Geographic patterns of postzygotic isolation between two closely related widespread dung fly species (Sepsis cynipsea and Sepsis neocynipsea; Diptera: Sepsidae)
Abstract
Identifying the contribution of pre- and postzygotic barriers to gene flow is a key goal of speciation research. The widespread dung fly species Sepsis cynipsea and Sepsis neocynipsea offer great potential for studying the speciation process over a range of opportunities for gene exchange within and across sister species (cross-continental allopatry, continental parapatry and sympatry). We examined the role of postcopulatory isolating barriers by comparing female fecundity and egg-to-adult viability of F1 and F2 hybrids, as well as backcrosses of F1 hybrids with the parental species, via replicated crosses of sym-, para- and allopatric populations. Egg-to-adult viability was strongly but not totally suppressed in hybrids, and offspring production approached nil in the F2 generation (hybrid breakdown), indicating yet unspecified intrinsic incompatibilities. Viable F1 hybrid offspring showed almost absolute male (the heterogametic sex) sterility while females remained largely fertile, in accordance with Haldane's rule. Hybridization between the two species in European areas of sympatry (Swiss Alps) indicated only minor reinforcement based on fecundity traits. Crossing geographically isolated European and North American S. neocynipsea showed similar albeit weaker isolating barriers that are most easily explained by random genetic drift. We conclude that in this system with a biogeographic continuum of reproductive barriers, speciation is mediated primarily by genetic drift following dispersal of flies over a wide (allopatric) geographic range, with some role of natural or sexual selection in incidental or direct reinforcement of incompatibility mechanisms in areas of European sympatry. S(ubs)pecies status of continental S. neocynipsea appears warranted.
1 INTRODUCTION
Speciation of genetically diverged populations is driven by ecological, spatial or temporal niche differentiation that ultimately leads to reproductive incompatibilities by natural selection or genetic drift (Coyne & Orr, 1997, 2004; Dobzhansky, 1951; Mayr, 1942; Schluter, 2000, 2001). Heterospecific mating may then be inhibited through morphological, behavioural or olfactory differences leading to strong prezygotic barriers, as predicted by the biological species concept (Futuyma, 1986; Mayr, 1940, 1982). Yet, closely related species may show incomplete prezygotic reproductive isolation, forming hybrid progeny with reduced fitness, hybrid sterility or low viability compared to their parental species as a result of postzygotic isolation (Hood, Egan, & Feder, 2012; Reed & Markow, 2004; Wasserman & Koepfer, 1977). Due to genetic interactions between sex chromosomes, partially recessive alleles of hybrid progeny can particularly reduce fitness in the heterogametic sex according to Haldane's rule (Coyne, 1985; Haldane, 1922; Turelli & Orr, 1995). On the other hand, F1 hybrids can show hybrid vigour by masking deleterious recessive alleles, which subsequently may (or not) result in reduced fitness of F2 progeny. This implicates epistasis in the process of hybrid breakdown, resulting in formation of viable F1 hybrid offspring but no F2 hybrids (Burton, Ellison, & Harrison, 2006; Dobzhansky, 1936; Endler, 1977; Muller, 1942).
The closely related sister species Sepsis cynipsea Linnaeus 1758 and Sepsis neocynipsea Melander & Spuler 1917 (Diptera: Sepsidae) offer great opportunity to investigate evolutionary mechanisms underlying reproductive barriers in recently diverged species. These two species are continentally widespread and have partly overlapping ranges. Sepsis cynipsea is the most common sepsid in north-central Europe, whereas S. neocynipsea abounds in North America, where it essentially occupies the ecological niche S. cynipsea has in Europe. Interestingly, however, S. neocynipsea also occurs at high elevation sites in Europe (Pont & Meier, 2002), such as in the Swiss Alps, where there is potential for natural hybridisation. The two species exhibit little differentiation at the mitochondrial barcoding cytochrome c oxidase subunit 1 (COI) and cytochrome b (Cytb) genes (Su, Kutty, & Meier, 2008), but substantial heterospecific genetic differentiation based on microsatellites in Europe (pairwise FST = 0.16) as well as across continents (FST = 0.22), with conspecific differentiation between European and North American S. neocynipsea intriguingly being of similar magnitude (FST = 0.16), and differentiation within the three lineages being much lower (FST ≤ 0.03; Baur, J., Giesen, A., Schäfer, M. A., Rohner, P. T., & Blanckenhorn, W. U., unpublished). Moreover, while morphologically well defined, the two species, and the continental populations, show strong similarities but also some differences in morphology, behaviour and ecology (Pont & Meier, 2002; Puniamoorthy, Ismail, Tan, & Meier, 2009; Rohner, Blanckenhorn, & Puniamoorthy, 2016). As expected by theory (Coyne & Orr, 1989, 2004; Turelli & Moyle, 2007; Yukilevich, 2012), precopulatory barriers between the two species became evident in several behavioural traits such as male mating attempts, female shaking to fend off males, or copulation frequency, which turned out strongest in areas of coexistence in Switzerland and are thus at least partly driven by character displacement and reinforcement (Giesen, Schäfer, & Blanckenhorn, 2017). Our behavioural studies demonstrated that these two species hybridize at least in the laboratory, suggesting that prezygotic isolation, though strong, is not absolute. Postcopulatory barriers to gene flow should play an additional role in separating the gene pools of the two species, barriers yet unquantified.
We here took advantage of this exceptional natural distribution to investigate the speciation process over a spatial continuum of genetic isolation, here referred to as biogeographic cross-types: European sympatry, European parapatry and cross-continental allopatry. We studied several standard female fecundity traits in con- versus heterospecific parental crosses, F1 and F2 hybrids, as well as backcrosses of F1 hybrid offspring with the parental species: female age at first reproduction, egg and offspring production, and egg-to-adult viability. As crosses were performed bidirectionally, we obtained information on male versus female fertility. We hypothesized that female fecundity and egg-to-adult viability would be significantly lowered in heterospecific parental, hybrid and backcrosses relative to the conspecific parental crosses, due to intrinsic incompatibilities such as difficulties with sperm transfer, unsuccessful fertilization or decreased hybrid viability. We further expected lower fecundity and viability in backcrosses with a hybrid male and a parental female when compared to the converse, in accordance with Haldane's rule (Haldane, 1922): The heterogametic sex of hybrids is often sterile because sex chromosomes tend to be frequently involved in reproductive isolation, resulting in genetic incompatibilities between species (Coyne & Orr, 1989; Turelli & Moyle, 2007). To explore ongoing speciation processes at various phases, we compared fecundity, fertility and viability of intercontinental conspecific S. neocynipsea crosses with that of interspecific crosses in sym-, para- and allopatry, expecting evidence of reinforcement in terms of most strongly reduced fecundity in areas of sympatry in the Swiss Alps (as found for precopulatory behaviour by Giesen et al., 2017). Most generally, we expected the contribution of random genetic drift to con- and heterospecific differentiation in these widespread species to be greatest in (distant) allopatry, and that of natural selection (by way of reinforcement, purging of genetic incompatibilities, etc.) to be greatest in areas of sympatry.
2 MATERIALS AND METHODS
2.1 Study organism
Sepsis cynipsea is the most abundant sepsid in north-central Europe, where it occurs in sympatry with the rare S. neocynipsea in some mountainous regions such as the Swiss Alps. In North America, where S. cynipsea does not occur, S. neocynipsea occupies a similar warm-adapted temperature niche as S. cynipsea in Europe (Pont & Meier, 2002). The mating system of S. cynipsea has been described in detail (Blanckenhorn, Morf, Mühlhäuser, & Reusch, 1999; Blanckenhorn et al., 2002; Parker, 1972a,b; Puniamoorthy et al., 2009; Ward, 1983; Ward, Hemi, & Rösli, 1992), whereas relatively little is known about S. neocynipsea (Eberhard, 1999; Puniamoorthy et al., 2009; Rohner et al., 2016). More detailed descriptions of the maintenance of the flies in our laboratory and their behaviour can be found in Giesen et al. (2017).
2.2 Crossing scheme
We caught gravid females from six sites (i.e., populations) to establish 5–15 iso-female lines (i.e., offspring of a single field-caught female) per population in the laboratory. Sympatric populations were collected in Switzerland (Zurich, CH1: 47°24′0.60″N, 8°34′23.97″E, sampled in June 2013 (2013.6); and Sörenberg, CH2: 46°49′23.72″N, 8°1′54.59″E, in 2013.7), where both species co-occur. We further obtained S. cynipsea from two parapatric European areas (Ludwigshafen, Germany, EU1: 49°28′41.25″N, 8°22′21.65″E, in 2014.7; Stirling, Scotland, EU2: 56°6′59.47″N, -3°56′12.83″W, in 2013.5), where S. neocynipsea only has been observed in adjacent regions (Ozerov, 2005; Pont & Meier, 2002). The other S. neocynipsea were collected from two allopatric North American populations (Fort Hall, Idaho, NA1: 43°1′59.69″N, −112°26′17.91″W; Lamar Valley, Wyoming, NA2: 44°52′6.67″N, -110°10′28.72″W, both in 2013.6), where S. cynipsea does not exist.
Flies from iso-female lines, which at the time had been kept for at least half a year (ca. eight generations) in the laboratory, were used in conspecific parental crosses within each of the four populations per species as the baseline. They were further used in two replicate reciprocal population crosses of European and North American S. neocynipsea (conspecific allopatric cross-continental crosses). Moreover, reciprocal heterospecific parental crosses of three biogeographic types were set up with two population replicates each: European sympatry, European parapatry and cross-continental allopatry (Table 1). One population replicate consisted of 15–20 replicate crossings of iso-female lines, which were conducted over extended periods of time (weeks to months).
| Biogeographical type | Crosses | Population replicates |
|---|---|---|
| European sympatry | CH S. cynipsea × CH S. neocynipsea | (CH1) × (CH1) |
| (CH2) × (CH2) | ||
| European parapatry | EU S. cynipsea × CH S. neocynipsea | (EU1) × (CH1) |
| (EU2) × (CH2) | ||
| Cross-continental allopatry | ||
| Interspecific | EU S. cynipsea × NA S. neocynipsea | (CH1) × (NA1) |
| (CH2) × (NA2) | ||
| Intraspecific | EU S. neocynipsea × NA S. neocynipsea | (CH1) × (NA1) |
| (CH2) × (NA2) | ||
Note
- CH: Switzerland; EU: Europe; NA: North America.
Hybrid F1 and F2 flies for our assessments were generated by randomly combining up to 20 flies of each sex from various iso-female lines of a given population and species to be paired with so treated flies from the other species and sex, always reciprocally. Matings in these settings were thus necessarily heterospecific (no choice). Potentially lower replicate numbers of F1 and F2 hybrid offspring were expected due to natural difficulties in obtaining hybrids. For backcrosses, we targeted a sample size of six replicates per pairing, as we set up two reciprocal cross-types (female hybrid with male parental – F1xP, and female parental with male hybrid – PxF1) to detect possible sex-specific effects.
We obtained fecundity and egg-to-adult viability measurements for (a) con- and heterospecific parental crossings (P; N ≈ 18 per crossing replicate), (b) F1 hybrid crossings using the offspring resulting from heterospecific crossings (N ≈ 10 per crossing replicate), (c) F2 hybrid crossings (N ≈ 6 per crossing replicate) and (d) backcrosses of F1 hybrid offspring with the parental species (BC; N ≈ 6, per crossing replicate). All crosses were done reciprocally, randomized over extended periods of time.
2.3 Fecundity and egg-to-adult viability assessment
One cross consisted of one female and two males (either con- or heterospecific). This grouping should simulate natural conditions, increasing hybridization probability according to Puniamoorthy (2014) and limiting the probability of total failure due to male sterility. Virgin flies were assigned randomly after adult eclosion to a round 50-ml glass vial containing fresh cow dung as oviposition substrate in a plastic rectangular dish (42 (L) × 21 (W) × 16 (H) mm2) plus some grains of sugar. We scored (a) female adult age when she first laid eggs into the dung [in days], (b) her first clutch size, (c) the number of emerged offspring, plus (d) female and (e) male mating partner head widths as measures of their body size (Blanckenhorn, Reusch, & Mühlhäuser, 1998; Rohner et al., 2016). From these observations, we further derived (f) the probability that females of a given cross-produced eggs (p(eggs)) and (g) offspring (p(offspring)) [both binary: Y/N]. Lastly, we calculated (h) egg-to-adult viability as the proportion of offspring emerging from a clutch. Note that given our set-up with lots of heterospecific crosses, we expected low overall numbers of fertile eggs and offspring numbers.
2.4 Statistical analyses
All traits were analysed separately, with female head width as covariate because body size typically affects fecundity measures (Honek, 1993), in SPSS Statistics version 23 using univariate generalized linear models, with binomial errors when outcome variables were binary or proportional (only significant covariates are reported in the Results). A given trait was analysed as a function of species (pure C: S. cynipsea, pure N: S. neocynipsea; hybrid CN or NC; or, analogously, NEU, NUSA, hybrids NEUNUSA and NUSANEU – females always named first), biogeographic type nested within species (sympatric versus parapatric versus allopatric), all as fixed factors, and population nested within biogeographic type and species as random effect. Additionally planned baseline comparisons were performed to compare the two parental species (C versus N), the continental S. neocynipsea populations (NEU versus NUSA), and the direction of heterospecific mating (CN versus NC; NEUNUSA versus NUSANEU). F1, F2 hybrids and backcrosses were analysed analogously. Lastly, we compared the (F1 or F2) hybrid offspring with their parental conspecific crosses.
3 RESULTS
3.1 Baseline comparison between S. cynipsea and S. neocynipsea
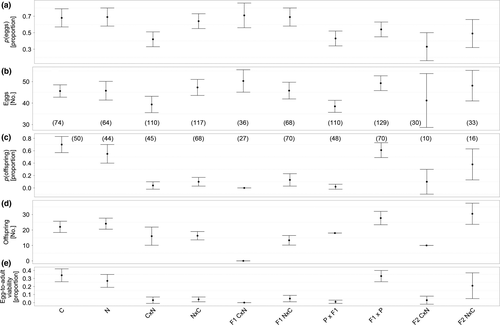
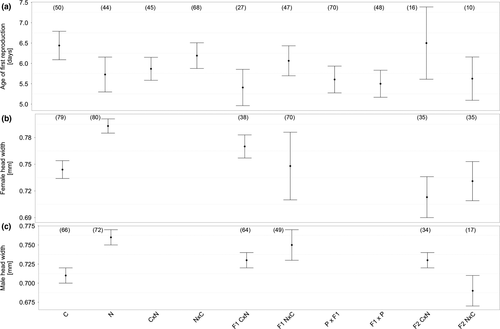
Most fecundity traits did not differ between the two species (Figure 1; Supporting Information Table S1). Mean egg-to-adult offspring viability was ~30% (χ2(1, 6) = 1.41, p = 0.235, Figure 1e). Females laid their first eggs after ca. 6 days (χ2(1, 6) = 2.04, p = 0.154, Figure 2a), with a mean clutch size of ~45 eggs (χ2(1, 6) = 1.34, p = 0.247, Figure 1b). In total, ca. 68% of all females laid eggs (p(eggs): χ2(1, 6) = 0.04, p = 0.846, Figure 1a). Offspring number was around ~22 individuals (χ2(1, 6) = 0.37, p = 0.542, Figure 1d). The only difference between the species was evident in the probability of producing offspring, which was higher for S. cynipsea (p(offspring) = 0.70 ± 0.13) than for S. neocynipsea (0.54 ± 0.15, χ2(1, 6) = 3.87, p = 0.049, Figure 1c), despite the latter species being larger (neocynipsea females: 0.79 ± 0.01 mm, males: 0.76 ± 0.01 mm; cynipsea females: 0.74 ± 0.01, males: 0.71 ± 0.01; χ2(1, 6) > 7.77, p < 0.001, Figure 2b,c).
3.2 Baseline comparison between continental S. neocynipsea
Differences between European and North American S. neocynipsea were only found in the female's age of first reproduction (χ2(1, 2) = 26.51, p < 0.001), with North American S. neocynipsea females (6.55 d ± 0.69) being older when first laying a clutch than those from Europe (4.91 d ± 0.18), even though females were of the same size (0.79 ± 0.01, χ2(1, 2) = 0.00, p = 0.999). All other traits were similar: both laid around ~45 eggs per clutch (χ2(1, 2) = 0.82, p = 0.365, Figure 3b), with p(eggs) around ~68% (χ2(1, 2) = 0.21, p = 0.646, Figure 3a). From these clutches ~23 offspring emerged (χ2(1, 2) = 0.15, p = 0.702, Figure 3d), p(offspring) being ~55% (χ2(1, 2) = 0.60, p = 0.440, Figure 3c). The egg-to-adult viability found on both continents was ~26% (χ2(1, 2) = 1.10, p = 0.295, Figure 3e).
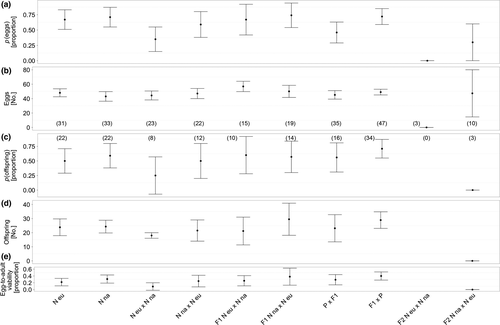
3.3 Intercontinental S. neocynipsea crosses over three generations
Although no differences in crossing direction across continents were detectable for S. neocynipsea, p(eggs) was higher when females from North America and males from Europe were crossed (~59%) relative to the reciprocal cross (~35%, χ2(1, 2) = 3.13, p = 0.007, Figure 3a). In the intercrossed F1 generation, only female age at first reproduction differed significantly in crossing direction, showing a difference of 1 day (χ2(1, 2) = 8.33, p = 0.004). Intercontinental F2 hybrids showed an asymmetry in hybridization direction with crosses of European females and North American males resulting in no eggs and no offspring whatsoever, although the overall sample size was very low (N = 3, Figure 3e). Backcrosses showed no significant differences across all measured traits (Supporting Information Table S1; Figure 3).
3.4 Fitness of heterospecific crosses and their hybrid offspring
In parental heterospecific crosses, the probability of producing eggs was affected by hybridisation direction (CN versus NC), with CN crosses (0.41 ± 0.09) having lower p(eggs) than NC crosses (0.64 ± 0.09, χ2(1, 6) = 8.05, p = 0.005; Figure 1a). Only this trait was also affected by biogeographic type (χ2(4, 6) = 32.96, p < 0.001), with sympatric crosses producing fewest eggs (p(eggs) ~27%), while parapatric (~65%) and allopatric (~70%) crosses produced many more. At the same time, p(eggs) marginally differed between con- (~68%) and heterospecific (~53%) parental crosses (χ2(1, 19) = 2.93, p = 0.087; Figure 1a). The same pairwise comparison revealed expectedly higher egg-to-adult viability in conspecific (~30%) than heterospecific (~4%) crosses (χ2(1, 14) = 50.70, p < 0.001, Figure 1e), as well as corresponding differences in the probability of producing offspring (conspecific ~63%; heterospecific ~8%; χ2(1, 15) = 10.15, p < 0.001; Figure 1c; Supporting Information Table S1).
Crossing of F1 hybrid offspring produced F2 hybrid offspring only in the NC direction, with CN crosses generating no offspring in most crosses (χ2(1, 3) = 5.10, p < 0.001, Figure 1d), and with corresponding differences in the probability of producing offspring (Figure 1c) and egg-to-adult viability (Figure 1e). Consequently, egg-to-adult viability from F1 hybrid crosses was strongly reduced relative to the conspecific parental crosses (χ2(1, 10) = 30.64, p < 0.001, Figure 1e). F1 hybrid females and males resulting from heterospecific CN and NC crosses differed in size, yielding a significant effect of crossing direction (females: χ2(1, 4) = 4.91, p = 0.027, Figure 2b; males: χ2(1, 4) = 6.56, p = 0.010, Figure 2c). Interestingly, F2 hybrids of both sexes were significantly smaller than the parental females (females: χ2(1, 6) = 60.11, p < 0.001, Figure 2b; males: χ2(1, 6) = 33.87, p < 0.001, Figure 2c).
We found the same hybridisation asymmetry in the F2 hybrid generation. Adults emerged only from one of 10 clutches in the CN direction, while p(offspring) ~38% resulted from NC crossings (no statistics calculable due to too many missing values; Figure 1c). This similarly affected offspring number (χ2(1, 2) = 7.58, p = 0.006, Figure 1d) and egg-to-adult viability (no statistics calculable; Figure 1e). Compared to the conspecific crosses (~30%), F2 hybrid viability was significantly decreased to ~10% (χ2(1, 6) = 4.99, p = 0.025, Figure 1e).
3.5 Haldane's rule in backcrosses of F1 hybrids with the parental species
To test for sex-specific deleterious hybridization effects, we reciprocally crossed F1 hybrids with the parental species. Egg-to-adult viability was reduced when hybrid males mated with parental females, but not when hybrid females were mated with parental males (χ2(1, 12) = 67.37, p < 0.001, Figure 1e); egg (χ2(1, 12) = 16.57, p < 0.001, Figure 1b), and offspring numbers including zeroes (χ2(1, 12) = 54.30, p < 0.001, Figure 1d) were similarly decreased for the former cross.
4 DISCUSSION
The traditional biological species concept only allows for interbreeding within species, while gene flow across species should be prevented by reproductive isolating mechanisms (Dobzhansky, 1970; Futuyma, 1986; Mayr, 1940, 1982; Simpson, 1961). Nevertheless, extensive speciation research since revealed that hybridisation between species is not uncommon (Anderson, 1949; Barton & Bengtsson, 1986; DeMarais, Dowling, Douglas, Minckley, & Marsh, 1992; Mallet, 2007; Nolte & Tautz, 2010; Rieseberg et al., 2003; Trier, Hermansen, Sætre, & Bailey, 2014). We here provided a thoroughly replicated, hierarchically subdivided case study of reproductive barriers in a pair of very closely related sepsid flies, by comparing three lineages (i.e., species and continental populations) at a continuum of stages in the speciation process (European sympatry, European parapatry, and cross-continental allopatry). The sister species Sepsis cynipsea and S. neocynipsea differ in their behaviour and morphology (Giesen et al., 2017; Pont & Meier, 2002), with minor genetic differentiation evident at the mitochondrial COI and Cytb gene sequences (Su et al., 2008). North American and European S. neocynipsea, by contrast, are traditionally regarded as the same species based on great similarities in morphology and behaviour, although sexual size dimorphism is reversed on the two continents (Rohner et al., 2016) and microsatellite nuclear markers indicate substantial continental differences in population genetic structure (FST = 0.16; see Introduction). We therefore expected clear postcopulatory isolating barriers between the species reinforced by natural selection in areas of European sympatry, but no to few such barriers between geographically isolated European and North American S. neocynipsea populations. Instead we found similar, that is, quantitatively but not qualitatively differing barriers between the three lineages, which are most easily explained by random genetic drift with only little reinforcement.
Forced interbreeding under laboratory conditions between S. cynipsea and S. neocynipsea here resulted in successful hybridization, however with the typically expected (op. cit.) strong fitness reductions due to hybrid breakdown in terms of fecundity, fertility and egg-to-adult survival, revealing clear genetic incompatibilities. Disruption was evident predominantly in one of the heterospecific hybridization directions (CN), with male F1 hybrids exhibiting sterility while female F1 hybrids continued to lay eggs with only somewhat reduced fertility, in accordance with Haldane's rule (Haldane, 1922). Interestingly, yet, our study also revealed similar “hybrid” fitness decrements when crossing continental (European versus North American) S. neocynipsea populations, which presumably have differentiated without gene exchange over long periods of evolutionary time, although more viable and fertile F1 offspring, but not necessarily F2 hybrid offspring, resulted from these intraspecific crosses. Comparing biogeographic cross-types over a range of opportunities for gene exchange (cross-continental allopatry, continental parapatry, sympatry) within and across sister species, with two population replicates each (Table 1), thus documents a continuum of reproductive barriers lacking clear qualitative “species” distinctions. Contrary to the precopulatory behavioural traits investigated by Giesen et al. (2017), we here obtained only little evidence for reinforcement by natural selection in sympatric areas of the two species in the Swiss Alps (in egg production). Instead, generally strong reproductive barriers between the two species in para- and allopatric situations were evident, with similar barriers between the isolated continental populations of the same species (S. neocynipsea). These incompatibilities may have evolved by genetic drift (the null hypothesis), but alternatively also as incidental by-products of (ecological) selection on other, unmeasured traits. In either case, we here uncovered incipient speciation in this fly system, effected by a spatiotemporal interplay of drift and selection (likely the most common mode of evolution), that probably justifies (sub)species status of continental S. neocynipsea at this point (cf. Ozerov, 2005; Pont & Meier, 2002).
4.1 Baseline comparison of the two species
Our study revealed minor differences in fecundity and fertility traits between S. cynipsea and S. neocynipsea, as could be expected due to their ecological similarities and their relatively low heterospecific genetic differentiation (Pont & Meier, 2002; Su et al., 2008). However, the species exhibit significant differences in body size, S. neocynipsea being larger than S. cynipsea, in agreement with a previous study (Rohner et al., 2016). All other fecundity and fertility traits showed few differences, especially when controlling for the body size differences, which contrasts with the more pronounced but nevertheless merely quantitative (rather than qualitative) differences in mating behaviour between the two species (Giesen et al., 2017). Roughly two-thirds of all females of both species laid a first clutch containing ~45 eggs after ~6 days. Thus, a considerable proportion of females was not able to produce any eggs and therefore might be sterile, again in accordance with previous studies of S. cynipsea (Blanckenhorn et al., 2002; Teuschl & Blanckenhorn, 2007). This might well be a consequence of our protracted laboratory breeding, as freshly caught field females are typically more fertile (~80%–90%; P.T. Rohner, personal communication). Egg-to-adult survival was also low (~30%), again possibly explained by our samples stemming from long-term laboratory iso-female lines. Despite some likely effects of inbreeding across all populations held in the laboratory for multiple generations, this low baseline fertility of our iso-female lines ultimately did not impede our ability to detect differences between con- and heterospecific crosses in the fecundity traits investigated, our main goal here, as these were substantial.
4.2 Heterospecific crosses resulting in F1 hybrid offspring
As expected, fertility of heterospecific crosses was strongly reduced to roughly 3.5% in both hybridization directions (CN versus NC), documenting clear mating and fertilization incompatibilities between the two species (Turelli, Barton, & Coyne, 2001). This reduction in F1 viability and fecundity is a typical hybridization pattern evident in several taxa (see Dowling & Secor, 1997; Fitzpatrick, Fordyce, & Gavrilets, 2009) and confirms our expectation of intrinsic postcopulatory incompatibilities between the species effected by processes such as disrupted sperm transfer (Arthur & Dyer, 2015), cryptic female discrimination of heterospecific sperm (Eberhard, 1991) or high mortality of hybrid offspring following zygote formation (Dowling & Secor, 1997; Mayr, 1942). Despite low fertility, egg numbers were similar in heterospecific and conspecific crosses, although the likelihood of producing eggs was significantly lowered for the CN (41%) relative to the NC hybridization direction (64%) and the conspecific crosses (68%). This suggests disruption of egg production by heterospecific males due to postmating, prezygotic barriers, for instance via incompatibilities between sperm and egg surface proteins preventing fertilization (Palumbi, 1999), toxicity of S. neocynipsea sperm for S. cynipsea eggs (Rice, 1996) or male failure to induce proper female ovulation (Sirot, Wong, Chapman, & Wolfner, 2015), all potentially reflecting sexual conflict. Moreover, egg production in the CN direction approached zero in sympatric relative to para- and allopatric crosses. This again supports our conjecture that heterospecific sperm possibly disrupts fertilization, which in sympatry might be reinforced by natural selection due to the continuous contact of the two species (Coyne & Orr, 1989, 2004; Turelli et al., 2001; Yukilevich, 2012), which we also found for some behavioural traits (Giesen et al., 2017).
4.3 Reduced hybrid fitness across generations with F2 hybrid breakdown
Similar to the results of the heterospecific crosses, intercrossed F1 and F2 hybrids had very low egg-to-adult viability. However, at least egg numbers of F1 hybrid intercrosses were not reduced but in fact somewhat higher than those of the con- and heterospecific crosses (Figures 1b and 3b), suggesting hybrid vigour (Todesco et al., 2016; Wolf, Takebayasi, & Rieseberg, 2001). Hybrid vigour admittedly can be expected when comparing conspecific crosses based on potentially inbred iso-female line populations with the resulting heterospecific crosses. Nevertheless, egg and offspring production was significantly reduced in intercrosses of F2 hybrids, demonstrating strong hybrid breakdown. Furthermore, emergence of F2 and F3 hybrid offspring approached zero, re-emphasizing fertilization difficulties similar to the heterospecific crosses (Dowling & Secor, 1997; Palumbi, 1999; Rice, 1996; Turelli et al., 2001). This again appears to document a reinforcement pattern, as sympatric crosses produced no F3 offspring at all, while some offspring resulted in parapatric crosses. Yet, no F3 offspring were produced in allopatric crosses either, and the overall sample sizes were low at this stage due to intrinsic incompatibilities between the species.
More interestingly, our data demonstrate an asymmetry in hybridization direction (CN versus NC) affecting offspring number and hence fertility of the F1 generation. If the mating partners stemmed from crosses of S. cynipsea females with S. neocynipsea males, they were not able to produce any adult F2 offspring, similar to the asymmetry in egg production of the parental generation. As the less abundant species is more likely to mate heterospecifically (Matute, 2014), S. neocynipsea females have a better chance to mate and produce fertile F1 hybrid offspring with S. cynipsea males than vice versa (Matsuda, Ng, Doi, Kopp, & Tobari, 2009; Sawamura, Sato, Lee, Kamimura, & Matsuda, 2016). This asymmetry was still evident in intercrosses of the F2 hybrids and explains our low sample sizes for the later hybrid generations, as it was very hard to obtain F2 and F3 hybrid offspring.
4.4 Haldane's rule—male sterility in F1 hybrid offspring
Our study highlights a breakdown of hybrid offspring production between S. cynipsea and S. neocynipsea in terms of decreased egg production and egg-to-adult viability (Figure 1), signifying strong but not absolute suppression of hybridization. In addition, hybrids performed differently depending on sex as predicted by Haldane's rule, according to which the heterogametic sex, here the male, is mostly sterile (Haldane, 1922). This was already visible in the first hybrid generation, but also in the backcrosses of F1 hybrid offspring with the parental species. Egg-to-adult viability, as well as egg and offspring numbers, revealed a certain degree of sterility when hybrid males were forced to mate with S. cynipsea or S. neocynipsea females, whereas female hybrids showed little to no such effects in the converse situation, clearly demonstrating hybrid female fertility (Figure 1b). Our results thus agree well with Haldane's rule and Darwin's corollary thereof as already documented in several other taxa (Dowling & Secor, 1997; Turelli & Moyle, 2007). However, while fitness of the heterogametic sex is almost entirely suppressed via genetic incompatibilities between hybrids and parental species, the homogametic sex sometimes also shows lowered fitness (e.g., in flycatchers: Alatalo, Eriksson, Gustafsson, & Lundberg, 1990). In this bird example, natural selection acted mainly via sterility of the heterogametic sex (in this case females), while reduced fitness of the homogametic sex (the males) was driven by sexual selection (Qvarnström, Alund, McFarlane, & Sirkiä, 2016). We can conclude that natural selection, either by directly acting on compatibility mechanisms or indirectly on other traits, is the main force driving selection on hybrids via reduced survival, with sexual selection as an additional precopulatory force as demonstrated by Giesen et al. (2017). A next step will be determining the extent of hybridization and its underlying evolutionary forces in nature using genomic tools.
4.5 Comparing the biogeographic cross-types
Strongest reproductive isolation is typically expected in sympatric species pairs following reinforcement and/or some sort of purging of genetic incompatibilities due to natural selection (Coyne & Orr, 2004; Turelli & Moyle, 2007; Via, 2001, 2009). Contrary to a number of precopulatory behavioural traits (Giesen et al., 2017), we obtained only weak evidence for such reinforcement in sympatric areas (the Swiss Alps) for the (postcopulatory) fecundity traits assessed here (egg production only). It thus appears that precopulatory behavioural traits, which are inherently plastic and can potentially evolve fast, are of overriding importance in preventing hybridisation in nature in this and other systems (Eberhard, 1996; Puniamoorthy, 2014; Puniamoorthy et al., 2009; Beysard, Krebs-Wheaton, & Heckel, 2015; Schmidt & Pfennig, 2016).
4.6 Cross-continental S. neocynipsea hybridization
We here also explored differences between continental S. neocynipsea populations featuring some morphological differences (Rohner et al., 2016). Although European and North American populations are not in contact in nature due to their allopatric distribution, they are recognized as the same species based on similar morphology and behaviour (Giesen et al., 2017; Pont & Meier, 2002). However, population genetic differentiation between the continents is substantial (op. cit.), indicating that allopatric speciation is ongoing, mediated by genetic drift and/or adaptation to new habitats, as we found substantial evidence for hybridization barriers (Figure 3) similar to those between the two species (Figure 1). North American S. neocynipsea required more time than European flies to lay their first clutch. This might merely reflect ecological or geographic differences, as body size was controlled for. Egg production and egg-to-adult viability were lower in crosses of North American females with European males (Figure 3). Therefore, sperm from cross-continental males also appears to disturb fertilization in some way (Arthur & Dyer, 2015), and/or incompatibilities are evolving, at least in one direction, perhaps as by-products of other ecological adaptations (Turelli & Moyle, 2007). Intercrossing of F2 offspring showed signs of hybrid breakdown too, but this was based on only very few replicates.
However, in contrast to the heterospecific situation in Figure 1, backcrosses between hybrid offspring and parental continental S. neocynipsea populations showed few fitness reductions in terms of egg-to-adult viability, such that both hybrid sexes appear to be equally reproductively fertile; genetic incompatibilities according to Haldane's rule were not detected at this level either (Figure 3). Overall, our cross-continental S. neocynipsea crosses indicate some degree of genetic differentiation similar to that between the two species (Figure 1), with associated incompatibilities and fitness decrements following hybridization. These incompatibilities are presumably due to genetic drift alone but potentially may also involve direct or indirect natural or sexual selection (Rohner et al., 2016), as flies from both continents still recognize each other as the same species behaviourally (Giesen et al., 2017).
5 CONCLUSIONS
To conclude, our laboratory study revealed strong but not ultimate postzygotic barriers between the closely related sister species S. cynipsea and S. neocynipsea, probably involving natural and/or sexual selection (Giesen et al., 2017; Via, 2009), with strong indications of similar isolating barriers developing among continental European and North American (i.e., allopatric) S. neocynipsea populations, possibly solely due to random drift. We uncovered strong hybrid breakdown in the F1, F2 and F3 generations across several fecundity and fertility traits revealing difficulties to form hybrid offspring, potentially due to barriers in sperm transfer, difficulties with zygote formation and/or other physiological or developmental problems. We further found hybridization asymmetry with viable female fertility and hybrid offspring but hybrid male (the heterogametic sex) sterility, in accordance with Haldane's rule. Hybridization between the two species in European areas of sympatry (here the Swiss Alps) is prevented to a minor extent primarily at the precopulatory level by reinforcement, sexual or natural selection (Giesen et al., 2017), whereas the lower fitness of naturally not occurring continental (allopatric) S. neocynipsea hybrids is presumably best explained by random processes (i.e., genetic drift). Both neutral and selective mechanisms thus can lead to the same outcome, a synthetic view ultimately driving speciation in this and probably many other systems (Coyne & Orr, 1997, 2004; Via, 2009). Whether there are genomic signs of hybridization in natural populations remains to be investigated.
ACKNOWLEDGEMENTS
We thank Manuela Bizzozzero, Martina Ramel and Christina Ebneter for help with collecting the data. Thanks also to the dung fly group at the University of Zurich for their support and help in maintaining fly cultures, and Patrick Rohner, Nalini Puniamoorthy, Anders Kjaersgaard and Cait Dmitriew for collecting samples. The University Research Priority Program (URPP) “Evolution in Action” of the University of Zurich, the Swiss National Science Foundation and the Georges and Antoine Claraz-Donation funded this research.
AUTHOR CONTRIBUTIONS
AG, MAS and WUB conceived the study, and all contributed to the statistical analysis and the writing of the manuscript. AG largely performed the experiments as part of her PhD, with some help from undergraduate students.




