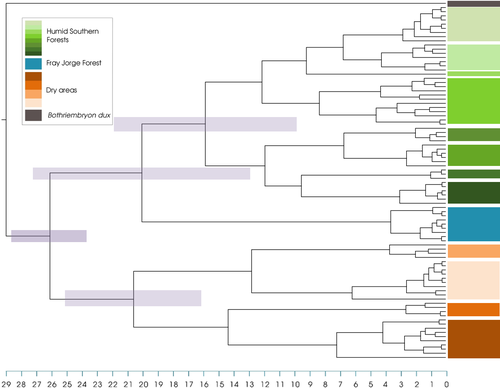
The time divergence estimates for the four most important nodes in the tree—most recent common ancestor (MRCA) of each of the two main clades, the MRCA for Plectostylus and FJ—can be seen in Figure 3 and in Table 2. The complete list of divergence times can be found in Supporting information Table S3.
| Node | Estimate (Mya) | 95% confidence interval (Mya) |
|---|---|---|
| MRCA to Plectostylus | 26.8463 | 30.67–22.82 |
| MRCA to arid clades | 21.1658 | 12.8–29.31 |
| MRCA to forest clades + FJ | 20.674 | 12.81–28.28 |
| MRCA to remaining forest clades | 16.3021 | 9.11–22.83 |
For the populations described for the desert and semi-desert areas, the date of the most recent common ancestor (MRCA) was calculated to be around 20.6 (12.81–28.28) Mya. In the case of the populations from the forests of central and southern Chile, the time for the MRCA was calculated to be 21 (12.8–29.31) Mya. Last, for the MRCA of the genus as a whole, our estimate is of 26 (30.67–22.82) Mya. All these dates are older than the Quaternary, therefore supporting the Paleogene/Neogene divergence hypothesis.
4 DISCUSSION
The isolation of the FJ, as well the MRCA of the remaining forest clades, was estimated to have occurred in the first half of the Miocene. This period is marked by a series of geological events that caused great climatic changes in the south of South America. The separation of South America and Antarctica was followed by the glaciation of Western Antarctica, which in turn was caused by the opening of the Drake Passage and the formation of the Antarctic Circumpolar Current (DeConto & Pollard, 2003). As a result of these events, the Humboldt Current was formed. At the same time, the uplift of the Andes would have raised the altitude of the Atacama Desert to approximately 2,750 m a.s.l. (Herold, You, Müller, & Seton, 2009). These events were the driving force behind a major climate change in South America. The uplift of the Andes changed the rain regime in the entire southern region of South America. The effect of blocking the rains of Amazonic origin (Houston & Hartley, 2003; Placzek et al., 2009) coupled with an intensification of the Humboldt Current and global cooling (Hartley, 2003) explain to great lengths the desertification of this region. Additionally, Sepulchre, Sloan, Snyder, and Fiechter (2009) stated that the uplift of the Andes directly influenced the Humboldt Current and the temperature of the surface of the ocean, reinforcing the desertification process. The hyper-aridity that characterizes the Atacama Desert today originated about 15 Mya (e.g., Alpers & Brimhall, 1988; Bissig & Riquelme, 2010) as a consequence of the changes described here. Under these conditions, the mixed forest that characterizes FJ would have been isolated in their current distribution, while the dry formations expanded. The whole process described above led ultimately to the creation which is now known as the dry diagonal of South America during the Pliocene, which in turn restricted the Miocene forests to the Pacific and Atlantic coasts of South America (Hinojosa & Villagrán, 1997).
The Forest clade has two main groups. The first is the clade formed by the animals from FJ relict. The second clade, sister to the FJ clade, is formed by all remaining forest populations that occur in central and southern Chile. Unlike the arid clade, in this group we do not observe a clear latitudinal distribution of the clades. This could be a consequence of the complex history that the Chilean forests have experienced in both the Paleogene/Neogene (Romero, 1978, 1986; Hinojosa & Villagrán, 1997) and the Quaternary (Villagrán, 2001; Villagrán et al., 2004). One important feature of the clades described here is that they do not match the currently recognized species for this genus in Chile. The species descriptions by Valdovinos and Stuardo (1988) depict wide latitudinal distributions for individual taxa. This goes against the literature on land snails that indicates that these animals tend to have a restricted distribution that is very commonly accompanied by very strict habitat requirements and low active dispersal capabilities that result in fragmented distributions (Pfenninger et al., 2003; Baur & Baur, 1993). Our phylogeny fits this description much better, with clades corresponding to geographically restricted populations. Therefore, one of the outcomes of this study is that the current taxonomy on the groups needs to be re-evaluated.
The extant distribution of the Orthalicoidea superfamily clearly shows a Gondwanan pattern (Herbert & Mitchell, 2009), with Plectostylus forming the subfamily Prestonellinae together with the genera Discoleus from Argentina, Bothriembryon from Australia and Prestonella from Southern Africa (Van Bruggen, Herbert, & Breure, 2016; see Breure, 1979 and Breure et al., 2010 for a historical perspective). The separation of the clades of our study organisms inhabiting arid and forest areas dates back to late Oligocene or early Miocene. According to fossil and paleopalynologic data, it was around this time that the mixed forests migrated north while being replaced by Antarctic forest on its original range (Romero, 1978, 1986; Gayó, Hinojosa, & Villagran, 2005). Since the two clades of land snails are sister taxa, it is not possible to infer if the ancestral of all the current species belonged to an arid or humid environment. Stratigraphic and sedimentological evidence shows that the contemporaneous Atacama Desert retained arid conditions for over 90 Mya (Hartley, Jolley, & Turner, 1992; Houston & Hartley, 2003). Closely related genera Discoleus and Bothriembryon inhabit both humid and arid environments in Argentina and Australia, respectively, making it difficult to extrapolate a possible ancestral state for Plectostylus. Therefore, it seems equally likely that the ancestral Plectostylus could have originated in a dry environment and colonized a humid one or vice versa.
The estimate made here for the MRCA of the arid clade puts this ancestor in the beginning of the Miocene. During this period, the paleofloras remained stable in comparison with the previous period, or at least did so in Chile. The most important event in this time frame is the final separation between Antarctica and South America, according to Hinojosa and Villagrán (1997), but this dating is disputed (Barker, 2001; Zhang, Yan, & Wang, 2010). As was the case with the Forest clade, the clades that form this group are geographically restricted and do not match the currently described species distribution. An in-depth taxonomic study will be necessary for this clade as well.
The data presented above describe the molecular phylogeny and timing of divergence for the genus Plectostylus, giving new insights into the historical dynamics of the landscapes in Chile and the origin of the FJ forest relict in northern Chile. According to our data, the animals in FJ belong to a clade that groups all of the Plectostylus that live in forest areas, instead of being closely related to the clade that represents the dry areas surrounding the relict. The time divergence estimates suggest that the isolation of the FJ forest relict has its origin in the early Miocene. This coincides with major geological events in South America and support the hypothesis created by Philippi (1884) and Schmithüsen (1956) of a Paleogene/Neogene origin for this relict. Finally, we would like to point that molecular genetic approaches for biogeographical and taxonomic issues concerning the evolution of Chilean biotas have been extremely underutilized; we believe that more effort should be made to elucidate the fascinating questions regarding the evolution in temperate areas of the South American continent.
ACKNOWLEDGEMENTS
We would like to thank Natalia Luchetti for help with figures, Fernando Carbayo for help in the field, Manuel Antunes Jr. for help in the laboratory, and Fernando P.L. Marques for the support during the collection and laboratory phases of the work. We also would like to thank Ian Brennan and Nick Matzke for reading earlier versions of this manuscript and Mercedes Okumura for the abstract in Spanish. This work became possible through grants FAPESP 2011/06298-6, PRODOC 0072/087 and CAPES 138705/2009-7.




