Life cycle, morphology of developmental stages of Metorchis ussuriensis sp. nov. (Trematoda: Opisthorchiidae), and phylogenetic relationships with other opisthorchiids
Abstract
The complete life cycle and developmental stages of the fluke, Metorchis ussuriensis sp. nov. (Trematoda, Platyhelminthes), are herein described. The results of the present experiments showed that, for flukes from the Primorsky Region in the Russian southern Far East, the first intermediate hosts are the snails Parafossarulus spiridonovi and Boreoelona ussuriensis, and the second intermediate hosts are freshwater fish, tadpoles, and snails. The definitive host in this experiment was Anas platyrhynchos dom. Morphometric parameters of M. ussuriensis sp. nov. demonstrate similarities with Metorchis taiwanensis, but the two species differ in the sizes of their bodies, sizes of suckers of adult worms, and sizes of cercariae, as well as respective positions of the finfold in cercariae. Phylogenetic reconstructions and genetic distances using the cox1 gene sequences support the conclusion that M. ussuriensis sp. nov. is well distinguished from all other species of the genus Metorchis, while sequences of internal transcribed spacers (ITS1 and ITS2) failed to separate M. ussuriensis sp. nov., Metorchis bilis, and Metorchis xanthosomus. In addition, we sequenced 1,402 bp of the 28SrRNA gene of M. ussuriensis sp. nov. being the first 28S sequences in the genus Metorchis. Comparison to other trematodes suggests that 28SrRNA could proof suitable for the differentiation of trematode species.
1 INTRODUCTION
Representatives of the genus Metorchis Looss, 1899, have been discovered in both the eastern and western hemispheres. Most Metorchis species parasitize the bile ducts of birds and mammals. Among these species, Metorchis bilis (Braun, 1890), Metorchis conjunctus (Cobbold, 1860), and Metorchis orientalis Tanabe, 1921, were detected in humans in Eurasia, North America, and East Asia, respectively (Behr, Gyorkos, Kokoskin, Ward, & MacLean, 1998; Cheng et al., 2005; Lin et al., 2001; Mordvinov, Yurlova, Ogorodova, & Katokhin, 2012). Other species of this genus can also be expected to be potential human parasites, since the majority of them, such as the three species mentioned above, are parasites of warm-blooded vertebrates. In the East Asian region, five species are found: M. orientalis, Metorchis taiwanensis Morishita, 1929, M. elegans Belogurov & Leonov, 1963, M. butoridi Oschmarin, 1963, and Metorchis kimbangensis Ha, 2005 (Belogurov & Leonov, 1963; Chen & Tang, 1981; Ha, 2005; Oshmarin, 1963). The latter was obtained from the bile ducts of the fish, Parasilurus asotus (Linnaeus, 1758), in Vietnam (Ha, 2005). There have been reports of M. pinquinicola Skrjabin, 1913, and Metorchis xanthosomus Creplin, 1846 from the Russian southern Far East (Alekseev, 1970; Oshmarin, 1963); however, this information has not yet been confirmed and a description of these worms is missing. The life cycles, with descriptions of developmental stages, have been studied for M. conjunctus, Metorchis intermedius Heinemann, 1937, and M. taiwanensis (Cameron, 1934 cited in Skrjabin & Petrov, 1950; Heineman, 1937; Vishkvartseva, 1969; Yang & Lai, 1997). It has been established that the first intermediate hosts for these species are snails from the family Bithyniidae (Amnicola limosa porata Say, 1817, Bithynia tentaculata (Linnaeus, 1758), and B. fuchsianus Moellendorff, 1894, respectively), the second intermediate hosts are freshwater fish or amphibians for M. taiwanensis, and the definitive hosts are birds and mammals. There have also been reports that Bithynia snails are first intermediate hosts, fish are the second intermediate hosts, and birds and mammals are the definitive hosts in the circulation of M. bilis and M. xanthosomus (Creplin, 1846), but these findings have not been experimentally confirmed (Filimonova, 1995, 1998; Razmashkin, 1978; Sitko et al., 2016). For M. orientalis, M. bilis, and M. xanthosomus, genetic data have confirmed the validity of these species. Moreover, according to genetic data, worms previously obtained from Europe, and identified as Metorchis albidus (Braun, 1893) and M. crassiusculus (Rudolphi, 1809), are synonymous with M. bilis (Sherrard-Smith et al., 2016; Sitko et al., 2016). For the rest of the Metorchis species, only morphological data are available, and the species status remains unresolved. Unfortunately, for this genus, a limited number of molecular markers have been used to determine the species status: internal transcribed spacers and 18S rRNA gene (18S) of nuclear DNA and cox1 and nad1 genes of mitochondrial DNA (Ai et al., 2010; Kang et al., 2008; Sherrard-Smith et al., 2016; Sitko et al., 2016).
During a parasitological investigation of the freshwater snails Parafossarulus and Boreoelona (Bithyniidae) in the Primorsky Region (Russia), we obtained cercariae belonging to the Pleurolophocerca group, which are typical for representatives of Opisthorchioidea Looss, 1899. Further investigations of the life cycle, morphology of developmental stages, and genetic data (ITS2 region, 28S rRNA, and cox1 mtDNA) showed that this trematode is a new Metorchis species. Results of these investigations are presented below.
2 MATERIALS AND METHODS
In this study, 10 adult worms, five rediae, 15 cercariae, and 10 metacercariae of M. ussuriensis sp. nov. were used for morphological analysis. Holotype No 104–Tr and paratypes No 105–113–Tr are held in the parasitological collection of the Zoological Museum (Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far East Branch of the Russian Academy of Sciences, Vladivostok, Russia); e-mail: [email protected]. Deposited 2016.11.20. 32, six and 22 individuals were analyzed using nucleotide sequences of ITS1-5.8S-ITS2 (ITS), 28S rRNA gene (28S), and cox1 gene, respectively (Table S1). The data were not published earlier.
2.1 Morphological analysis
The study was carried out using the freshwater snails, Parafossarulus spiridonovi Zatravkin, Dovgalev et Starobogatov, 1989, and Boreoelona ussuriensis Ehrmann, 1927, from Magdikovoe lake in the middle reaches of the Bolshaya Ussurka River, Primorsky Region, Russia, which emitted the cercariae from the Pleurolophocerca group. To establish potential second intermediate hosts of this trematoda, experiments on infection by cercariae detected in the snails were conducted with the following animals: the snails B. ussuriensis, tadpoles of the frog Rana dybowskii (Guenther, 1876), and the fish Perccottus glenii Dybowski, 1877, and Rhynchocypris percnurus mantschuricus (Berg, 1907). Boreoelona snails were grown in laboratory conditions from clutches, while the tadpoles and young fish were caught from artificial pond, where these animals were free from any metacercariae (the presence of metacercariae was examined on 30 specimens of fish and tadpoles). Two experiments were conducted to identify the second intermediate hosts. The animals were infected with Pleurolophocerca from one Parafossarulus snail in one experiment and from one Boreoelona snail in another. For each of the two experiments, 30 snails, 18 tadpoles, and eight fish were used. For the period of infection, the animals were placed separately in 0.5 L vessels (snails) or 1.5 L vessels (tadpoles and fish). For 3 days, the water from Petri dishes containing snails, emitting Pleurolophocerca, was poured into the vessels with animals. After the infection period, the animals were kept in separate aquaria at a water temperature of 18–22°C. To determine whether the animals were infected by metacercariae, one sample of snails, tadpoles, and fish from both experiments was dissected on days 10 and 21 after infection. On day 30 after infection, 10 snails, four tadpoles, and two fish from each experiment were examined to determine infection intensity. The remaining animals, in equal numbers from each experiment, were fed, in both cases, to two rats and four ducklings. In addition, 50 metacercariae from each experiment were fed, in both cases, to two chickens using a pipette. On day 8 postinfection, one rat, one chicken, and two ducklings from each experiment were dissected. No trematodes were found in rats or chickens. In the gallbladder of one duckling from the first experiment (the first intermediate host was the Boreoelona snail), two adult flukes were detected, while in one duckling from the second experiment (the first intermediate host was the Parafossarulus snail), eight worms were detected. On day 9, no parasites were obtained from ducklings or rats, which were infected with trematodes from the first intermediate host Boreoelona, while in the gallbladder of one of two ducklings infected by trematodes from the first intermediate host Parafossarulus, 12 mature worms were found (the rats were not infected). In addition, the naturally infected fish P. glenii and R. percnurus mantschuricus from Magdikovoe lake were fed to two mice and two ducklings. The mice did not become infected, while 30 adult worms, morphologically identical to samples from previous experiments, were obtained in the gallbladder of one of two ducklings. The behavior of the cercariae and the infection process of the second intermediate hosts (the penetration of cercariae into tadpoles) were observed using a stereo microscope. In addition to experimental studies, the naturally infected animals from Magdikovoe lake were examined for infection with M. ussuriensis sp. nov. A total of 500 specimens of each snail species (P. spiridonovi and B. ussuriensis) were examined for the presence of rediae. 30 specimens of each fish species (P. glenii and R. percnurus mantschuricus) were examined for the presence of metacercariae.
The rediae and metacercariae were measured in live specimens, while cercariae were fixed in hot 4% formalin before measurements. The detection of sensillae on the cercariae body was performed using the method by Ginetsinskaya and Dobrovolsky (1963). The position of sensillae was described according to Richard (1971). Adult flukes were fixed in 70% ethanol and stored in 96% ethanol. Whole-mounts of flukes were prepared by staining with alum carmine, dehydrated in a graded ethanol series, cleared in clove oil, and mounted in Canada balsam.
All measurements were made in millimeters (mm).
2.2 Genetic analysis
Genomic DNA was isolated from individual specimens using the HotSHOT method (Truett et al., 2000). The DNA solution was stored at −20°C. The complete nucleotide sequences of ITS region were amplified by the polymerase chain reaction using the following primers: forward BD1 (5′-GTC-GTA-ACA-AGG-TTT-CCG-TA-3′ (Morgan & Blair, 1995)), and reverse 28S4R (5′-TAT-TTA-GCC-TTG-GAT-GGA-GTT-TAC-C-3′) designed in the Lasergene Primer Select program using the following sequences: JF823989 (Clonorchis sinensis), AF382059 (Pseudomurraytrema sp.) and HM172627 (Paragonimus westermani). The partial cox1 gene sequences were amplified using the forward 5cF22 (5′-TAG-ACT-ATC-TGT-CTT-CAA-AAC-A-3′ (Chelomina, Tatonova, Hung, & Ngo, 2014)) and reverse CO1-Rv (5′-AAC-AAA-TCA-TGA-TGC-AAA-AGG-TA-3′ (Katokhin et al., 2008)) primers. The partial sequences of 28S were amplified using the following primers: forward dig12 (5′-AAG-CAT-ATC-ACT-AAG-CGG-3′) and reverse 1500R (5′-GCT-ATC-CTG-AGG-GAA-ACT-TCG-3′) (Tkach, Littlewood, Olson, Kinsella, & Swiderski, 2003). The reaction mixture of 20 μl total volume contained 0.25 mM of each primer, 1× Taq buffer, 2 mM MgCl2, 0.8 mM dNTP, 0.5 units of Taq polymerase (Thermo Fisher Scientific, Vilnius, Lithuania) and approximately 10 ng or 15 ng of total DNA in water (for nuclear and mitochondrial markers, respectively). PCR was performed on a GeneAmp 9700 thermocycler (Applied Biosystems, USA) with the following conditions: initial denaturation (1 min, 94°C), and then 35 cycles: denaturation (15 s, 94°C), annealing (30 s, 55°C), synthesis (2 min, 72°C). Annealing temperature for the mitochondrial region was lowered to 52°C. PCR was completed by additional synthesis for 5 min at 72°C. PCR products were sequenced using ABI 3130 Genetic Analyzer (Applied Biosystems; at the Federal Scientific Center of the East Asia Terrestrial Biodiversity FEB RAS). The external primers, BD1, 28S4R, and the internal primer, R1i15 (5′-CCA-TTC-TGA-CAG-CAC-ATC-CCG-T-3′ (Tatonova, Chelomina, & Besprozvannykh, 2012)), were used for ITS sequencing. For sequencing of the mitochondrial gene, we used only external primers. Primers 300F (5′-CAA-GTA-CCG-TGA-GGG-AAA-GTT-G-3′), 900F (5′-CCG-TCT-TGA-AAC-ACG-GAC-CAA-G-3′), ECD2 (5′-CTT-GGT-CCG-TGT-TTC-AAG-ACG-GG-3′) (Tkach et al., 2003), 1200R (5′-GCA-TAG-TTC-ACC-ATC-TTT-CGG-3′) (Waeschenbach, Cox, Littlewood, Porter, & Taylor, 2009) were used for sequencing of 28S amplicons.
The 32 complete ITS sequences (1,080 bp), six partial 28S sequences (1,402 bp), and 22 partial cox1 gene (1,473 bp) sequences were deposited into the GenBank database (Tables 1 and S1). The nucleotide sequences were assembled manually and aligned by the Clustal W option in MEGA version 5.03 (Tamura et al., 2011). The p-distances between species were also analyzed with the same program. The level of nucleotide and haplotype diversity for cox1 gene sequences of M. ussuriensis sp. nov. was detected using Arlequin version 3.11 (Excoffier, Laval, & Schneider, 2006).
| DNA region/Species | Locality of Metorchis species | Hosts | n, references | GenBank accession number | ||
|---|---|---|---|---|---|---|
| First intermediate | Second intermediate | Definitive | ||||
| ITS1-5.8S-ITS2, complete | ||||||
| Metorchis ussuriensis sp. nov. | Magdikovoe lake, Russia | Unknown | Perccottus glenii, Rhynchocypris percnurus mantschuricus | Duckling | 29, this study | KP222468–KP222496 |
| Boreoelona ussuriensis | P. glenii , R. percnurus mantschuricus | Duckling | 1, this study | KP222497 | ||
| Parafossarulus spiridonovi | P. glenii, R. percnurus mantschuricus | Duckling | 2, this study | KY075778, KY075779 | ||
| ITS1, partial | ||||||
| Metorchis bilis | Spain | Unknown | Unknown | Unknown | 1 (Kang et al., 2008) | EU038154 |
| Metorchis xanthosomus | Unknown | Unknown | Unknown | Circus aeruginosus | 1, unpublished | JQ716400 |
| Metorchis orientalis | China | Unknown | Pseudorasbora parva | No | 6 (Ai et al., 2010) | HM347223–HM347228 |
| ITS2, partial | ||||||
| Opisthorchiidae | ||||||
| Metorchis orientalis | China | Unknown | Pseudorasbora parva | No | 3 (Ai et al., 2010) | HM347223–HM347225 |
| Metorchis albidus (M. bilis) | United Kingdom | Unknown | Unknown | Lutra lutra | 1 (Sherrard-Smith et al., 2016) | JF710316 |
| Metorchis bilis | Czech Republic | Unknown | Unknown | Aquila heliaca, Phalacrocorax carbo | 3 (Sitko et al., 2016) | KT740976, KT740978, KT740979 |
| Metorchis xanthosomus | Slovakia | Unknown | Unknown | Buteo rufinus | 3 (Sitko et al., 2016) | KT740980–KT740982 |
| Poland | Unknown | Unknown | Anas platyrhynchos | 1 (Sitko et al., 2016) | KT740983 | |
| Pseudamphistomum truncatum | Czech Republic | Unknown | Unknown | Aythya fuligula | 1 (Sitko et al., 2016) | KT740977 |
| Denmark | Unknown | Unknown | Mustela vison | 1 (Skov, Kania, Jørgensen, & Buchmann, 2008) | EU483072 | |
| Opisthorchis felineus | 1 (Brusentsov et al., 2013) | JN646477 | ||||
| Opisthorchis viverrini | 1 (Parvathi et al., 2008) | AY584735 | ||||
| Clonorchis sinensis | 1 (Tatonova et al., 2012) | JQ048598 | ||||
| Amphimerus sp. | 1 (Calvopiña et al., 2015) | AB678442 | ||||
| Heterophyidae (Outgroup) | ||||||
| Euryhelmis zelleri | 1 (Heneberg et al., 2015) | KM594177 | ||||
| Cryptocotyle lingua | 1, unpublished | KJ641524 | ||||
| Metagonimus miyatai | 1 (Pornruseetairatn et al., 2016) | HQ832615 | ||||
| Metagonimus yokogawai | 1, unpublished | KJ631740 | ||||
| Metagonimus takahashii | 1 (Thaenkham, Blair, Nawa, & Waikagul,2012) | HQ832618 | ||||
| Haplorchis pumilio | 1 (Mei et al., 2015) | KP165437 | ||||
| Haplorchis popelkae | 1 (Snyder & Tkach, 2009) | EU883584 | ||||
| 28S gene, partial | ||||||
| Opisthorchiidae | ||||||
| Metorchis ussuriensis sp. nov. | Magdikovoe lake, Russia | Unknown | Perccottus glenii, Rhynchocypris percnurus mantschuricus | Duckling | 4, this study | KY075772–KY075775 |
| Boreoelona ussuriensis | P. glenii, R. percnurus mantschuricus | Duckling | 1, this study | KY075776 | ||
| P. glenii, R. percnurus mantschuricus | Duckling | 1, this study | KY075777 | |||
| Clonorchis sinensis | 1 (Tantrawatpan et al., 2014) | JF823989 | ||||
| Opisthorchis viverrini | 1 (Thaenkham, Nawa, Blair, & Pakdee, 2011) | JF823990 | ||||
| Amphimerus ovalis | 1 (Olson et al., 2003) | AY116876 | ||||
| Heterophyidae (Outgroup) | ||||||
| Euryhelmis costaricensis | 1 (Sato, Ihara, Inaba, & Une, 2010) | AB521799 | ||||
| Metagonimoides oregonensis | 1 (Belden et al., 2012) | JQ995473 | ||||
| Cryptocotyle lingua | 1 (Olson et al., 2003) | JQ995473 | ||||
| Metagonimus yokogawai | 1 (Pornruseetairatn et al., 2016) | AY222228 | ||||
| Metagonimus takahashii | 1 (Pornruseetairatn et al., 2016) | HQ832636 | ||||
| Metagonimus miyatai | 1 (Pornruseetairatn et al., 2016) | HQ832633 | ||||
| Procerovum varium | 1 (Thaenkham et al., 2010) | HM004182 | ||||
| cox1 gene, partial | ||||||
| Opisthorchiidae | ||||||
| Metorchis ussuriensis sp. nov. | Magdikovoe lake, Russia | Unknown | Perccottus glenii, Rhynchocypris percnurus mantschuricus | duckling | 19, this study | KY075772 |
| Boreoelona ussuriensis | P. glenii, R. percnurus mantschuricus | duckling | 1, this study | KY075775 | ||
| Parafossarulus spiridonovi | P. glenii, R. percnurus mantschuricus | duckling | 2, this study | KY075776 | ||
| Metorchis xanthosomus | Germany | Unknown | Unknown | Unknown | 1 (Ai et al., 2010) | FJ423740 |
| Metorchis bilis | Germany | Unknown | Unknown | Unknown | 1 (Ai et al., 2010) | FJ423739 |
| Pseudamphistomum truncatum | Europe (Denmark, United Kingdom, Sweden, Czech Republic, Germany) | Unknown | Unknown | Lutra lutra, Neovison vison | 6 (Sherrard-Smith et al., 2016) | KP869078, KP869079, KP869081–KP869084 |
| Metorchis albidus (M. bilis) | Europe (Germany, United Kingdom, Denmark, Czech Republic, France) | Unknown | Unknown | Lutra lutra or Neovison vison | 9 (Sherrard-Smith et al., 2016) | KP869069–KP869077 |
| Metorchis orientalis | China | Unknown | Unknown | duck | 1 (Na et al., 2016) | KT239342 |
| Outgroup | ||||||
| Opisthorchis felineus | 1 (Shekhovtsov, Katokhin, Kolchanov, & Mordvinov, 2010) | EU921260 | ||||
| Clonorchis sinensis | 2 (Chelomina et al., 2014) | KJ204567, KJ204606 | ||||
| 1 (Cai et al., 2012) | JF729303 | |||||
- n, number of sequences.
For phylogenetic reconstructions, the following aligned sequences were used: 274, 1,277 and 348 bp for ITS2, 28S, and cox1, respectively. Since M. bilis, M. albidus, and M. crassiusculus are one species according to Sitko et al. (2016) and because cox1 sequences published by these authors overlap with the data from this study by 100 nucleotides, we only used sequences for M. albidus by Sherrard-Smith et al. (2016) (synonym of M. bilis according to Sitko et al. (2016)) for phylogenetic analysis. The nucleotide sequences used as outgroups in phylogenetic reconstructions are listed in Table 1. The BI (Bayesian Inference) method in MrBayes version 3.1.2 (Ronquist & Huelsenbeck, 2003) was used for reconstruction of interspecific phylogenetic trees. According to the Akaike information criterion in Modeltest version 3.7 (Posada & Krandall, 1998), the transversional model (TVM) with invariable sites plus G-distributed rates (TVM + I + G) was optimal for determining genetic distances for the ITS2 region. The general time reversible model with invariable sites plus G-distributed rates (GTR + I + G) was optimal for 28S sequences, and the Tamura-Nei model with invariable sites plus G-distributed rates (TrN + I + G) was optimal for the cox1 gene. The BI analysis was performed using 900,000, 100,000, and 600,000 generations of MCMC chains for ITS2 region, 28S, and cox1 gene, respectively. This number of generations was sufficient, because the SD value fell below 0.01. Of the samples, 25% were excluded to construct the consensus trees.
Euthanasia of laboratory animals was carried out in accordance with the Committee on the Ethics of Animal Experiments of the Federal Scientific Center of the East Asia Terrestrial Biodiversity, Russia.
3 RESULTS
3.1 Morphological description
Metorchis ussuriensis sp. nov
Definitive host
Anas platyrhynchos dom. (experimentally).
Site
The gallbladder.
Intensity of infection
Two to twelve individuals.
First intermediate host
Parafossarulus spiridonovi and Boreoelona ussuriensis.
Second intermediate host
Parafossarulus spiridonovi, Boreoelona ussuriensis (Bithyniidae Gray, 1857, in experiment and nature); Perccottus glenii (Odontobutidae Hoese et Gill, 1993, in experiment and nature), R. percnurus mantschuricus (Cyprinidae Rafinesque, 1815, in experiment and nature); tadpoles of Rana dybowskii (Ranidae Rafinesque, 1814, experimentally).
Type-locality
Magdikovoe lake in the basin of the Bolshaya Ussurka River (tributary of the Ussuri River), Primorsky Region, the Russian southern Far East; 45°57′ N, 133°53′ E.
Type-deposition
Holotype No 104–Tr, paratypes No 105–113–Tr.
This material is held in the parasitological collection of the Zoological Museum (Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far East Branch of the Russian Academy of Sciences, Vladivostok, Russia); e-mail: [email protected]. Deposited 2016.11.20.
Etymology
The specific name refers to the name of the Ussuri River.
3.1.1 Adult worm (material examined: 10 specimens)
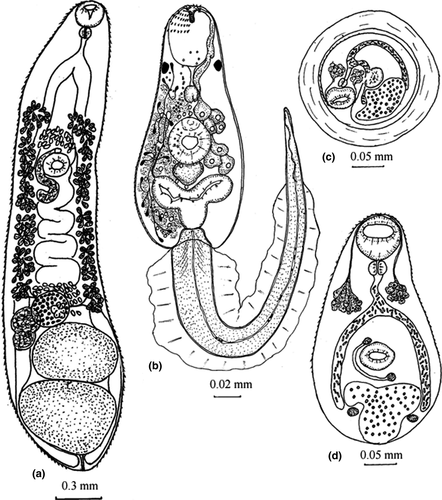
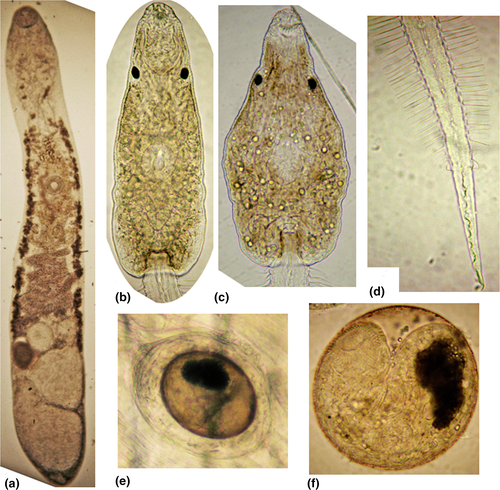
Body elongated, with tapered anterior and expanded posterior ends (Figures 1, 2, Figure S1; Table 2). Tegument with fine spines. Oral sucker subterminal. Diameter of ventral sucker slightly less than diameter of oral sucker, on border between first and second thirds of body length. Prepharynx absent, pharynx small, spherical, esophagus short. Intestinal bifurcation at short distance from anterior end of body. Ceca wide, dorsally from testes and reaches posterior end of body, where cecal branches adjacent to each other. Testes two, entire, tandem, close to each other, near posterior extremity, either on median line of body, or slightly diagonal, anterior testis left from median line of body. Seminal vesicle tubular, coiled. Ovary globular, pretesticular either left from anterior testis or on median line. Seminal receptacle right from ovary, Mehlis’ gland left from ovary. Uterus intercecal, between genital pore and ovary. Genital pore at short distance before ventral sucker. Vitellarium forms two lateral fields between postbifurcal region and ovarian level. Eggs small, numerous, embryonated, operculated, with knob. Excretory vesicle Y-shaped.
| M. ussuriensis sp. nov. this study n = 10 | M. elegansa* | M. butoridib* | M. orientalisc n = 10 | M. taiwanensisc n = 15 | M. taiwanensisd* | M. bilise n = 30 | M. xanthosomuse n = 30 | ||
|---|---|---|---|---|---|---|---|---|---|
| Holotype | Range | ||||||||
| BL | 3.19 | 2.42–3.93 (3.02) | 3.2–4.5 | 4.10–4.70 | 3.00–6.81 | 3.49–5.81 (4.38) | 2.5–3.5 (3.10) | 1.80–3.14 (2.41) | 2.06–3.85 (2.76) |
| BW | 0.62 | 0.34–0.79 (0.55) | 0.46–0.54 | 0.65–0.70 | 0.61–1.64 | 0.42–1.45 (0.78) | 0.40–0.55 (0.52) | 0.69–1.00 (0.81) | 0.57–1.43 (0.92) |
| LWR | 1: 5.2 | 1: 4.06–8.50 (1:5.5) | – | – | 1:2.99–4.49 (1:3.8) | 1:4.26–11.2 (1:5.97) | – | 1:2.1–4.4 (3.0) | 1:2.2–4.4 (3.1) |
| FL | 1.08 | 0.83–1.42 (1.06) | – | – | 0.66–1.81 | 0.83–1.68 | – | 0.51–0.94 (0.73) | 0.69–1.43 (0.93) |
| HL | 2.11 | 1.53–2.71 (1.96) | – | – | – | – | – | 1.09–1.77 (1.41) | 1.06–2.23 (1.64) |
| BFLR | 1: 2.96 | 1: 2.3–3.2 (1: 2.85) | – | – | – | – | – | – | – |
| HFLR | 1:1.51 | 1:1.34–2.23 (1:1.85) | – | – | – | – | – | 1:1.5–3.0 (2.0) | 1.2–2.2 (1.8) |
| OSL | 0.18 | 0.16–0.19 (0.18) | 0.25–0.25 | 0.22–0.30 | 0.27–0.38 | 0.16–0.29 (0.22) | 0.18–023 (0.20) | 0.16–0.25 (0.21) | 0.17–0.37 (0.24) |
| OSW | 0.19 | 0.17–0.19 (0.18) | 0.25–0.32 | 0.30–0.34 | 0.30–0.38 | 0.1992–0.2822 (0.26) | 0.20–0.23 (0.20) | 0.20–0.25 (0.23) | 0.20–0.37 (0.26) |
| VSL | 0.17 | 0.14–0.18 (0.15) | 0.21–0.27 | 0.25–0.34 | 0.23–0.33 | 0.20–0.28 (0.24) | 0.15–0.18 (0.17) | 0.15–0.22 (0.19) | 0.20–0.32 (0.25) |
| VSW | 0.17 | 0.14–0.17 (0.15) | 0.19–0.25 | 0.25–0.34 | 0.22–0.33 | 0.20–0.27 (0.23) | 0.15–0.18 (0.17) | 0.15–0.23 (0.19) | 0.20–0.36 (0.26) |
| VOSLR | 1:0.82–0.98 (1:0.87) | – | – | – | – | – | 1:1.00–1.38 (1:1.11) | 1:0.79–1.16 (1:0.96) | |
| VOSWR | 1:0.78–0.94 (1:0.84) | – | – | – | – | – | 1:1.00–1.40 (1:1.18) | 0.93–1.16 (1:0.99) | |
| PL | 0.07 | 0.05–0.07 (0.06) | 0.30 | 0.08–0.10 | 0.07–0.08 | 0.07–0.10 (0.09) | – | 0.08–0.10 (0.07) | 0.06–0.13 (0.08) |
| PW | 0.06 | 0.05–0.07 (0.06) | 0.26 | 0.08–0.09 | 0.07–0.08 | 0.05–0.08 (0.07) | – | 0.05–0.09 (0.07) | 0.06–0.15 (0.07) |
| EsL | 0.08 | 0.03–0.10 (0.06) | – | – | 0.08–0.18 | 0.03–0.12 | – | 0–0.02 (0.01) | 0.03–0.08 (0.06) |
| OL | 0.24 | 0.12–0.24 (0.17) | 0.12–0.16 | 0.18–0.20 | 0.12–0.33 | 0.18–0.32 (0.2251) | 0.15–0.18 (0.17) | 0.09–0.20 (0.14) | 0.09–0.30 (0.19) |
| OW | 0.25 | 0.12–0. 27 (0.20) | 0.10–0.11 | 0.18–0.23 | 0.17–0.40 | 0.15–0.33 (0.24) | 0.15–0.18 (0.17) | 0.09–0.22 (0.16) | 0.17–0.32 (0.25) |
| ATL | 0.39 | 0.24–0.44 (0.35) | 0.22–0.37 | 0.35 | 0.25–0.83 | 0.33–0.83 (0.50) | 0.25–0.35 (0.30) | 0.22–0.51 (0.34) | 0.24–0.74 (0.45) |
| ATW | 0.56 | 0.27–0.56 (0.38) | 0.20–0.31 | 0.26 | 0.32–0.98 | 0.33–0.61 (0.47) | 0.30–0.38 (0.35) | 0.27–0.57 (0.38) | 0.30–0.83 (0.50) |
| PTL | 0.50 | 0.29–0.58 (0.41) | 0.28–0.36 | 0.36–0.44 | 0.25–0.90 | 0.33–0.83 (0.52) | 0.30–0.35 (0.32) | 0.23–0.45 (0.32) | 0.23–0.78 (0.44) |
| PTW | 0.67 | 0.30–0.67 (0.43) | 0.22–0.38 | 0.34 | 0.32–1.08 | 0.40–0.65 (0.51) | 0.33–0.43 (0.37) | 0.35–0.63 (0.56) | 0.26–1.22 (0.55) |
| SRL | 0.33 | 0.17–0.33 (0.24) | – | – | – | – | – | – | – |
| SRW | 0.19 | 0.13–0.23 (0.181) | – | – | – | – | – | – | – |
| VFL | 1.37–1.39 | 0.91–1.98 (1.37) | – | – | – | – | – | 1.00–1.71 (1.37) | 0.91–2.29 (1.56) |
| VFR | – | – | – | – | – | 1.06–1.86 (1.39) | 0.92–2.49 (1.55) | ||
| AEV | 0.69 | 0.43–0.89 (0.75) | 0.60 | – | – | – | – | – | – |
| AEU | 0.82 | 0.66–1.16 (0.91) | – | – | – | – | – | – | – |
| EL | 0.027–0.031 | 0.027–0.031 (–) | 0.024–0.028 | 0.025–0.027 | 0.029–0.032 | 0.026–0.031 | 0.023–0.028 | 0.027–0.031 (0.029) | 0.027–0.034 (0.029) |
| EW | 0.015–0.019 | 0.015–0.019 (–) | 0.012–0.014 | 0.014–0.015 | 0.014–0.017 | 0.136–0.017 | 0.014–0.016 | 0.016–0.019 (0.018) | 0.017–0.022 (0.021) |
- n, number of specimens; *, number of specimens is unknown; BL, body length; BW, body width; LWR, length/width ratio; FL, forebody length; HL, hindbody length; BFLR, body/forebody length ratio; HFLR, hindbody/forebody length ratio; OSL, oral sucker length; OSW, oral sucker width; VSL, ventral sucker length; VSW, ventral sucker width; VOSLR, ventral/oral sucker length ratio; VOSWR, ventral/oral sucker width ratio; PL, pharynx length; PW, pharynx width; EsL, esophagus length; OL, ovary length; OW, ovary width; ATL, anterior testis length; ATW, anterior testis width; PTL, posterior testis length; PTW, posterior testis width; SRL, seminal receptacle length; SRW, seminal receptacle width; VFL, vitellarium fields left; VFR, vitellarium fields right; AEV, anterior end of vitellarium; AEU, anterior end of uterus; EL, eggs length; EW, eggs width; a, Belogurov and Leonov (1963); b, Oshmarin (1963); c, Chen and Tang (1981); d, Yang and Lai (1997); e, Sitko et al. (2016).
Remarks
Two samples had a mirror arrangement of the genital system organs: testes, ovary, seminal receptacle, and Mehlis’ gland.
3.1.2 Redia (material examined: five specimens)
Body sac-shaped, 1.65–1.87 × 0.19–0.23, pharynx 0.036–0.039 in diameter, cecum short.
3.1.3 Cercaria (material examined: 15 specimens)
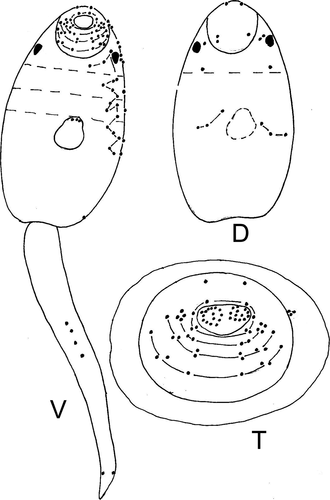
Body oval or trapezoidal, with yellow-brown pigment, covered by fine spines from anterior end to middle part (Figures 1-3, S1; Table 3). Largest spines arranged at anterior end, formed three groups consisting of two rows separated by narrow space. Circumoral spines can be retracted into cavity of oral sucker. Forebody 0.10–0.14. Two pigment eye-spots on both sides of body, on dorsal side, on level of posterior border of oral sucker or at short distance from it. Vacuole-like inclusions from posterior end of body to level of posterior border of oral sucker. Oral sucker subterminal, prepharynx present, pharynx spherical, esophagus and cecal branches underdeveloped. Ventral sucker and primordium of genital pore form ventrogenital complex. Primordium of the genital system dorsal to ventral sucker, from middle of ventral sucker to excretory vesicle. Penetrating glands 14, 7 in each body side, on the level of ventral sucker. Ducts of these glands opened at anterior end of body according formula 3 + 4 + 4 + 3. Cystogenous glands from level of pharynx to posterior end of body. Tail inserted in tail socket, with finfold. Finfold consists of two parts: first lateral, from tail base to 4/5 of tail length, second dorsoventral, on remaining part of tail. Excretory vesicle Y-shaped with thick walls. Excretory formula: 2[(3 + 3)+(3 + 3 + 3)] = 30. Formula of sensory structures: 26–28 sensillae on internal wall of oral sucker; CI = 1–2V1, 1–2V2, 1D; CII = 4V1, 1V2, 1V3, 1D; CIII = 1V1, 1V2, 1V3, 1–2V4 (1V1, 2V2, 1–2V3), 1D; CIV = 1V1, 1–2V2, 1V3, 3D; AI = 3V, 5–6L, 1D; AII = 3–5V, 2L; AIII = 4V, 0–4L; M = 5V, 2L, 3D; P = 1–2L; S = 3; U = 5L.
| M. ussuriensis sp. nov. | M. taiwanensis (Yang & Lai, 1997) | ||
|---|---|---|---|
| from Parafossarilus spiridonovi | from Boreoelona ussuriensis | ||
| Body | 0.173–0.200 × 0.081–0.11 | 0.165–0.184 × 0.095–0.11 | 0.120–0.175 × 0.050–0.063 |
| Oral sucker | 0.033–0.045 × 0.028–0.039 | 0.034–0.036 × 0.034–0.039 | 0.012–0.015 × 0.015 |
| Ventral sucker | 0.033–0.039 × 0.031–0.034 | 0.036–0.042 × 0.028–0.034 | 0.008–0.012 × 0.012 |
| Pharynx | 0.014–0.016 × 0.012–0.020 | 0.016–0.019 × 0.019–0.022 | – |
| Forebody | 0.081–0.092 | 0.075–0.084 | – |
| Tail | 0.27–0.33 × 0.030–0.033 | 0.27–0.35 × 0.025–0.034 | 0.300–0.425 × 0.025–0.030 |
The present studies showed that cercariae from Boreoelona ussuriensis vs. cercariae from Parafossarulus spiridonovi have small differences: brown vs. yellow body pigment; numerous large vs. few small vacuole-shaped inclusions (Figure 2).
3.1.4 Metacercaria (material examined: 10 specimens)
Cyst of metacercaria globular or oval, 0.21–0.27 × 0.21–0.24. Cyst wall 0.02–0.07 (Figures 1, 2, S1). Body of metacercaria extracted from cyst 0.34–0.35 × 0.18–0.20, covered by spines. Oral sucker 0.056–0.072 × 0.050–0.084. Prepharynx absent, pharynx 0.023–0.028 in diameter, esophagus 0.039–0.041 in length. Cecal branches reach level of middle of excretory vesicle, filled with rod-shaped granules. Ventral sucker 0.039–0.045 × 0.045–0.056. Numerous glands on level of esophagus and on both sides of it, ducts of these glands opened on anterior end of body. Primodiums of testes 0.012 × 0.018, on both sides of excretory vesicle; ovary primodium 0.012–0.013 in diameter, between ventral sucker and excretory vesicle. Uterus primodium is visible, on level of ventral sucker, right from median line of body terminating in genital pore 0.011 × 0.012. Excretory vesicle Y-shaped, filled with dark granules.
3.2 Life cycle
Our results show that snails, fish, and frogs can be second intermediate hosts of M. ussuriensis sp. nov. All snails, tadpoles, and fish examined were infected with metacercariae of M. ussuriensis sp. nov. with an intensity of 2–30 metacercariae in snails (n = 10), 20–35 metacercariae in fish (n = 2) and 24–60 in tadpoles (n = 4). Trematodes were located in the muscular foot and tentacles of snails, in the muscle of fish, and in the muscle and tissues of internal organs of tadpoles. It has been observed that cercariae just emitted from the snails actively move in the water column, without a resting phase, and do not show infective activity when they come into contact with the second intermediate host. These characteristics last for approximately 30–40 min. After this, the active movements of cercariae alternate with phases of rest, interrupted by a display of rheotaxis. In this period, if cercariae come into contact with the host, it attaches to the host using the oral sucker. Trematodes invade tadpoles directly at the places of attachment to the host body, while in the case of fish, cercariae move on the body for a short time (10–25 s), likely to find a place for penetration into internal tissues. If cercariae do not find a place for penetration, they separate from the animal. Cercariae penetrate fish over a period of 15–25 min, and tadpoles over 5–10 min. On day 21 postinfection of the second intermediate host, the metacercariae were well developed larvae. In the definitive host, Anas platyrhynchos dom., trematodes become mature on day eight.
Among the first intermediate hosts, Parafossarulus spiridonovi are likely to play a major role in the circulation of M. ussuriensis sp. nov. in natural conditions. Infection rate for this snail with rediae of M. ussuriensis sp. nov. reaches 4%, while for Boreoelona ussuriensis, this value is only 0.6%. The infection rates of the fish Perccottus glenii and R. percnurus mantschuricus were 100% with an intensity of invasion of up to 200 metacercariae, while the snails P. spiridonovi and B. ussuriensis were infected with an intensity of up to 20 metacercariae of M. ussuriensis sp. nov.
3.3 Genetic data
3.3.1 Intraspecific variation
Sequences of the partial 28S (1,402 bp) were identical for M. ussuriensis sp. nov. The complete sequence length of ITS region for this species was 1,080 bp. The 5.8S gene (160 bp) and the ITS2 region (291 bp) sequences were conservative. Analysis of the 32 ITS1 (629 bp) complete sequences indicated 99.9% identity and the presence of one C → T transition in position 492 bp. Only two genotypes were found by this nuclear marker. One was represented by a single ITS1 sequence, and the other included 31 samples.
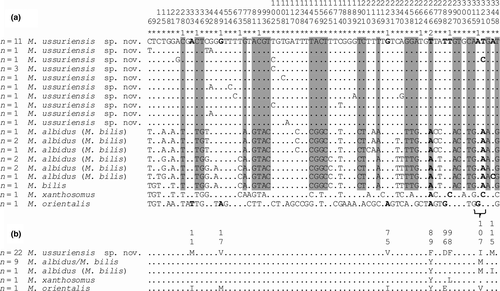
Analysis of the 22 partial sequences of the cox1 gene revealed a higher variability level. The sequence length was 1,473 bp, which comprises 93% of the full-size gene (positions 100–1,572 bp; 5′-end was detected using complete genome of Metorchis orientalis, KT239342, 3′-end was detected by the presence of a stop codon TAG). Sequences differ by 39 variable sites, nine of which were parsimony-informative. The majority of mutations were transitions (8 of A → G, 6 of G → A, 9 of C → T, 15 of T → C) and only one was a transversion (A → C). Only two nucleotide substitutions were non-synonymous. The T → C transition at position 977 bp led to a Val → Ala amino acid substitution, whereas the C → T substitution at the 1,148 bp position caused the Tre → Met substitution. In this population, we have detected low nucleotide (π = 0.00342 ± 0.00045) and high haplotype (Hd = 0.970 ± 0.028; 18 haplotypes) diversities for the cox1 gene.
3.3.2 Phylogenetic relationships
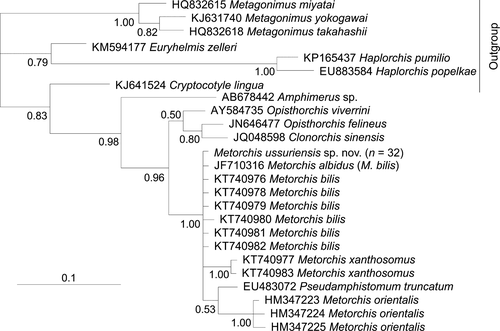
Prior to this study, 28S data were absent for the genus Metorchis in GenBank. Sequences aligned for phylogenetic analysis in this study are 1,277 bp in length, differentiate M. ussuriensis sp. nov. from other trematodes and form a common cluster with another species of the family Opisthorchiidae (Opisthorchis viverrini and Clonorchis sinensis) with high posterior probability (Figure 4). The genetic distances between M. ussuriensis sp. nov. and these two species are situated in the 0.007–0.016 range. The distances between species of the genus Metagonimus (family Heterophyidae) are in the 0.009–0.012 range. The genetic distances between other genera of both families have 3–11 times higher values (0.047–0.051 and 0.044–0.077, respectively, for Opisthorchiidae and Heterophyidae).
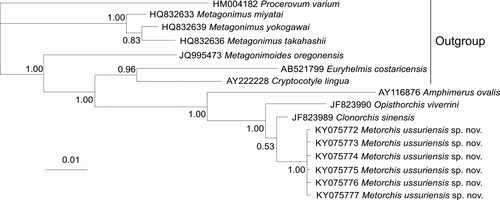
In the phylogenetic analysis based on 274 bp ITS2 sequences, M. ussuriensis sp. nov. form a common cluster with M. bilis (M. albidus), M. orientalis, M. xanthosomus, and Pseudamphistomum truncatum with high posterior probability (Figure 5). Within this cluster, M. ussuriensis sp. nov. and M. bilis (M. albidus) do not form a distinct branch. The genetic distances are zero between these species, but p distance did not take into account the presence of a 3-bp indel, which differentiates these species. The 3-bp insertion, which is found in all M. ussuriensis sp. nov. samples, is absent in other Metorchis species. When comparing the full-length sequence of ITS2 region (288 bp for M. albidus/bilis and 291 bp for M. ussuriensis sp. nov.), we found that these species also have a nucleotide substitution at position 280 bp (transversion C ↔ G). The minimum value of p-distances was detected between these two species and P. truncatum (d = 0.016). Distances between other members of the family Opisthorchiidae ranged between 0.023 and 0.096.
Genetic distances between M. ussuriensis sp. nov., M. xanthosomus, and M. bilis, based on ITS1 region sequences, were lower (0.004–0.010) than between these species and M. orientalis (0.027–0.30). The distance between M. ussuriensis sp. nov. and M. bilis (0.010) was lower than between these two species and M. xanthosomus (0.004 and 0.006, respectively).
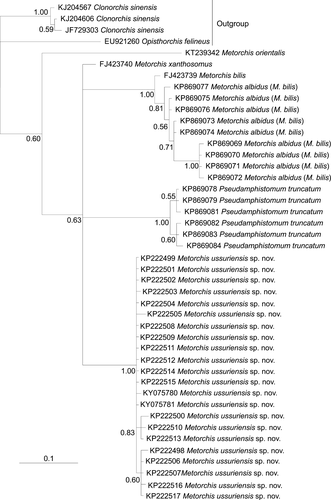
The interspecific phylogenetic reconstruction based on the 348 bp cox1 gene sequences (Figure 6) separated all M. ussuriensis sp. nov. samples in a cluster with high posterior probability. P. truncatum also formed distinct clusters, while M. albidus (M. bilis) and M. bilis sequences were joined together. Within these groups of sequences, the genetic distances ranged between 0.005 and 0.020, while the distances between the groups were higher (0.086–0.112) (Table 4). Most of the genetic differences (n = 25) between M. ussuriensis sp. nov. and M. albidus/M. bilis were fixed substitutions. Nucleotide substitutions were predominantly (86%) in third codon positions (Figure 7a). Yet, non-synonymous substitutions were found in all codon positions (Figure 7b). M. ussuriensis sp. nov. and M. albidus/M. bilis differed by three amino acids.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Metorchis ussuriensis sp.n. | 0.014 | 0.016 | 0.016 | 0.017 | 0.018 | 0.018 | |
| 2 | Metorchis xanthosomus | 0.085 | 0.015 | 0.016 | 0.015 | 0.017 | 0.017 | |
| 3 | Metorchis albidus (M. bilis) | 0.107 | 0.086 | 0.017 | 0.017 | 0.018 | 0.017 | |
| 4 | Pseudamphistomum truncatum | 0.112 | 0.104 | 0.127 | 0.019 | 0.019 | 0.019 | |
| 5 | Clonorchis sinensis | 0.131 | 0.093 | 0.135 | 0.144 | 0.018 | 0.017 | |
| 6 | Metorchis orientalis | 0.144 | 0.135 | 0.147 | 0.153 | 0.136 | 0.019 | |
| 7 | Opisthorchis felineus | 0.150 | 0.121 | 0.134 | 0.156 | 0.127 | 0.155 |
The alignments of the sequences are available in the Supporting Information (Data S1–S3).
4 DISCUSSION
Adult worms obtained as a result of experimental investigations are in accordance with descriptions of the genus Metorchis by all morphological characters. M. ussuriensis sp. nov. differs from three other species (M. elegans, M. butoridi, and M. orientalis) that parasitize warm-blooded animals, described in the East Asian region: First, it has a significantly smaller maximum body length; second, it has smaller suckers and pharynx (Table 2); and third, it has entire testes, whereas the other three species have lobed testes. Adult M. ussuriensis sp. nov. samples have a greater similarity to M. taiwanensis from China by morphological and metric parameters (Table 2). In China (Fuzhou), Chen and Tang (1981) obtained two Metorchis species in the gallbladder of Anas platyrhynchos dom., one of which belongs to M. taiwanensis according to the opinion of the authors. In the study of the parasite life cycle, Yang and Lai (1997) also found adult M. taiwanensis in Anas platyrhynchos dom. in China. Yet, the flukes from the study by Chen and Tang (1981) were larger than the M. taiwanensis in the study by Yang and Lai (1997) in a number of metrics (body, oral and ventral suckers, ovary, testes) (Table 2). For metric parameters, M. ussuriensis sp. nov. has the most similarities to M. taiwanensis obtained by Yang and Lai (1997). However, adult worms of M. ussuriensis sp. nov., in contrast to M. taiwanensis described by Yang and Lai (1997), are smaller in size of their oral suckers and larger in the size of their eggs. At the same time, cercariae of M. ussuriensis sp. nov. differ from M. taiwanensis by having a slightly larger body size and considerably larger oral and ventral sucker sizes (Table 3). Furthermore, they differ in structure and location of the tail membrane. M. ussuriensis sp. nov. has a membrane consisting of two separate parts, one of which is laterally located, and the other is dorsoventrally located, while the membrane of M. taiwanensis is a single complete membrane and laterally located. In addition, Morishita and Tsuchimora (1925; cited in Skrjabin & Petrov, 1950) obtained adult M. taiwanensis worms from the bile ducts of domestic ducks, and Tao (1948; cited in Skrjabin & Petrov, 1950) found adult M. taiwanensis in the bile ducts of experimentally infected chickens and quail. The results of Chen and Tang (1981), Yang and Lai (1997) for M. taiwanensis and in this study for M. ussuriensis sp. nov. (albeit small sample sizes were investigated) show that these trematodes are located only in the gallbladder of ducklings and do not infect chickens. Considering the above-described morphometric parameters of trematodes, as well as the localization features of adult worms and adaptation to the definitive hosts, we attributed the trematodes Metorchis found in the Russian southern Far East to a new species, M. ussuriensis sp. nov.
According to genetic data obtained by Sitko et al. (2016), M. albidus and M. crassiusculus are synonymies of M. bilis. Thus, all nucleotide sequences of these three species are discussed as M. bilis in this study. According to data based on cox1 gene sequences, M. ussuriensis sp. nov. is a new species, since the reconstruction based on cox1 gene sequences separated all M. ussuriensis sp. nov. samples in a cluster with high statistical support. Moreover, the values of distances between M. ussuriensis sp. nov. and other Metorchis species (M. bilis, M. xanthosomus and M. orientalis) based on the mitochondrial marker correspond to the distances between the other opisthorchiids and to the average values of interspecific divergence of trematodes from different families using the cox1 gene (Vilas, Criscione, & Blouin, 2005). In addition, M. ussuriensis sp. nov. differs from M. bilis, M. xanthosomus, and M. orientalis in most metrics, as well as the length/width body ratio and ventral/oral sucker ratio (Table 2). Besides the determination of the phylogenetic status for the Metorchis representative in the Russian southern Far East, we analyzed the levels of nucleotide and haplotype diversities for the cox1 gene, which indicate long-term isolation of a relatively small population (Avise, 2000), as was previously revealed for Clonorchis sinensis from the same geographic territory (Chelomina et al., 2014). Almost all M. ussuriensis sp. nov. samples for genetic analysis were obtained from experiments with naturally infected second intermediate hosts, and this explains the number of different haplotypes in one population.
In contrast to the markers mentioned above, in the phylogenetic tree based on ITS2 sequences, M. ussuriensis sp. nov. and M. bilis form a common branch, which indicates the identity of the investigated samples: Based on these findings, it could be assumed that M. ussuriensis sp. nov. and M. bilis are one species. However, this reconstruction does not consider the presence of a 3-bp insertion in the M. ussuriensis sp. nov. nucleotide sequence. The same insertion is unusual for ITS2 of trematodes, because this region is usually conserved within a species, and indels may influence the secondary structure of rRNA (Tatonova et al., 2012). The distances based on ITS2 sequences showed that only M. orientalis differs from other Metorchis species on the level of distances between different opisthorchiid genera, while the genetic distances between M. ussuriensis sp. nov. and both Pseudamphistomum truncatum and M. xanthosomus had lower values. Low distances between a representative of another genus (P. truncatum) and M. xanthosomus and large differences in morphology demonstrate that this genetic marker is unsuitable for species identification and for phylogenetic reconstructions among the genus Metorchis and the family Opisthorchiidae in general. In addition, the ITS2 region does not separate representatives of other families of trematodes (Després, Kruger, Imbert-Establet, & Adamson, 1995; Pornruseetairatn et al., 2016). Similar to ITS2 sequences, the distances between the ITS1 sequences of M. ussuriensis, M. bilis, and M. xanthosomus are in the range of genetic distances within species of the family Opisthorchiidae, while distances between these species and M. orientalis were 3–7.5 times higher, but did not reach the values between the genera of the family (Kang et al., 2008; Tatonova et al., 2012). Furthermore, according to data based on the ITS1 region, M. xanthosomus is closer to both M. ussuriensis and M. bilis, than M. ussuriensis and M. bilis to each other.
Besides the ITS1 and ITS2 regions, Sitko et al. (2016) used 18S and nad1 genes for species identification. Yet, 18S showed an absence of genetic distances within the genus Metorchis. The use of the nad1 gene seems inappropriate, since the rate of mutation accumulation within the genus is not uniform and varies over a wide range, from 0.19 to 0.52. Therefore, we cannot estimate the level of differences (species, genus, or other).
The 28S gene is more suited to the genetics of trematodes including Opisthorchioidea (Olson, Cribb, Tkach, Bray, & Littlewood, 2003; Shumenko, Tatonova, & Besprozvannykh, 2017). Unfortunately, 28S sequences for other Metorchis species are not available in GenBank. The genetic distances between M. ussuriensis sp. nov. and other representatives of the family Opisthorchiidae (Opisthorchis, Clonorchis) are similar to the distances between species of the genus Metagonimus (family Heterophyidae) (Pornruseetairatn et al., 2016; Shumenko et al., 2017), while the genetic distances between other genera of each family mentioned above are 3–11 times higher. These values indicate that the representatives of Metorchis, Opisthorchis, and Clonorchis may belong to one genus, despite some differences in morphology (Bray, Gibson, & Jones, 2008). This assumption is consistent with data based on ITS2 sequences, because the values of genetic distances between different genera based on this marker are also equal to the distances within Metagonimus (Pornruseetairatn et al., 2016; Shumenko et al., 2017). It should be noted that the description of morphological features is not always sufficient to identify species of trematodes. Moreover, often species that are similar and even identical according to morphological criteria are well separated into independent species using molecular data, for example, representatives of the family Heterophyidae, Metagonimus yokogawai Katsuradai, 1912, and M. suifunensis Shumenko et al., 2017 (Shumenko et al., 2017). Nevertheless, the genera mentioned above include a large number of species, for which molecular data have not yet been obtained. Also, the family Opisthorchiidae includes other genera, which await taxonomic clarification. Thus, for the final decision, more data, first of all, molecular, are needed for other representatives of the family.
5 CONCLUSION
The study of the life cycle and morphology of the developmental stages of the Metorchis samples from the Russian southern Far East showed that these specimens belong to a new species, M. ussuriensis sp. nov. Our study also indicates that the ITS1 and ITS2 regions, as well as the 18S and nad1 genes, are not suitable for species differentiation of representatives of the genus Metorchis, while the sequences of the cox1 gene are suitable for separating the species, and their use confirmed the species status of M. ussuriensis sp. nov. However, a lack of data for the probably most appropriate and widely used marker for species identification of trematodes (the 28S) prevented the conclusive determination of the species composition of the genus Metorchis.
ACKNOWLEDGEMENTS
The study was partially funded by FEB RAS (Far Eastern Branch, Russian Academy of Sciences) project No 15-1-6-014. The authors declare no conflict of interest.




