Phylogenetic relationships within a patagonian clade of reptiles (Liolaemidae: Phymaturus) based on DNA sequences and morphology
Abstract
Phymaturus is a clade of lizards that occurs at moderate to high elevations in western Argentina and the adjacent central region of Chile, as well as in various volcanic plateaus of the Patagonian region of Argentina. This genus had previously been divided into two groups: the patagonicus and the palluma groups. In this study, we analyzed relationships within the patagonicus group. The data set was built for 23 species plus nine other terminal taxa of undetermined taxonomic status. In total, 10,631 bp (ND4, Cytb, 12S, COI, five protein coding nuclear genes and seven anonymous nuclear loci) and 254 morphological characters were analyzed in a combined data set for 35 ingroup taxa and nine outgroups. We also ran separate DNA sequence and morphological data sets. We identified four main clades, and revealed congruencies and incongruences with previous studies. The indistinctus clade is recovered as the most basal within the patagonicus group in the strict parsimony analysis, while the somuncurensis clade is the most basal under Bayesian inference. The previously recovered calcogaster clade resulted paraphyletic in both analyses and part of their species are included in a redefined somuncurensis clade. We found low support at basal nodes provoked in part by contradictory evidence shown by rogue taxa. We show the phylogenetic information given by each partition/marker and how they contribute to relationships found in the total evidence analysis. We discuss the phylogenetic position of Phymaturus manuelae, Phymaturus tenebrosus, and Phymaturus patagonicus.
1 INTRODUCTION
The genus Phymaturus comprises lizards of a very conservative mode of life, they are all saxicolous, herbivorous and viviparous lizards found in Patagonian and Andean landscapes of Argentina and Chile. Contrary to its sister genus Liolaemus, Phymaturus is strictly herbivorous (see occurrence of herbivory in Liolaemus in Espinoza, Wiens, & Tracy, 2004; Valdecantos, Arias, & Espinoza, 2012) and all species of Phymaturus are viviparous (see about viviparism of Liolaemus in Schulte, Macey, Espinoza, & Larson, 2000; Cei, Videla, & Vicente, 2003; among others). Within Phymaturus, two groups are recognized (Etheridge, 1995): the palluma and the patagonicus groups, whose monophyly was confirmed by several independent studies (Espinoza et al., 2004; Lobo & Quinteros, 2005; Lobo, Abdala, & Valdecantos, 2012; Morando, Avila, Pérez, Hawkins, & Sites, 2013; Pyron, Burbrink, & Wiens, 2013).
The Phymaturus patagonicus group is composed of 25 taxa (Table 1) distributed entirely in Argentina. It extends from the region called Payunia (Mendoza and Neuquén provinces) to southern Chubut Province, adjacent to Colihue Lake (Cei & Castro, 1973; Lobo, Abdala, & Valdecantos, 2012; Morando et al., 2013). The earliest studies on this group were the original descriptions of P. patagonicus (Koslowsky, 1898; and Phymaturus spurcus Barbour, 1921). Half a century later, diverse populations from Mendoza, and northern and southern Patagonia were described as Phymaturus indistinctus, Phymaturus somuncurensis, Phymaturus zapalensis, Phymaturus payuniae, and Phymaturus nevadoi, which are currently considered to be subspecies of P. patagonicus (Cei & Castro, 1973; Cei & Roig, 1975). In those studies, morphological descriptions and serological comparisons were included. Pereyra (1992) studied several morphological characters, described numbers and morphology of chromosomes and allozymes for six species of Phymaturus, and included only one member of the patagonicus group (P. payuniae). Etheridge (1995) recognized all names available for the group in the literature as full species: P. patagonicus, P. nevadoi, P. payuniae, P. indistinctus, P. somuncurensis, and P. zapalensis. Scolaro and Cei (2003) described Phymaturus calcogaster in Chubut Province, including this taxon in the palluma group, and at first, cited a site north of Esquel (western Chubut Province) as the type locality, later correcting both the group assignation and type locality (patagonicus group, Laguna de las Vacas, Scolaro, Tappari, & Cei, 2005). Lobo and Quinteros (2005) redescribed P. patagonicus and resurrected P. spurcus, previously synonymized with P. patagonicus by Burt and Burt (1931), and at the same time brought the composition of the group to eight species (P. patagonicus, P. spurcus, P. zapalensis, P. payuniae, P. nevadoi, P. indistinctus, P. somuncurensis, and P. calcogaster). Over the last 10 years, several species were added to the composition of the P. patagonicus group: Lobo and Quinteros (2005) described Phymaturus tenebrosus, Phymaturus excelsus, and Phymaturus spectabilis for western Río Negro Province; Scolaro and Ibargüengoytía (2007, 2008) described Phymaturus ceii and Phymaturus manuelae from Río Negro Province; Scolaro, Ibargüengoytía, and Pincheira-Donoso (2008) described Phymaturus agilis as syntopic of P. spectabilis, while Lobo, Cruz, and Abdala (2012), finding no statistical differences, identified P. agilis as a brown morph of P. spectabilis (and also showed a female giving birth to both patterns). Scolaro and Tappari (2009) described Phymaturus desuetus from a site adjacent to Ingeniero Jacobacci (Río Negro) based on only one specimen, but no other individual of this taxon has been reported to date. Scolaro and Pincheira-Donoso (2010) described Phymaturus videlai and Phymaturus castillensis, from southern Chubut; Lobo, Abdala, and Valdecantos (2010) described Phymaturus felixi and Phymaturus etheridgei (Chubut and Río Negro). Avila, Pérez, Pérez, and Morando (2011) discovered Phymaturus sitesi and Phymaturus delheyi from eastern Neuquén Province, species living syntopically with species/populations of the palluma group. Scolaro, Méndez de la Cruz, and Ibargüengoytía (2012) added Phymaturus sinervoi (Río Negro) and a year later Phymaturus camilae from Chubut Province (Scolaro, Jara, & Pincheira-Donoso, 2013). Avila, Pérez, Minoli, and Morando (2014) recorded Phymaturus yachanana from Eastern Chubut Province and Lobo and Nenda (2015) described Phymaturus cacivioi from northwestern Río Negro Province, a species that exhibits melanistic individuals. According to Morando et al. (2013), within the patagonicus group there are several unnamed populations, most of them requiring a detailed morphological study. Cryptic diversity within Phymaturus is not uncommon, as was revealed recently in Morando et al. (2013) and Lobo, Barrasso, Hibbard, and Basso (2016), also this phenomenon occurs in its sister genus Liolaemus (see for example studies of Torres-Pérez et al., 2007; Medina, Avila, Sites, & Morando, 2017). In a recent study, González-Marín, Morando, & Avila (2016), studied the species of the calcogaster clade (P. yachanana, P. camilae, P. calcogaster, and P. patagonicus) using linear and geometric analysis, and presented results that confirm their specific status as they were originally described based on traditional morphology. This study is also consistent with DNA evidence that considers these species to be independent lineages (Morando et al., 2013). In another recent publication, a new species for the patagonicus group was described as Phymaturus rahuensis by González-Marín, Pérez, Minoli, Morando, & Avila (2016) (sp. 16 in Morando et al., 2013), assigned to the payuniae clade. The latest contribution to date is a new completely melanic species from western Chubut Province, named Phymaturus curivilcun (Scolaro, Corbalán, Tappari, & Obregón Streitenberger, 2016).
| Species/authors | Type locality |
|---|---|
| Phymaturus cacivioi Lobo & Nenda 2015 | 12.6 km SW of Mencué, Rio Negro |
| Phymaturus calcogaster Scolaro & Cei 2003 | Laguna de la Vaca, Chubut |
| Phymaturus camilae Scolaro, Jara & Pincheira-Donoso 2013 | Sacanana bridge, Chubut |
| Phymaturus castillensis Scolaro & Pincheira-Donoso 2010 | La Juanita, Chubut |
| Phymaturus ceii Scolaro & Ibargüengoytía 2007 | ca. Chasicó, Río Negro |
| Phymaturus curivilcun Scolaro, Corbalán, Tappari & Obregó Streitenberger 2016 | Paraje El Mirador, Chubut |
| Phymaturus delheyi Avila, Pérez, Pérez & Morando 2011 | Tromen volcano, Neuquén |
| Phymaturus desuetus Scolaro & Tappari 2009 | S. Ingeniero Jacobacci, Río Negro |
| Phymaturus etheridgei Lobo, Abdala & Valdecantos 2010 | ca. Molihue, Río Negro |
| Phymaturus excelsus Lobo & Quinteros 2005 | ca. Ojo de Agua, Río Negro |
| Phymaturus felixi Lobo, Abdala & Valdecantos 2010 | 108 km S Paso de Indios, Chubut |
| Phymaturus indistinctus Cei & Castro 1973 | Las Pulgas, Chubut |
| Phymaturus manuelae Scolaro & Ibargüengoytía 2008 | ca. Comallo, Río Negro |
| Phymaturus nevadoi Cei & Roig 1975 | Cerro del Nevado, Mendoza |
| P. patagonicus Koslowsky 1898 | Dolavon to Paso de Indios, Chubut |
| Phymaturus payuniae Cei & Castro 1973 | Volcán Payún, Mendoza |
| Phymaturus rahuensis González Marín, Pérez, Minoli, Morando & Avila 2016 | 25 km E Rahue, Neuquén |
| Phymaturus sinervoi Scolaro, Méndez de la Cruz, Ibargüengoytía 2012 | Cari Laufquen, Rio Negro |
| Phymaturus sitesi Avila, Pérez, Pérez & Morando 2011 | Sierra Auca Mahuida, Neuquén |
| Phymaturus somuncurensis Cei & Castro 1973 | Somuncurá Plateau, Río Negro |
| Phymaturus spectabilis Lobo & Quinteros 2005 | ca. Ingeniero Jacobacci, Río Negro |
| Phymaturus spurcus Barbour 1921 | Huanulan, Río Negro |
| Phymaturus tenebrosus Lobo & Quinteros 2005 | ca. Cerro Alto, Río Negro |
| Phymaturus videlai Scolaro & Pincheira-Donoso 2010 | Buen Pasto (ca. Sarmiento), Chubut |
| Phymaturus zapalensis Cei & Castro 1973 | Laguna Teru (ca. Zapala), Neuquén |
- Phymaturus agilis Scolaro et al. 2008 is considered a synonym of P. spectabilis Lobo and Quinteros 2005, see Lobo et al. (2012). The type locality (ca. Esquel, Chubut) given in the type description of P. calcogaster Scolaro and Cei 2003 was corrected by Scolaro et al. (2005). The original description of P. desuetus Scolaro and Tappari 2009 was based on a unique specimen, no new report of this taxon was provided in literature.
Regarding biological and/or ecological characteristics of the patagonicus group, several contributions have been made over the last 10 years. Boretto et al. (2007), Boretto, Jahn, Fornés, Cussac, and Ibargüengoytía (2012) analyzed different aspects related to the reproductive biology of Phymaturus. Ibargüengoytía et al. (2005), Ibargüengoytía et al. (2008) and Cruz et al. (2009) studied thermoregulation in this genus (including members of the patagonicus group). Piantoni, Ibargüengoytía, and Cussac (2006) studied age and growth of a population of P. patagonicus (as P. tenebrosus). Debandi, Corbalán, Scolaro, and Roig-Juñent (2012) developed environmental niche models (ENMs) using Maxent software. Corbalán and Debandi (2013) studied the basking behavior in P. payuniae and Phymaturus roigorum and more recently, trophic segregation and daily patterns of activities (Corbalán & Debandi, 2014). Ibargüengoytía et al. (2016) tested the running performance of two species of Phymaturus related to volcanic eruptions and ashes deposition. Despite the amount of new knowledge with respect to different biological aspects of this group, there is still a lack of rigorous comparative analyses that would be useful in answering evolutionary questions.
With regard to phylogenetic relationships within the patagonicus group there are four previous studies: Lobo and Quinteros (2005), Lobo, Abdala, et al. (2012), Morando et al. (2013), and Corbalán, Debandi, Scolaro, and Ojeda (2016). Lobo and Quinteros (2005) analyzed a morphological data set of 133 characters and 22 terminal taxa of the whole genus, (11 species belonging to the patagonicus group). Later, Lobo, Abdala, et al. (2012) included 206 characters and 36 terminal taxa (17 species for the patagonicus group). Morando et al. (2013) sampled 27 of the 38 currently recognized species of Phymaturus and 22 candidate species using two mitochondrial genes, four protein coding nuclear genes, and seven anonymous nuclear loci, presenting the first comprehensive phylogenetic hypothesis with DNA sequences for the genus Phymaturus. Recently, Corbalán et al. (2016) studied the mitochondrial locus cytochrome oxidase I (COI) for 18 described species and two unnamed populations to test if this DNA marker would be able to discriminate species of the patagonicus group. Based on the genetic marker they analyzed, they questioned the specific status of P. excelsus and P. spectabilis.
In this study, we present a total evidence analysis (TEA) of the P. patagonicus group including all information available (DNA sequences and morphology) and new original data to find out: (i) which monophyletic, well-supported groups are recovered, (ii) the morphological features that distinguish each clade, and (iii) the cladistic information that each DNA and morphological partition provides to the TEA.
2 MATERIALS AND METHODS
Only three species of those recognized in literature were not included in this study, due to lack of information (no morphology nor DNA sequence data): P. desuetus, P. sinervoi, and P. curivilcun.
2.1 Morphological characters
We studied the morphology of 427 individuals representing 21 recognized species (most species of the group with the exception of P. camilae, and P. rahuensis). Data of these materials can be found in Appendix 1. The morphological data matrix includes 254 characters. In the present study, character lists of Lobo, Abdala, et al. (2012) and Lobo et al. (2016) were revisited across the patagonicus group – 38.7% of those characters involves informative characters within the patagonicus group (98 characters). Continuous characters were coded and scored following the range method of Goloboff, Mattoni, and Quinteros (2006) as in previous studies (Lobo, Abdala, et al., 2012; Lobo & Quinteros, 2005; Lobo et al., 2016). Fifty-three characters are continuous and 200 characters are discrete: 28.8% of characters are of color pattern, 22.5% squamation, 19.8% morphometric, 11.5% skeletal, and 17.4% miscellaneous (scale organs, precloacal pores, axial muscles, sulcus spermaticus pigmented, visceral characters, chromosomes, fecundity, salt excretion, etc.). All skeletons are wet preparations made following the standard clearing and staining techniques described in Wassersug (1976), whereas R. Etheridge skeletal collection (REE-SDSU) are dry skeletons. Binary polymorphisms were treated as ‘scaled’ polymorphic species, with an intermediate state ‘1’ in an ordered series between species without that state (0) and species with that state (2) (Wiens, 2000). Multistate polymorphisms were scored in each case, indicating all states present for each taxon. We selected 11 measures following Laurent (1986) and eight measures from Lobo, Abdala, et al. (2012). Continuous characters were analyzed and ranges were made for each individual character for each species. TNT (Goloboff, Farris, & Nixon, 2003; Goloboff, Farris, Källersjö, et al., 2003) uses ‘Farris optimization’ (Farris, 1970) to estimate distances and costs among ranges: when ranges between two terminals overlap, TNT assumes zero cost. In this study, we entered ranges for continuous data into the program, considering means ± SDs as ranges for any continuous character. We standardized every continuous character by dividing the minimum and maximum value of each species by the maximum value found among species, thus forming a new range for each species that varies between 0 and 1. Then, we multiplied this range by ten. In this way, the costs of transformations among states of any continuous character were estimated by trying to make them proportional to those of a discrete character.
2.2 DNA data sets
In this study, we added 26 sequences of Phymaturus to those recorded at the Genbank: a fragment of NADH dehydrogenase subunit 4 gene (ND4) for 20 terminals and a fragment of cytochrome b gene (Cytb), 12S ribosomal RNA gene (12S) and oocyte maturation factor Mos gene (C-mos) for P. videlai and P. castillensis; our DNA sequence data contain 20 of the 24 species currently recognized within the P. patagonicus group. The genomic DNA was extracted from 96% ethanol-preserved tissue samples (liver or muscle) using the phenol/chloroform method (Sambrook & Russell 2001). The DNA markers were amplified following standard polymerase chain reaction (PCR) procedures in reactions of 25 μl: 1 μl sample (approximate 10 ng/μl), 1 μl each primer (10 μmol/L), 17.5 μl distilled water, 2.5 μl buffer (10×), 1.5 μl MgCl (50 nmol/L), 0.5 μl de dNTPs (50 nmol/L), and 0.25 μl de Taq polymerase. Thermal profiles were: 2 min initial denaturation at 94°C, 40 cycles of 30 s at 94°C, 30 s of annealing at 43°C for Cyt b, 58°C for 12S, 54°C to C-mos and ND4, and 2 min at 72°C of extension, followed by a final extension of 6 min at 72°C. The primers used were G73 (5′-GCGGT AAAGC AGGTG AAGAAA-3′) and G78 (5′-AGRGT GATRW CAAAN GARTA RATGTC-3′), for ~ 420 bp of C-mos nuclear fragment (Saint, Austin, Donnellan, & Hutchinson, 1998); ND4 (5′-CACCT ATGAC TACCA AAAGC TCATG TAGAAGC-3′) and Leu (5′-CATTA CTTTT ACTTG GATTT GCACCA-3′), for ~ 740 bp of ND4 fragment (Arévalo, Davis, & Sites, 1994); 12e (5′-GTRCG CTTAC CWTG TTACG ACT-3′) and tPhe (5′-AAAGC ACRGC ACTGA AGATGC-3′), for ~ 800 bp of 12S fragment (Wiens, Reeder, & Montes de Oca, 1999); and GLUDGL (5′-TGACT TGAAR AACCA YCGTTG-3′) and CB3–3′ (5′-GGCAA ATAGG AARTA TCATTC-3′), for ~ 800 bp of Cytb (Palumbi, 1996). Sequencing reactions were run using Big Dye Terminators 3.1 in an ABI 3130 Genetic Analyzer (Applied Biosystems). All samples were sequenced in both directions and the contigs were made using DNA BASER 3 (Heracle BioSoft, Pitesti, Romania). We also included 39 sequences of Cytb, 16 of cytochrome c oxidase 1 (COI), 39 of 12S, 38 of C-mos, 34 of neurotrophin 3 (NTF3), 35 of pinin (PNN), 38 of prolactin receptor gene (PRLR) and 217 sequences of seven anonymous nuclear loci (Phy38, Phy41, Phy60, Phy64, Phy84, Phy87, Phy89) that were recorded and uploaded by Morando, Avila, and Sites (2003), Breitman, Avila, Sites, and Morando (2011), Morando et al. (2013), Olave, Avila, Sites, and Morando (2014), Lobo et al. (2016), and Corbalán et al. (2016). In total, we used 460 sequences of Phymaturus, and 46 loci were treated as missing data. Five species of Liolaemus and five of the Phymaturus belonging to the Phymaturus palluma group were added as outgroups, as detailed in Appendix 2.
The composition of DNA sequence data is described in Appendix 2. Sequences were edited with BioEdit (Hall, 1999). Each gene was aligned with ClustalW (Thompson, Higgins, & Gibson, 1994) and run in BioEdit under default parameters, and subsequently concatenated with SequenceMatrix 1.7 (Vaidya, Lohman, & Meier, 2011). In total, 10,631 bp were analyzed.
2.3 Phylogenetic analyses
A total of 10,885 characters and 44 terminal taxa were analyzed. We included nine outgroups: Liolaemus archeforus, Liolaemus buergeri, Liolaemus kingii, Liolaemus lineomaculatus, and Liolaemus petrophilus, and species of the palluma group: Phymaturus mallimaccii, P. palluma, Phymaturus punae, and Phymaturus vociferator. The ingroup was formed by 35 taxa including almost all taxa described in the literature, as well as the candidate species of Morando et al. (2013). We were not able to obtain morphological or DNA sequence information of P. curivilcun, P. desuetus and P. sinervoi. Analyzed character blocks are shown in Table 2; for each one, the number of informative character/positions, number of obtained trees, average support, and nodes of the TEA recovered are reported.
| Block | Source of data | Number of charactersa | Informative characters | Tree length | Number of trees | Averageb support | Congruencec nodes recovered |
|---|---|---|---|---|---|---|---|
| All (SP) | 10,885 | 654 | 4,126.419 | 1 | 66.9 | ||
| 4–18 | DNA (SP) | 10,631 bp | 556 | 2,926 | 3 | 65.2 | 21/32 (65.6%) |
| 4–18 | DNA (BI) | 10,631 | 556 | – | 1 | – | 28/32 (87.5%) |
| 1–3 | Morphology | 254 | 98 | 63.18266d | 1 | 24.8 | 6/21 (28.5%) |
| 4 | 12S | 862 bp | 76 | 828 | 50 | 51.8 | 8/32 (25%) |
| 5 | Cytb | 829 bp | 130 | 862 | 2 | 55.1 | 14/32 (43.7%) |
| 6 | C-mos | 555 bp | 4 | 56 | 29 | 19.9 | 0/32 (0.0%) |
| 7 | NTF3 | 541 bp | 2 | 15 | 3 | 10.6 | 1/30 (3.3%) |
| 8 | PNN | 1,004 bp | 5 | 88 | 5 | 23.9 | 2/30 (6.6%) |
| 9 | PRLR | 536 bp | 12 | 81 | 4,676 | 16.6 | 1/30 (3.3%) |
| 10 | PHY38 | 735 bp | 10 | 61 | 4 | 17.1 | 1/30 (3.3%) |
| 11 | PHY41 | 613 bp | 17 | 40 | 3 | 19.0 | 1/30 (3.3%) |
| 12 | PHY60 | 936 bp | 22 | 87 | 864 | 23.9 | 2/30 (6.6%) |
| 13 | PHY64 | 631 bp | 37 | 91 | 2,268 | 16.7 | 1/30 (3.3%) |
| 14 | PHY84 | 616 bp | 36 | 92 | 1,680 | 16.9 | 1/30 (3.3%) |
| 15 | PHY87 | 737 bp | 8 | 29 | 5 | 9.0 | 0/29 (0.0%) |
| 16 | PHY89 | 632 bp | 18 | 65 | 15 | 15.7 | 0/29 (0.0%) |
| 17 | ND4 | 746 bp | 102 | 606 | 9 | 41.3 | 8/21 (38.1%) |
| 18 | COI | 658 bp | 77 | 247 | 1 | 23.8 | 6/15 (40.0%) |
| Nuclear | 7,536 bp | 167 | 873 | 1,076 | 38.1 | 3/32 (9.4%) | |
| Mit | 3,095 bp | 389 | 2,255 | 4 | 66.7 | 12/32 (37.5%) |
- SP, Strict parsimony; BI, Bayesian Inference.
- a Total length of each alignment matrix.
- b Average of tree support measured applying symmetric resampling (frequency differences).
- c Percentage of nodes those are congruent with the total evidence analysis. When several fundamental trees are obtained, comparison is made with the combinable components consensus tree (semi-strict).
- d Fit value result of the analysis applying “implied weights”.
We performed several phylogenetic analyses: a TEA (applying strict parsimony), the same analysis but considering gaps as a fifth state, a Bayesian analysis (only for the DNA sequence data), a strict parsimony analysis for DNA data, and a parsimony analysis for each partition of this study (Table 2). (i) The TEA was performed by applying strict parsimony in TNT (Tree Analysis using New Technology; Goloboff, Farris, & Nixon, 2003; Goloboff, Farris, Källersjö, et al., 2003). We used TNT as it is the only software that allows for the analysis of continuous and discrete character blocks at the same time, with continuous characters scored as ranges. We made a ‘traditional search’ applying tree bisection and reconnection (TBR), with 10,000 replications (saving 20 trees per replication). (ii) An additional analysis of total evidence was performed in the exact same way of the first one but gaps treated as fifth state. (iii) The DNA sequence partition (all markers together) was also analyzed by Bayesian Inference in order to compare our results with alternative hypotheses reported by Morando et al. (2013). To find the best-fitting model for the ingroup, we used the Akaike information criterion in jModelTest 2 (Darriba, Taboada, Doallo, & Posada, 2012) without a partition matrix. The selected model was GTR + G + I. Bayesian analyses were conducted with BEAST 1.8 (Drummond, Suchard, Xie, & Rambaut, 2012) using the tree prior Yule process, with a randomly generated starting tree. Default values were used with the ‘Auto Optimize’ option. We computed 50,000,000 generations, sampled every 5,000 generations, after which we examined the stationarity of parameters using TRACER 1.5. All ESS values were >200. The maximum clade credibility tree was computed with TREE ANNOTATOR 1.8, and the first 20% of the samples were discarded as burn-in. (iv) A strict parsimony analysis for the all the DNA sequence data evidence was made using TNT. (v) Separate analyses of the different DNA data sets (15 markers; Table 2) were performed using strict parsimony with gaps treated as missing entries (TNT). For analyzing the morphology data set, we used the implied weights method (Goloboff, 1993). This method allows for weighting against homoplasy and is recommended for morphological characters because it improves clade support (Goloboff, Carpenter, Arias, & Miranda Esquivel, 2008). Furthermore, in a previous study (Lobo, Abdala, et al., 2012), congruence with a previous molecular genetic analysis (Espinoza et al., 2004) was increased by using this method. In Lobo et al. (2016), we found that when running morphology using implied weights, the resulting topology recovers more nodes of the TEA analysis than when not using this method. The criterion of weighting against homoplasy has been previously presented by Farris (1969, 1983), and further discussion and analysis of this criterion are found in Goloboff (1995, 1997).
Support for individual nodes was assessed with symmetric resampling (Goloboff, Farris, & Nixon, 2003; Goloboff, Farris, Källersjö, et al., 2003) using 1,000 replicates, with 33% removal and reporting frequency differences (GC).
3 RESULTS
3.1 The total evidence analysis
The combined analysis recovered only one tree of 4,126.419 steps in length (Figure 1). The indistinctus clade was the most basal within the patagonicus group (Symmetric Resampling = 96), and the monophyly with support of the spurcus clade was also recovered (SR = 79), but manuelae does not belong to this clade as it was in the morphological analysis of Lobo, Abdala, et al. (2012). In both analyses (gaps as missing entries and gaps as fifth state), the indistinctus and payuniae (SR = 93) clades were recovered and with the highest supports.
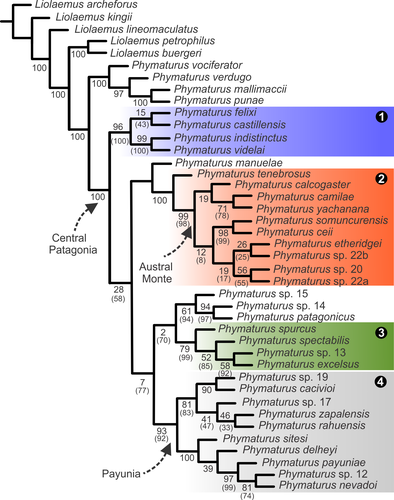
The somuncurensis clade was also recovered and part of Morando's calcogaster group (P. yachanana, P. camilae and P. calcogaster) form a sister taxon of the rest of the somuncurensis clade. It can be clearly seen that together all these species form a natural group: a more specious somuncurensis clade containing P. calcogaster, P. yachanana, P. camilae, and P. tenebrosus as well (SR = 100). Phymaturus patagonicus, sp. 14 and sp. 15 of candidate species of Morando et al. (2013) are not related to this group in any analysis. The calcogaster group (sensu Morando et al., 2013) is paraphyletic according to this study. In two of the three Morando's analyses, some terminals of their calcogaster group are found nested within of the currently redefined somuncurensis clade (see below) and others are recovered related to the spurcus clade.
The position of P. patagonicus and the other two Morando terminals (P. sp. 15 and P. sp. 16) remain enigmatic, they are recovered related to the spurcus clade, but the support is very weak. The support of the spurcus clade in the gaps as fifth state analysis was quite weak, yet better in the gaps as missing entries analysis (79%). Phymaturus manuelae is sister taxon of the somuncurensis clade but lacking support. Basal relationships among these four clades remain not well-supported and require further studies. Phymaturus manuelae is a floating taxon that introduces incongruences in the analysis. In fact, when we inactivated this terminal, all deep nodes (basal relationships) improved their support significantly (see Figure 1).
The P. indistinctus clade (node 1) is formed by P. castillensis, P. felixi, P. indistinctus, and P. videlai. It is supported by five continuous characters (char. 7: increase number of scales in contact with interparietal; char. 14: decrease number of scales in contact with nasal; char. 26: increase interorbit distance/head length ratio in females; char. 30: increase of abdominal width/SVL ratio in males; char. 35: increase of the number of precloacal pores in males) four discrete characters (char. 127: 1–>2 belly color of females changing from yellow to orange; char. 164: 0–>1 pattern on dorsum of neck and occipital regions formed by black transversal bars (“star” pattern); char. 171: 1–>0 lack of dorsal melanism on the neck; char. 218: 1–>0 neural spines inconspicuous) and 65 DNA changes: 12S (10 changes); Cytb (13 changes); NTF3 (only one change); PNN (only one change); PRLR (two changes); Phy41 (two changes); Phy60 (two changes); Phy64 (17 changes); Phy84 (five changes); ND4 (three changes); COI (nine changes). Previously, Roig-Juñent, Domínguez, Flores, and Mattoni (2006) had described endemism areas for the “South American arid lands”. Phymaturus clades geographical distributions match very well with that division, as was remarked in a previous article (Lobo et al., 2016). The indistinctus clade is endemic of central Patagonia (Figure 2, Roig-Juñent et al., 2006); the all species recognized at this time live in the south-central province of Chubut.

The somuncurensis clade (node 2) is formed by P. calcogaster, P. camilae, P. ceii, P. etheridgei, P. somuncurensis, P. sp. 20, P. sp. 22a, P. sp. 22b, P. tenebrosus, and P. yachanana. All species of the somuncurensis clade are distributed in Austral Monte. The somuncurensis clade exhibits few morphological apomorphies, as one continuous character (Char. 10: increase in number of the upper ciliaries scales); two discrete characters (char. 169: 01–>2 internasal region becomes concave in the middle (Lobo, Abdala, et al., 2012; Figure 3f); char. 225: 0–>1 pigal scales of the posterior half of the precloacal region similar size in both sexes) and 46 DNA changes: 12S (15 changes); Cytb (30 changes); Phy84 (only one change).
The spurcus clade (node 3) includes P. excelsus, P. sp. 13, P. spurcus, and P. spectabilis, and lives in a restricted area in the northwestern Austral Monte. It is supported by four continuous characters (char. 12: the supralabial scale upturned from its file tends to be one of the posterior ones); char. 13: increase of subocular fragmentation (number of subocular scales); char. 14: increase of scales in contact to nasal; eight discrete characters: (char. 122: 0–>1 interparietal color: white remarked from the rest of dorsal head coloration; char. 130: 0–>2 number of ocelli in dorsal patterns (6–8); char. 131: 0–>1 occurrence of light brown morphs (not sexually dimorphic); char. 165: 1–>2 number of enlarged scales on the anterior border of the auditory meatus (fixed); char. 218: 1–>0 neural spines inconspicuous (among the axial musculature); char. 223: 0–>1 Granular scales among dorsal tibials; char. 231: 1–>0 lack of ontogenetic change of dorsal ocelli; char. 235: 0–>1 scale organs on rostral scale absent) and 16 DNA changes: 12S (only one change); Cytb (six changes); PRLR (only one change); Phy38 (only one change); Phy84 (two changes); ND4 (seven changes); COI (five changes).
The payuniae clade (node 4) includes P. delheyi, P. cacivioi, P. nevadoi, P. sp. 12, P. rahuensis, P. sitesi, P. sp. 17, P. sp. 19, P. payuniae, and P. zapalensis.
It is formed by two subclades, the northern subclade (Mendoza and Neuquén provinces), including P. delheyi, P. nevadoi, P. payuniae, P. sp. 12 and P. sitesi and the southern subclade (Neuquén and Río Negro provinces), including P. cacivioi, P. rahuensis, P. sp. 17, P. sp. 19, and P. zapalensis. Only P. cacivioi and P. sp. 19 are inhabitants of a different endemism area, the Austral Monte. The payuniae clade was recovered with several apomorphies, six continuous characters (char. 5: increase of lateral number of neck scales; char. 9: decrease number of superciliaries; char. 19: decrease of head length/SVL males ratio; char. 28: increase internasal distance/head length ratio in females; char. 32: increase of females tibia length/snout-vent length ratio; char. 52: tends to be the fifth superciliary scale imbricated in both extremes and three discrete characters; char. 120: 0–>1 acquisition of a dark lateral (flank) band; char. 177: 1–>0 loss of ventral pattern of tails with scattered small dark spots; char. 192: 2–>1 occurrence in certain individuals of unicuspid premaxillary teeth and 14 DNA changes: 12S (only one change); Cytb (three changes); Phy64 (two changes); Phy87 (five changes); Phy89 (two changes); ND4 (only one change).
The southern subclade of the payuniae clade is supported by the following apomorphies: char. 12: the upturned supralabial tends to be the fifth or sixth; char. 14: decrease the number of scales in contact with the nasal scale; char. 127: 1–>2 in males belly color changes from yellow to orange; char. 214: 1–>2 the external wall of rectum turns deeply striated; char. 248: 0–>1 occurrence of irregularly divided row of precloacal pores (not in the middle) and 12 DNA changes: 12S (four changes); Cytb (three changes); and ND4 (five changes). The northern subclade of the payuniae clade is supported by the following apomorphies: char. 6: decrease in the number of gular scales char. 9: decrease of the number of superciliaries; char. 21: head width/SVL ratio in males decrease; char. 23: head height/SVL ratio in males increase; char. 146: 1–>2 dorsum and sides of heads in females become brown/gray with thin white spots; char. 211: 0–>1 tails spotted white similar to dorsum of trunk; and 46 DNA changes: 12S (10 changes); Cytb (14 changes); ND4 (five changes); PRLR (two changes); Phy38 (two changes); Phy41 (two changes); and ND4 (11 changes).
Our TEA topology shows central Patagonia as the most common area of distribution for members of the patagonicus group. The basal indistinctus clade inhabits this area, and two significant diversifications happened in the history of the group: one endemic of the Austral Monte area and the other endemic of the Payunia area (Figure 1). Until investigators perform an ancestral areas analysis or other biogeographic explicit methods, this observation will remain as hypothetical.
3.2 The total evidence analysis performed considering gaps as a fifth state
The topology recovered (length 4,031.324) has lower values of support compared to the former analysis. In this case, P. manuelae and the spurcus clade are the most basal members of the patagonicus group, but they lack support. The somuncurensis clade, payuniae and indistinctus clades are well-supported as in the “gaps as missing entries analysis”, but the spurcus clade has very weak support (30%). Relationships among the four clades in this run also exhibit low values of support.
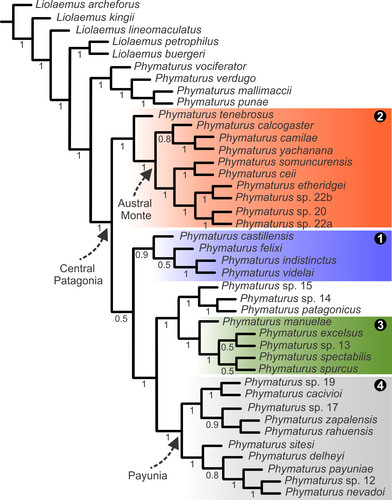
3.3 Bayesian inference (Figure 3)
In this hypothesis, the somuncurensis clade is the most basal, but again the support of basal nodes is weaker than terminal ones. The indistinctus clade is basal to the clade formed by P. patagonicus (and more closely related candidate species) and the spurcus and payuniae clades. In this topology, P. felixi is sister taxon of the pair formed by P. indistinctus and P. videlai. Phymaturus manuelae is included in the spurcus clade and P. spectabilis is sister taxon of P. spurcus. The payuniae clade exhibits exactly the same topology of the TEA analysis. In this hypothesis, the calcogaster clade is not recovered, contrary to the concatenated nuclear loci analysis of Morando et al. (2013).
3.4 Only DNA analysis (strict parsimony)
DNA sequences superimpose over morphology, the “only DNA analysis” recovered almost the complete topology of the combined analysis (except two nodes). Three trees of maximum parsimony that varies in the position of P. manuelae were found (length = 2,926), in two trees as sister taxon of the clade formed by the spurcus clade, patagonicus and the payuniae clade, and in another tree as sister taxon of the somuncurensis clade. Additionally, P. calcogaster was recovered as sister taxon of the pair P. yachanana- P. camilae, or related to the rest of the somuncurensis clade, leaving P. tenebrosus and those two species in a basal position. Average support was almost the same as the combined analysis, but there were differences in support in certain nodes of the topology. Its support was slightly better in deep nodes, but still weak. Support of the spurcus clade was lower than in the combined analysis (that exhibit additional apomorphies from the morphological dataset). In that run, the indistinctus clade as basal clade for the entire patagonicus group was much better supported (60% versus 28%). As shown in Table 2 and Figure 4, Cytb, COI and ND4 are the most informative datasets and when the three were analyzed separately, several nodes were recovered (43.7%, 40%, and 38.1%, respectively) of the TEA. 12S also provided useful information (25%) but nuclear markers had a very low contribution (see Table 2).
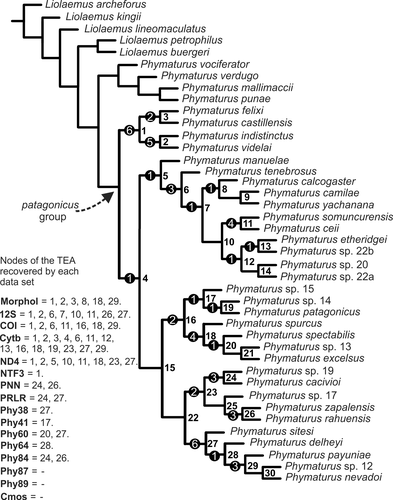
3.5 All partitions analyzed separately (Figure 4)
Several additional analyses to those indicated above were performed; all partitions were analyzed separately and a resume of that analyses are shown in Table 2. In Figure 4, we indicate over the TEA topology how each independent data set contributes to node recovery.
3.6 Biogeography
Morrone (2015) describes the Andean region as divided into four subregions. One is the Patagonian subregion, formed by one Patagonian province and five subprovinces: Central Patagonian, Fueguian, Payunia, Subandean, and Western Patagonian. The indistinctus clade is endemic of the Chubut District of central Patagonia while the somuncurensis clade is distributed in the Austral Monte area (Roig-Juñent et al., 2006). This last area is included in the Monte Province of Morrone (2015). The two subclades recovered here for the payuniae clade are distinctive of the two districts of Payunia (Morrone, 2015): Payunia Norte and Payunia Sur, respectively. The spurcus clade matches most of its distribution with Western Patagonia in Río Negro Province (Morrone, 2015; Roig-Juñent & Debandi, 2004; and Roig-Juñent et al., 2006) and in part in the extreme north of central Patagonia. On a smaller scale, in 150 square km in northwestern Río Negro Province, species of different subclades coexist and the borders of those proposed endemism areas are not very clear. Phymaturus cacivioi is nested within the southern subclade of the payuniae clade. If we propose a perfect match with recognized districts, the Payunia Sur district should be considered as reaching a slender zone of northwestern Río Negro Province (see map Lobo & Nenda, 2015). It is also difficult to assign an area to P. manuelae (discussed above) and P. sinervoi. This last species described for the Cari Laufquen plateau (Scolaro et al., 2012) is not far from P. cacivioi and P. ceii distributions, species living on the border between Austral Monte and Payunia. According to Corbalán et al. (2016), P. sinervoi is more closely related to P. etheridgei (COI parsimony analysis) or to P. ceii and P. somuncurensis (COI ML analysis). The distribution areas of P. etheridgei and P. camilae are also difficult to assign to any endemism because they inhabit the area close to the border between Austral Monte and central Patagonia.
4 DISCUSSION
4.1 Phylogenetic relationships
In this study, we performed individual runs of every block of data with the aim to recognize at which level those different sources of evidence provide phylogenetic information for this group of reptiles and/or are more influential in building that tree (Figure 4, Table 2). In our group under study, we are now able to remark that only a few of those sets included in the study are significantly informative. Mitochondrial markers (Cytb, COI, ND4, and 12S), when analyzed independently, exhibit greater support and also recover a larger number of nodes of the TEA (Table 2). Logically, when these genes are analyzed alone, a similar topology to the total evidence run is recovered. In our case, the TEA did not exhibit a lower average value of support than the separated analyses (Table 2). We use symmetric resampling and report frequency differences (GC) Goloboff, Farris, & Nixon (2003), Goloboff, Farris, Källersjö, et al. (2003). In our example, when we apply resampling only taking absolute differences into account (equivalent to regular jackknife or bootstrap reports) no node of the TEA is recorded below 50% support, thus masking the incongruence we find among gene trees (see nodes of the TEA recovered in individual runs in Table 2). Values of support reported here for the combined analysis (Figure 1) put on the table how reliable certain nodes are. In this case, 12/31 nodes (38.7%) exhibit significant contradictions among the different combined datasets.
Our analysis combining all available evidence (DNA sequences and morphology) resulted in a tree that exhibits significant congruence with BEST (all genes) and Bayesian Inference (all genes) of Morando et al. (2013). In this section, we comment on those nodes and relationships that resulted incongruent, as they will most likely need to be studied further and more in depth in the future. In our analysis (Figure 3), the indistinctus clade is recovered as the most basal, and the somuncurensis clade as sister taxon of the pair formed by the spurcus and payuniae clades. In the Bayesian Inference (all genes) of Morando et al., (2013), the recovered spurcus clade (as named here) was not found as a natural group, due to the fact that a couple of their members were more related to the rest of the patagonicus group. Their calcogaster clade here is split (not monophyletic) in Bayesian topology, similar to our TEA. In the BEST- all genes analysis of Morando et al. (2013), the spurcus clade is recovered monophyletic, and forms a politomy with the indistinctus clade and a clade formed by all other members of the patagonicus group. In addition, the calcogaster clade is recovered monophyletic (but with lower values of posterior probabilities than all others nodes of the tree). The different arrangement of basal relationships between the Morando et al. (2013) study and the present one (which is the most basal group, spurcus or indistinctus clade) is probably affected by how both analyses were rooted. In our case, we included several Liolaemus species representatives of the major clades of the genus sister taxon of Phymaturus, in addition to species of the P. palluma group. In Morando et al. (2013, p. 700), each group (patagonicus and palluma) was analyzed separately using one taxon of the other group for rooting the tree: P. patagonicus as outgroup of the palluma group and P. mallimaccii (a member of a terminal lineage within the palluma group) as outgroup of the patagonicus group. Using different outgroups clearly affects results. When running our data set making inactive those data sets not included in Morando et al. (2013) (consisting of morphology, COI, and ND4), and using P. mallimaccii as outgroup, we found a different basal arrangement, as this time the somuncurensis clade was the most basal subclade. We recovered a topology quite similar to our Bayesian tree of the present study (Figure 3), with only a few differences (the relative position within their clades of P. castillensis, P. felixi, P. spurcus, and P. excelsus). Only in the Bayesian – all genes analysis of Morando et al. (2013, Figure 5), P. cacivioi (= sp. 18) and P. sp. 19 are sisters to the payuniae clade. In any case, if the definition of the payuniae clade is changed to just one node more basal in that tree, these species would be included, but in all other analyses performed by the authors, these sister species are not recovered as sisters of the payuniae clade. In the BEST- all genes analysis (see Figure 6 in Morando et al., 2013), this pair of species is recovered outside the payuniae clade, thus forming a polytomy with that group, the somuncurensis clade and P. tenebrosus. In our analysis (Figures 1 and 3), P. cacivioi and P. sp. 19 are well-nested within the payuniae clade as sister taxa of P. zapalensis + P. rahuensis + P. sp. 17. This southern subclade is well-supported (81%) by several apomorphies (five morphological and twelve DNA changes).
Our Bayesian Inference recovered a tree with a few differences from our TEA (performed using strict parsimony) that were previously mentioned in the results section. With respect to hypotheses found by Morando et al. (2013), substantial incongruences exist, and our Bayesian analysis resembles our TEA analysis (parsimony) more than those trees.
Morphological characters turned out to be less informative in the patagonicus group than for the palluma group (see Lobo et al., 2016) when they were analyzed as a separated dataset. In Lobo, Abdala, et al. (2012), most clades obtained within the patagonicus group were also recovered in Morando et al. (2013), using exclusively DNA information. The increase in the number of terminals included with less morphological data scored may be the most logical explanation as to why morphology in this analysis was less informative than in the palluma group study (Lobo et al., 2016). Only nine characters of the 45 new ones described for Phymaturus (Lobo et al., 2016) are informative for the patagonicus group (20%). At the same time, the composition of the group was doubled: 35 terminal taxa versus 17 studied in Lobo, Abdala, et al. (2012). Half of these other 18 terminals have no morphological characters scored, and several have only partial information. The morph data set of Lobo, Abdala, et al. (2012) is a subsample of the actual composition of the group, but with fully scored morphology. In any case, morphology in the case of the patagonicus group looks to be naturally less informative than that of the palluma group. In the first publications analyzing phylogenetic relationships (Lobo & Quinteros, 2005), the patagonicus group exhibited significantly less support than its sister group (subsample of characters at that time of 133 characters). In fact, the patagonicus group turned out not to be monophyletic in certain runs. The total number of morphological characters studied is 254; informative characters within patagonicus group are 97, while 202 resulted informative for the palluma group.
As was pointed out in Lobo et al. (2016), applying implied weights to the morphological data sets increases the congruence of this individual analysis with the TEA. In this case, the same effect is found: no node of the TEA is recovered running morphology on its own unless we use implied weights. As we show in Figure 4, morphology (implied weights) recovers a similar number of nodes of the TEA as mitochondrial genes, with the exception of Cytb partition which was the most informative one.
4.2 Rogue taxa and contradictory evidence
In these analyses, neither with the TEA run nor with the Bayesian analysis were we able to get well-supported basal nodes. This is the main reason why we are hesitant to formulate a taxonomic proposal of group, clades, subclades and lineages as we did for the palluma group (Lobo et al., 2016). Curiously, a very enigmatic species, P. manuelae (“enigmatic” for many reasons: low abundance in the field, strange combination of morphological characters and contradictory DNA information) lowers support when present. According to Wilkinson (1994, 1996) rogue taxa are those that assume a different position in resultant trees bringing lower support values and provoking polytomies in consensus trees. Aberer and Stamatakis (2011) considered that the occurrence of rogue taxa would be caused because different phenomena: general lack of phylogenetic signal (because of an excessive proportion gaps in the alignment, or either too high or too low mutation rates), ambiguous phylogenetic signal because of mislabeled or erroneous sequences (specifically chimeric sequences) or horizontal gene transfer. Most investigators choose pruning rogue taxa from their analyses (Aberer, Krompass, & Stamatakis, 2013), as we experimented with in this study with P. manuelae. We do not have an explanation of what happened with P. manuelae but have some remarks below. Phymaturus manuelae is recovered in the TEA and found as sister taxon of P. tenebrosus and the whole somuncurensis clade, yet without support. Our Bayesian tree recovered this species as the most basal of the spurcus clade (Figure 3). This species was found as the most basal species of the spurcus clade in the morphological study of Lobo, Abdala, et al. (2012) and Lobo and Nenda (2015). This taxon can exhibit dorsal ocelli, more often in females, but its number between shoulder and thighs (nine or more) is higher as in species of the indistinctus clade or certain somuncurensis clade members (the spurcus clade exhibit larger and fewer dorsal ocelli). Phymaturus manuelae do not exhibit brown morphs and white interparietal scale like the spurcus clade. Analyzing all partitions separately, P. manuelae is found basal to all the patagonicus group species in two nuclear markers (PNN, Phy84), two mitochondrial loci (12S and Cytb) and the nuclear markers C-mos, Phy87, and PRLR locate this species in the spurcus clade. The updated morphological data set also put this taxon in the spurcus clade. Contradicting these hypotheses, ND4 and NTF3 include P. manuelae in the somuncurensis clade or related to species of that group. This contradictory information makes this species a floating terminal, consequently affecting the support of deep nodes in the tree (Figure 1). In fact, when P. manuelae is taken out of the analysis, the support of those nodes improves.
We proved that by not including this taxon we would get better support in the TEA run (Figure 1). In any case, at this time we prefer to be conservative and not present a taxonomic proposal. In the TEA analysis (Figure 1), P. patagonicus is recovered with two other terminal taxa (sp. 14 and sp. 15 of Morando et al., 2013), altogether closely related to the spurcus clade but with very weak support, recovering the same relationship with the highest support under the Bayesian Inference (Figure 3). This species is found around a restricted area close to Dolavon, near the Chubut river (43°28′S, 66°09′W) in eastern Chubut Province, while the spurcus clade is distributed in western-northwestern Río Negro, at 377 km in straight line (and between them there are populations and species of the somuncurensis clade). Geographically, this taxon is distributed more closely to members of the somuncurensis clade (P. calcogaster, P. yachanana, P. somuncurensis). But is there any evidence linking P. patagonicus to the somuncurensis clade? If we analyze the information provided by the different data sets studied (independent analyses of each marker), three mitochondrial markers relate P. patagonicus to the spurcus clade (12S, Cytb, and COI). One marker (ND4) recovers it nested within the payuniae clade, but seven other nuclear markers (C-mos, PNN, Phy38, Phy41, Phy60, Phy84, Phy89) recover this species nested within the somuncurensis clade or in certain trees. When the somuncurensis clade is not recovered, P. patagonicus is found closely related to species of that clade. The analysis of the morphology data set alone recovers P. patagonicus as the basal species of the somuncurensis clade. If we accept the relationship between the spurcus clade and P. patagonicus, because of the geographic gap that exists between them, it is necessary to postulate further explanations including a long-range dispersal (which is unlikely because the restricted habitat preferences of these animals) or the extinction of other species between them. If we consider P. patagonicus as related to the somuncurensis clade, because of its adjacency, no further evidence would need to be provided. The information provided by nuclear markers and morphology, rather than mitochondrial information, supports this relationship.
The phylogenetic position P. tenebrosus is also slightly controversial. Even when the TEA analysis recovers this taxon as the most basal of the somuncurensis clade and with the highest support, there are certain inconsistencies that suggest the need for further research. In Lobo, Abdala, et al. (2012), it is included in clade D (most of those species make up the payuniae clade of Morando et al., 2013). In Lobo and Nenda (2015), P. tenebrosus is also recovered within the payuniae clade. Current information collected from morphology put P. tenebrosus as sister taxon of P. cacivioi, since P. ceii is basal to them. Phymaturus tenebrosus exhibit (polymorphic) dark lateral band which is an apomorphy of the payuniae clade (but homoplastic found also in P. ceii and P. somuncurensis within the somuncurensis clade). The occurrence of brown morphs is an apomorphy of the spurcus clade, a character state that is also shared by P. tenebrosus. In addition, certain females of P. zapalensis, a member of the payuniae clade, can show a brown coloration similar to other species (Figure 5). Data partitions that recover P. tenebrosus within the somuncurensis clade or relate to different members of that clade are 12S, COI, Cytb, ND4, Phy38, PRLR, related or included in the payuniae clade by: Phy41, Phy84, and Phy89. Only C-mos recovers P. tenebrosus related to the spurcus clade. Both species—P. manuelae and P. tenebrosus—inhabit a complex biogeographic area, where four recognized endemism areas were identified (Morrone, 2015; Roig-Juñent et al., 2006), and meet altogether: the extreme south of Payunia (reaching southern Neuquén Province and the Limay river), Austral Monte which includes the Somuncurá plateau and adjacent areas, western Patagonia, and the extreme northwest of central Patagonia. Roig-Juñent et al. (2006) and Morrone (2015) built this proposal based on plant, arthropod and vertebrate distributions and it is clear that Phymaturus species distributions match widely with their delimitations, with the exceptions discussed above.

4.3 The taxonomic status of certain terminals, populations, and species included in this study
Because a recent publication on the patagonicus group involved the taxonomic status of certain species included in our analysis (Corbalán et al., 2016), we found the need to provide a short discussion to establish why we used this taxonomic composition and why we included those terminals in our present analysis. Corbalán et al. (2016) analyzed sequences of the COI obtained in the barcoding initiative (Barcode of Life Data Systems). Since they found shared haplotypes among certain terminal taxa, short genetic distances and individuals mixed up in their trees (NJ, MP and ML), they suggest the possibility that those populations are co-specific. They used only one marker (COI), which gives little evidence for assessing the taxonomic status of populations, according to Dupuis, Roe, and Sperling (2012) and Miralles and Vences (2013); there is always a discordance between species trees and gene trees (a differentiation made several years ago, Nei, 1987; Pamilo & Nei, 1988; Maddison, 1997; Page & Charleston, 1997; among others). Corbalán et al. (2016) considered in their conclusions that P. excelsus and P. spectabilis should be taken as synonyms of P. spurcus based on low COI distance and shared haplotypes. Yet, in equivalent results in other cases they do not come to the same conclusions: P. sinervoi individual with 0% distance with P. ceii and low distance between P. ceii and P. somuncurensis. When we review our complete data set, we find results that contradict and generate new questions. We found that P. excelsus, P. spectabilis, and P. spurcus have low distance or shared haplotypes in all mitochondrial markers (12S, COI, Cytb, ND4), as well as in various nuclear markers, but present differences in some nuclear loci. Between P. spurcus – P. excelsus and P. spurcus – P. excelsus, we found uncorrected pairwise distance of 0.0016 in Phy38 and 0.03 in Phy60, the same distance that exists between spurcus-payuniae (species belonging to different clades). In Phy87 the distance is 0.0016, equal to the pair spurcus-somuncurensis, but, for example, the pair spurcus-payuniae shows 0% divergence; in Phy84 the pair spurcus-excelsus shows 0.0019 of distance, equal to that found between spurcus-payuniae or spurcus-patagonicus or spurcus-somuncurensis (all of them belonging to different clades). These discordances must be studied in detail to explain which processes (e.g., introgressions, incomplete lineage sorting) are acting in the evolutionary history of this species group.
With the aim to provide more information regarding this categorization and to extend it to the whole group, it is important to say that brown morphs of P. spectabilis and P. excelsus are quite different (Figure 5). The P. spectabilis brown morph (without ocelli) exhibit a typical “spray” pattern along the dorsum that make a pair of dorsal longitudinal bands visible, in similar fashion to the typical pattern found in members of the Liolaemus elongatus group, while the brown morph of P. excelsus is homogeneous brown with light coloration, suggesting the formation of ocelli in the same dorsal places as the ocellated form. The brown morph of P. excelsus is very similar to P. spurcus, but no ocellated individuals of P. spurcus have ever been found. If J. Scolaro found newborns of P. spurcus with P. excelsus ocellated patterns (commented in Corbalán et al., 2016) such important evidence is necessary in their article as photos, collection numbers, corresponding haplotypes. We believe that full revisionary research involving morphological studies and the use of additional DNA markers across the distribution of these species is needed before proposing changes to the taxonomic composition of the patagonicus group.
ACKNOWLEDGEMENTS
We thank three anonymous referees for very useful suggestions. We thank S. Nenda, S. Valdecantos, A. Laspiur, J. Grosso, L. Cotichelli, S. Quinteros, D. Slodki, M. Quipildor, T. Hibbard, and C. Abdala for helping us in the field or with laboratory work, Dallas Jordan and Alex Gerrish for their revision of the language of this article. This study was supported by grants (FL) from CONICET Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina (PIP 0871, 0303) and CIUNSA Consejo de Investigaciones de la Universidad Nacional de Salta, Argentina. We thank the following colleages (and museums) for allowing FL to study specimens under their care over the last decade: E. Pereyra (Instituto de Biologia Animal, Universidad Nacional de Cuyo, Mendoza), F. Videla (IADIZA, Mendoza), E. Lavilla and S. Kretzschmar (Instituto de Herpetología, Fundación Miguel Lillo, Tucumán), J. Faivovich and S. Nenda (Museo Argentino de Ciencias Naturales, Buenos Aires), H. Núñez (Museo Nacional de Historia Natural, Santiago), A. Scolaro (IDEAUS, Pto. Madryn), R. Etheridge and T. Reeder (San Diego State University), J. Hanken and J. Rosado (Museum of Comparative Zoology, Harvard), J. Wiens (Carnegie Museum of Natural History, Pittsburgh), and J. McGuire (Museum of Vertebrate Zoology, Berkeley). We thank the fauna offices of Mendoza, Neuquén, Río Negro, and Chubut provinces for providing authorizations for our field research.
APPENDIX 1
Specimens of Phymaturus examined (427 individuals) representing 21 recognized species. Institutional acromyns: FML, Herpetological collection of Fundación Miguel Lillo, Tucumán, Argentina; IADIZA, Instituto Argentino de Investigaciones de Zonas Áridas, Mendoza, Argentina; IBA, Instituto de Biología Animal, Universidad Nacional de Cuyo, Mendoza, Argentina; JAS-DC, José Alejandro Scolaro–Diagnostic collection; MACN, Herpetological Collection of Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina; MCN-UNSa, Museo de Ciencias Naturales, Universidad Nacional de Salta, Salta, Argentina; MCZ, Museum of Comparative Zoology, Harvard University, Cambridge, USA; REE–SDSU, Richard E. Etheridge skeletal collection, San Diego State University, San Diego, California, USA; UNCo-PH. Universidad Nacional del Comahue, San Carlos de Bariloche, Río Negro, Argentina. Abbreviations: Department (Dept), National Route (RN), Provincial Route (RP).
Phymaturus cacivioi (n = 24)
MCN-UNSa 3895 (Holotype) male, 12.6 km SW of Mencué on RP No. 67 (40°30′53.90″S, 69°42′25.00″W, 1,140 m), El Cuy Dept, Río Negro Province, Argentina. Paratypes: MCN-UNSa 3888, MCN-UNSa 3890, MCN-UNSa 3894, MACN 44731 (ex MCN-UNSa 3896), MACN 44732 (ex MCN-UNSa 3897), MACN 44733 (ex MCN-UNSa 3898), MACN 44735 (ex MCN-UNSa 3935), MACN 44737 (ex MCN-UNSa 3938) females, MCN-UNSa 3936, MCN-UNSa 3889, MCN-UNSa 3891, MCN-UNSa 3892, MCN-UNSa 3899, MCN-UNSa 3901, MCN-UNSa 3902, MACN 44730 (ex MCN-UNSa 3893), MACN 44736 (ex MCN-UNSa 3937), MACN 44734 (ex MCN-UNSa 3900) males. MCN 3903–05 juveniles. All with same data as holotype.
Phymaturus calcogaster (n = 16)
MACN 39990–91 (paratypes), JAS-DC 799, 803,1096–97: Laguna de las Vacas, Telsen Dept, Chubut Province, Argentina; JAS-DC 1154–55, Bajo Amarillo, Telsen Dept, Chubut Province, Argentina. MCN-UNSa 4295–98, 4301–04, Laguna de las Vacas, southwestern end of the lake (42°29′54.60″S, 67°21′07.03″O, 651 m), Telsen Dept, Chubut Province, Argentina.
Phymaturus castillensis (n = 15)
IBA 869–1, 869–2, 869–3, NW Lago Colhué Huapi, Sierra Castillo, 1,000 m, Sarmiento Dept, Chubut Province, Argentina. MCN-UNSa 3960–64, 3967–69 3975–78 from Estancia La Juanita, Sierra del Castillo, near RP No. 24, 58 km NW of Sarmiento, Sarmiento Dept, Chubut Province, Argentina (45°08′11.30″S, 69°10′10.40″W, 405 m).
Phymaturus ceii (n = 21)
MCN-UNSa 910–18, RP No. 8, 17 km S of San Antonio del Cuy, 25 de Mayo Dept, Río Negro Province, Argentina. MACN 44738 (ex MCN-UNSa 3914), MACN 44739 (ex MCN-UNSa 3918), MACN 44740 (ex MCN-UNSa 3921), MACN 44741 (ex MCN-UNSa 3923), MACN 44742 (ex MCN-UNSa 3928), MACN 44743 (ex MCN-UNSa 3941), MCN-UNSa 3913, 3916, 3920, 3939–40, 3942, on RP No. 6 (40°20′47.1″S, 68°58′50.3″W, 1,194 m), El Cuy Dept, Río Negro Province, Argentina.
Phymaturus delheyi (n = 15)
MLP 2609 (holotype) MLP 2610–11, rocky environments of the northern Tromen Volcano massif, along Butacó Creek, on RP No. 37 (36°59′S, 69°59′W, 1,810 m), Pehuenches Dept, Neuquén Province, Argentina. MCN-UNSa 4932, 4970–74, 76, 80–84, RP No. 37 crossing of Arroyo Butacó (36°59′S, 70°00′W). Pehuenches Dept, Neuquén Province, Argentina.
Phymaturus etheridgei (n = 17)
FML 23495 (holotype) FML 23496–501 (paratypes), MCN-UNSa 4305, 07–08, 10, on RP No. 76, between Ingeniero Jacobacci and Moligüe (41°34′47.2″S, 69°23′33.0″W, 818 m), 25 de Mayo Dept, Río Negro Province, Argentina. FML 8435, MCN-UNSa 3109–13, 43 km N of Moligüe (41°35.880′S, 69°22.628′W), 25 de Mayo Dept, Río Negro Province, Argentina.
Phymaturus excelsus (n = 9)
MCN-UNSa 1582 (Holotype), MCN-UNSa 1590, RP No. 6, 1 km NW from Ojo de Agua (41°32′30″S, 69°51′33″W, 1,141 m), Ñorquinco Dept, Río Negro Province, Argentina. MCN-UNSa 1386 and 1388 (paratypes), MCN-UNSa 1385, 1387 from Ojo de Agua, Ñorquinco Dept, Río Negro Province, Argentina. MCN-UNSa 1587–88, no data.
Phymaturus felixi (n = 18)
MCN-UNSa 1280 (Holotype), MCN-UNSa 1279, 1281–83 (paratypes) RP No. 24 108 km S Paso de Indios, Paso de Indios Dept, Chubut Province, Argentina. MCN-UNSa 3979–91, RP No. 24 84.5 km S to Paso de Indios (44°27′10.5″S, 69°17′48.3″W, 734 m), Paso de Indios Dept, Chubut Province, Argentina.
Phymaturus indistinctus (n = 24)
IBA 666-1, (Holotype), IBA 666-2–3, 2 km W Lago Munsters, Las Pulgas (700–800 m), Sarmiento Dept., Chubut Province, Argentina. MCN-UNSa 1274–77, Las Pulgas, Sarmiento Dept, Chubut Province, Argentina. MCN-UNSa 3943–55. RP No. 20, 19 km W to Los Manantiales (45°27′S, 69°42′W, 669 m).
Phymaturus manuelae (n = 7)
UNCo-PH 201–02 (paratypes), JAS-DC 1251, 26 km W Comallo, adjacent to RN No. 23, Pilcaniyeu Dept, Río Negro Province, Argentina. MCN-UNSa 3929–30, 3932–33, between Pilcaniyeu and Las Bayas on RN1S40 (ex- RN No. 40; 41°12′11.1″S, 70°41′30.9″W, 1,014 m), Pilcaniyeu Dept, Río Negro Province, Argentina.
Phymaturus nevadoi (n = 17)
IBA 999 (three individuals, type series) Agua de la India Muerta, Macizo Nevado (1,750 m), Malargüe Dept, Mendoza Province, Argentina. MCN-UNSa 3647, 3652–64 on RP No. 186 (35°55′44.8″S, 68°32′36.7″W, 1,711 m), Malargüe Dept, Mendoza Province, Argentina.
Phymaturus patagonicus (n = 35)
MLP 778 (lectotype), MLP 777 (paralectotype), Chubut Province, Patagonia, Argentina. FML 10077–85, 1 km W from juction of RP No. 53 and RP No. 90, 2.2 km SW Meseta El Sombrero, Paso de Los Indios Dept, Chubut Province, Argentina. IADIZA 80, 40 km W Dolavon, 350 m, Gaiman Dept, Chubut Province, Argentina. IBA 783, IBA 785, 20 km W from Sombrero, Paso de Los Indios Dept, Chubut Province, Argentina. IBA 787, IBA 789, MCN-UNSa 1284–86, 40 km W Dolavon, Gaiman Dept, Chubut Province, Argentina. MCN-UNSa 1250–58, 1261, hills in front of El Sombrero, Paso de Los Indios Dept, Chubut Province, Argentina. SDSU 1980, 40 km WSW Dolavon, Gaiman Dept, Chubut Province, Argentina.
Phymaturus payuniae (n = 45)
IBA 769-2, 769-4–8, 769–10, 76912, 769–17, 769–20, 769–24, 769–26 (type series), Payún Plateau (2,000 m), 5 km from Volcán Payún Malargüe Dept, Mendoza Province, Argentina. IADIZA 87-8–9, 20 km SE Volcán Payún (1,800 m) Malargüe Dept, Mendoza Province, Argentina. MCZ 152079–81, basaltic rocks of the Payún Plateau, Malargüe Dept, Mendoza Province, Argentina. REE-SDSU 2330–32, 2339, SDSU 1981–84, 10 km SW base of Volcán Payún, Mendoza Province, Malargüe Dept, Argentina. MCN-UNSa 3648–51, 3665–79, on RP No. 183, 16 km S to Payún vulcano (36°40′20.8″S, 69°16′10.9″W, 1,737 m).
Phymaturus sitesi (n = 24)
MLP 2605 (holotype) 2606–08, rocky cliffs on the northeastern slope of Sierra de Auca Mahuida mountain (37°43′S, 68°55′W, 1,983 m), near Cerro de las Antenas, Auca Mahuida Natural Protected Area, Pehuenches Dept, Neuquén Province, Argentina. MCN-UNSa 4757–4774, 4792–93 Area Natural Protegida Auca Mahuida, from RP No. 6 (37°42′06.3″S, 68°51′29.5″W, 1,569 m) Pehuenches Dept, Neuquén Province, Argentina.
Phymaturus somuncurensis (n = 29)
IBA 470, IBA 472 (type series), MACN 37436–40, MCZ 156909, 170443–44, Laguna Raimunda, Meseta de Somuncurá, 9 de Julio Dept, Río Negro Province, Argentina. FML 1038, Laguna Raimunda, Meseta de Somuncurá (1,400 m) 9 de Julio Dept., Río Negro Province Argentina. IADIZA 212, Meseta de Somuncurá, Cerro Corona, 9 de Julio Dept., Río Negro Province, Argentina. IBA 507, 4, Laguna Raimunda, Meseta de Somuncurá, Río Negro Province, 9 de Julio Dept., Argentina. MACN 37431–35, 2 km N Casco Cecchi, Meseta de Somuncurá, 9 de Julio Dept, Río Negro Province Argentina. REE-SDSU 2433–35, N from Laguna Raimunda, Meseta de Somuncurá. 9 de Julio Dept, Río Negro Province, Argentina. SDSU 1780–83, 2 km N Laguna Raimunda, Meseta Somuncurá, 9 de Julio Dept, Río Negro Province, Argentina. MCN-UNSa 4550 (SJ 25) (41°12′13.95″S, 66°53′31.94″W, 1,060 m), Meseta Somuncurá. 9 de Julio Dept, Río Negro Province, Argentina.
Phymaturus spectabilis (n = 27)
MCN-UNSa 1203 (holotype), MCN-UNSa 1204–15 (paratypes), on RP No. 6, 28 km S Ingeniero Jacobacci, 25 de Mayo Dept, Río Negro Province, Argentina. FML 23502–15 RP No. 6, 27 km S of intersection with RP No. 23 (41°25′S, 69°45′W, 924 m), 25 de Mayo Dept, Río Negro Province, Argentina.
Phymaturus spurcus (n = 16)
MCZ 14791 (Holotype), MCZ 14914–15 (paratypes) Huanuluan, Pilcaniyeu Dept, Río Negro Province, Argentina. MCN-UNSa 1238–40, 1244– 49, hills opposite of Estancia Huanuluan, RN No. 23, 22 km W from Ingeniero Jacobacci, 25 de Mayo Dept, Río Negro Province, Argentina. MVZ 188904–07, along rim rock 4 km S and 1 km E Alto from Escorial (1,100 m), Ñorquinco Dept, Río Negro Province, Argentina.
Phymaturus tenebrosus (n = 18)
MCN-UNSa 1271 (Holotype), MCN-UNSa 1264–70, 1272–73 (paratypes), RN No. 40, 20 km S Cerro Alto; Pilcaniyeu Dept, Río Negro Province, Argentina. MCN-UNSa 1591–95, 1597–99, RN No. 23 between San Carlos de Bariloche and Pilcaniyeu, Pilcaniyeu Dept, Río Negro Province, Argentina.
Phymaturus videlai (n = 8)
FML 21240–43, 126 km N Alto Río Senguer, 7 km N intersection of RN No. 40 and RN No. 26, Río Senguer Dept, Chubut Province, Argentina. MCN-UNSa 4203–04, 07, near Buen Pasto, 85 km NW of Sarmiento (45°04′11″S, 69°25′25″W, 700 m), Sarmiento Dept, Chubut Province, Argentina.
Phymaturus yachanana (n = 5)
MCN-UNSa1334. Eight kilometer north from junction of RP No. 8 and RP No. 4, Sierra Colorada, Telsen Dept, Chubut Province, Argentina. MCN-UNSa 3281, MCN 4314, 4319–20, 8 km north of junction between RP No. 8 and RP No. 4 (on RP No. 8–42°41′40.9″S, 65°49′17.7″W), Telsen Dept, Chubut Province, Argentina.
Phymaturus zapalensis (n = 37)
IBA 792, 4 (type series), Laguna Teru, Zapala Dept, Neuquén Province, Argentina. IBA 866-1, 998-3, 2, 55 km S Piedra del Aguila, Collón Curá Dept, Neuquén Province, Argentina. MCN-UNSa 1600–02, RN No. 40, 1 km S from Salitral (39°40.600′S, 70°36.925′W, 994 m), Catán Lil Dept, Neuquén Province, Argentina. MVZ 188908–10, 8 km N and 4 km E from Junín de los Andes on rocks along Río Malleo (800 m), Huiliches Dept, Neuquén Province, Argentina. MVZ 232508–12, RP No. 46, 9.5 km S and 5 km from Cerro Chachil, (1,580 m), Catán Lil Dept, Neuquén Province, Argentina. MVZ 232513, 0.5 km W from Primeros Pinos (1,600 m), Pirunches Dept, Neuquén Province, Argentina. MVZ 232514, Puesto de Control, 3.5 km N of Laguna Blanca, Laguna Blanca National Park (39°02′32″S, 70°21′52″W, 1,300 m), Zapala Dept, Neuquén Province, Argentina. MVZ 232515–16, RP No. 46, Zapala Dept, Neuquén Province, Argentina. SDSU 1985–88, S shore Laguna Blanca; Zapala Dept, Neuquén Province, Argentina. SDSU 1989–90, S shore Laguna Blanca (1,275 m) Zapala Dept, Neuquén Province, Argentina. MCN-UNSa 3844–53, 9.5 km S from Laguna Blanca on RP No. 46 (39°08′02.40″S, 70°25′45.80″W, 1,387 m) Catán Lil Dept, Neuquén Province, Argentina.
APPENDIX 2
List of all the species, voucher numbers, and GenBank accession numbers of the sequences employed in this study; new sequences obtained in this study are marked in bold. Clarifications: P. sp. 16 of Morando et al. (2013) is named here as P. rahuensis González-Marín, Morando, et al. (2016); P. sp. 18 of Morando et al. (2013) is named here as P. cacivioi Lobo & Nenda (2015); P. sp. 21 of Morando et al. (2013) is named here as P. yachanana Avila et al. (2014); P. felixi_b of Morando et al. (2013) is named here as P. felixi. *Asterisks mark species with fragments belonging to different vouchers specimens, with superscript are indicate the voucher corresponds to each GenBank accession. The accession numbers AY173912, KF967760, KF967803, KF967809, KF967837, JF272897, JF272908, KF967611, and KF967641 could be aligned only when reverse complement transformation was used; previously published sequences downloaded from GenBank were published in the following articles: Morando et al. (2003), Avila, Morando, Perez, & Sites Jr. (2004), Breitman et al. (2011), Olave et al. (2014), Lobo et al. (2016), and Corbalán et al. (2016).
Institutional acronyms: BYU located at Brigham Young University, Provo, UT, USA; CH-IADIZA Instituto Argentino de Investigaciones de las Zonas Áridas, Mendoza, Argentina; Herpetological collection LJAMM-CNP located at Centro Nacional Patagónico, Chubut, Argentina; MACN, Herpetological Collection of Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina. MCN-UNSa, Museo de Ciencias Naturales, Universidad Nacional de Salta, Salta, Argentina; SDSU, Richard E. Etheridge skeletal collection, San Diego State University, San Diego, CA, USA.




