Cranial variation amongst independent lineages of the alligator snapping turtle (Macrochelys temminckii)
Contributing authors: Caleb D. McMahan ([email protected]), James L. Dobie ([email protected]), Craig Guyer ([email protected])
Abstract
Recent interest in diagnoses and relationships between lineages of the alligator snapping turtle (Macrochelys temminckii) present conflicting patterns of molecular variation across the taxon's range. This study uses geometric morphometric techniques to test molecular hypotheses. We analyse alligator snapping turtle cranial variation amongst populations (i.e. drainages) with the hypothesis that populations of turtles recovered as monophyletic by previous molecular studies are more similar to each other in cranial shape. Dorsal, lateral and ventral cranial shape analyses corroborate the uniqueness of populations recovered by molecular genetic hypotheses. Additionally, analyses reveal near equal separation between drainages that were assigned to monophyletic clades by previous phylogenetic studies. These results reveal the potential for more independent lineages that have yet to be diagnosed, and unique cranial shapes are described for our three most heavily sampled drainages.
Introduction
The alligator snapping turtle (Macrochelys temminckii; Troost, 1835) is one of the most charismatic of freshwater turtle species. Contributing to its magnetism is a remarkable size (up to approximately 100 kg) and a unique tongue lure for fish capture (Roman et al. 1999). The species is distributed along the southern Gulf Coast from Florida to Texas and as far north as Missouri and Indiana in the Mississippi/Ohio River drainages, where it is constrained to slow-moving freshwater systems and associated backwaters and ponds (Roman et al. 1999; Boundy and Kennedy 2006). Whilst typically perceived as a single species, geographic variation in morphology is known and this variation may be drainage specific (Pritchard 1989). Based on mitochondrial DNA haplotypes examined from 12 Gulf Coast drainages, Roman et al. (1999) documented three genetic clusters of M. temminckii: a western and central clade that are sister to one another and sister to a basal Suwannee River clade. These authors also suggested that cranial morphology might correlate with genetic variation. Echelle et al. (2010) examined microsatellite data using a neighbour-joining algorithm and re-examined the mitochondrial haplotypes from Roman et al. (1999) with more geographic coverage and a phylogenetic analysis. This analysis recovered a basal Suwannee River clade, and a paraphyletic assemblage of samples from the central clade including the Trinity River, and a monophyletic western clade but lacking the Trinity River.
An improved analysis of morphological variation within M. temminckii is warranted, given emerging phylogeographic patterns based on molecular data. A valuable tool for assessing such variation is geometric morphometrics, a set of analyses that continue to gain popularity and utility in ecological and evolutionary shape quantification. Contributions from Kendall (1977), Strauss and Bookstein (1982), Bookstein (1989), Rohlf (1990), Rohlf and Marcus (1993) and Adams et al. (2004) have developed into a methodology to isolate shape from size and extract information pertaining to an organism's geometric structure. Here, we use thin plate spline and multivariate geometric morphometric approaches to assess cranial shape variation across the geographic range of M. temminckii and identify unique cranial shapes for lineages on independent evolutionary trajectories. We test whether cranial shape variation amongst populations (i.e. drainages) corresponds to the three hypothesized lineages (a Suwannee, central and western clade; data from Roman et al. 1999 with analysis from Echelle et al. 2010). We predicted that specimens from drainages within a given clade would be more similar in cranial shape than comparison of specimens from drainages in different clades.
Materials and Methods
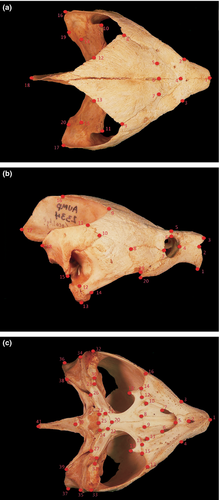
One hundred and eighteen M. temminckii skulls from the Auburn University Museum (AUM), Florida Museum of Natural History (UF-Herpetology) and Tulane University (TU) collections were examined (Appendix 1). The dorsal, right lateral and ventral surfaces of each skull were photographed. No keratinized rhamphotheca existed on included specimens. All skulls were placed on a black sheet overlaying a bed of rice to stabilize the skulls during photography. Photographs were taken with a Panasonic Lumix digital camera and a scale bar; specimen IDs were included in every photograph. Specimens with missing structures were excluded in subsequent analyses for relevant views. Homologous landmarks were chosen to evenly cover all functional areas of the skull. Areas that were not clearly identifiable on all specimens were not used as landmarks. Further, both left and right sides of the skull were quantified in dorsal and ventral views to provide depiction of shape change that is as field applicable as possible.
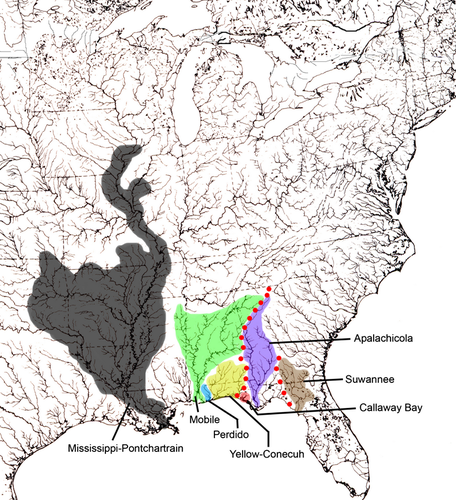
All photographs were digitized using TPSDig2 (Rohlf 2004). Twenty homologous landmarks on the dorsal surface (Fig. 1a), twenty on the lateral surface (Fig. 1b) and 41 on the ventral surface (Fig. 1c) were assigned (described in Appendix 2). Only 27 ventral landmarks were used ordination statistics to avoid redundancy and a specimen to variable bias. Digitized images were combined, and grouping files were obtained using TPSUtil (Rohlf 2000). TPSRegr (Rohlf 2009) was used to obtain consensus, aligned data, centroid size and weight matrix files. We constructed a classifier file by transposing the weight matrix in Microsoft Excel (Professional Edition 2003), and classifier variables were assigned to specimens. Each corresponded to the collection drainage of each specimen. These included eight drainages; Apalachicola, Callaway Bay, Mobile, Suwannee, Perdido, Mississippi and Pontchartrain (combined), Yellow and Conecuh (combined) and an unknown drainage in Texas (Fig. 2). Callaway Bay, Mobile, Perdido and Texas drainages suffered from low sample size resulting in no or meaningless variance. These classifiers were left as unknowns to avoid CVA biases.
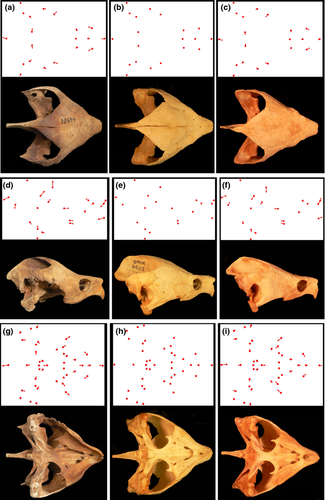
Canonical variates analyses (CVA), principal component analyses (PCA) and Procrustes anovas were performed using MorphoJ software (Klingenberg 2011) to visualize cranial variation in M. temminckii amongst drainages. Confidence ellipses (95%) were assigned to all ordinations. Centroid size variation amongst all drainages was also analysed to ensure no statistical difference in sizes of specimens amongst drainages. No ontogenetic biases based on size class existed in our sample. The anatomical sources of cranial variation relative to a consensus specimen were interpreted using transformation grids calculated in TPSRegr (Rohlf 2009) (Fig. 3). Lines extending away from a consensus landmark depict the direction and extent of shape variation. A 3x exaggeration was used to aid shape change visually.
Results
Morphological variation amongst drainages is evident dorsally in the relative length of the supraoccipital, length of the prefrontal, overall width of the cranium and width of the posterior parietal (Fig. 3). Suwannee specimens present a long supraoccipital, blunt prefrontal, wide cranium and wide posterior parietal relative to other drainages (Fig. 3a). Pontchartrain/Mississippi drainage specimens are characterized by a short supraoccipital, long and narrow prefrontal, narrow cranium and narrow posterior parietal (Fig. 3c), relative to all other drainages. The Apalachicola drainage presents specimens with an intermediate morphology on all above-mentioned characters and serves as a near-consensus morphology between drainages to the east and west (Fig. 3b).
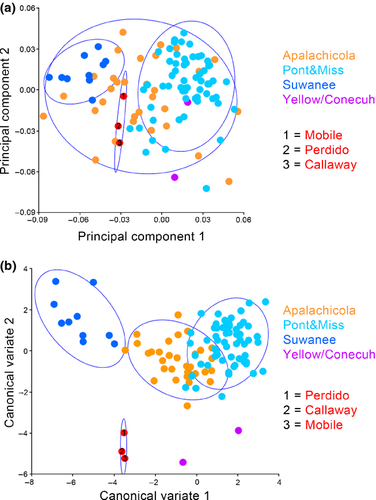
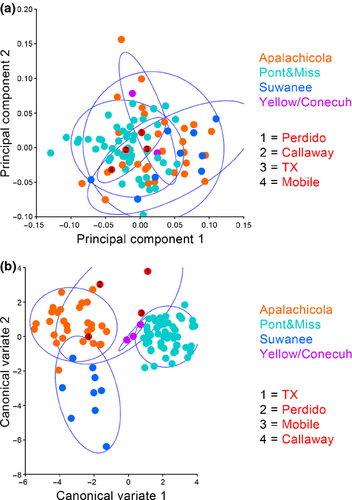
Ordination analyses for the dorsal morphology elucidate morphological separation between Suwannee, Pontchartrain/Mississippi and Apalachicola drainage specimens. PCA on the same dataset depicts clear separation in morphospace between Suwannee and Pontchartrain/Mississippi drainages and additional separation between those drainages and all other Gulf Coast drainages except Apalachicola whose confidence ellipse encompasses all other drainages (Fig. 4a). A Procrustes anova recovers significant variation in dorsal cranial shape amongst drainages (p = <0.0001). Centroid size was also significantly different amongst drainages (p = 0.609). The CVA performed on the dorsal cranial landmarks amongst drainages shows distinct separation in morphospace between the Suwannee and Pontchartrain/Mississippi drainages, whilst the Apalachicola drainage overlaps the Pontchartrain/Mississippi cluster. Perdido, Mobile, Callaway and Texas specimens cluster together and are unique in morphospace, most closely resembling Yellow/Conecuh specimens (Fig. 4b).
Laterally, morphological variation amongst clades is evident in the relative length of the supraoccipital, height of the parietal and length of the prefrontals (Fig. 3). Suwannee drainages present a long supraoccipital, high parietal hump and short prefrontal relative to the Pontchartrain/Mississippi and Apalachicola drainages (Fig. 3d). The Pontchartrain/Mississippi specimens present a short supraoccipital, no parietal hump and long prefrontals (Fig. 3f). Again, the Apalachicola drainage depicts average shape change in these characters and is similar to the consensus for all turtles (Fig. 3e).
Principal component analysis of lateral cranial landmarks reveals limited separation amongst the Suwannee, Pontchartrain/Mississippi and Apalachicola drainages and all other Gulf Coast drainages including a Texas specimen of unknown locality (Fig. 5a). The CVA, however, depicts clear separation amongst the Pontchartrain/Mississippi, Apalachicola and Suwannee drainages with minor overlap between Suwannee and Apalachicola clusters along CV1. The Callaway and Perdido specimens cluster with Apalachicola whilst the mobile specimen groups with the Yellow/Conecuh and Pontchartrain/Mississippi clusters. The Texas specimen appears to have distinct morphologies, and Yellow/Conecuh specimens appear to be potentially unique but similar to the Pontchartrain/Mississippi cluster (Fig. 5b). Procrustes anova recovers significant variation in lateral cranial shape amongst drainages (p = <0.0001). Centroid size was not significantly different amongst drainages (p = 0.544).
Ventrally, relative length of the prefrontal, overall width of the cranium, length of the supraoccipital, length of the basisphenoid and angle of the maxilla are characters that present morphological variation amongst drainages (Fig. 3). Suwannee drainages are characterized by blunt prefrontals, wide cranium, long supraoccipital, short and wide basisphenoid and start of maxillary widening (Fig. 3g). Specimens from western drainages possess a long, narrow prefrontal, a narrow cranium, a short supraoccipital, a long basisphenoid and an acute distal projection of the maxilla relative to Apalachicola and Pontchartrain/Mississippi drainages (Fig. 3i). Apalachicola specimens possess characters with intermediate morphologies between the western and eastern drainages (Fig. 3h).
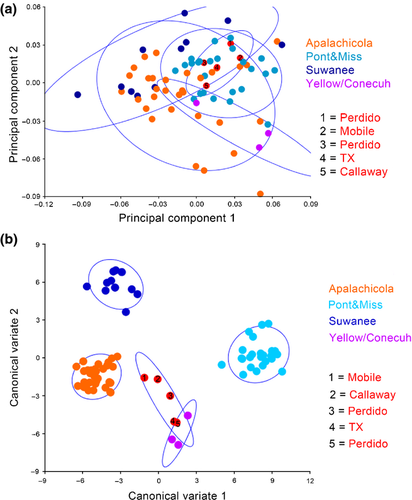
Principal component analysis of ventral cranial landmarks reveals moderate separation between the Pontchartrain/Mississippi and Suwannee drainages. Specimens from the Apalachicola drainage overlap all other drainages. All other Gulf Coast drainages are scattered throughout the ordination grouping within Apalachicola and Pontchartrain/Mississippi clusters, except the Yellow/Conecuh which is isolated from Suwannee and exhibits significant separation from Pontchartrain/Mississippi (Fig. 6a). The CVA depicts relatively extreme separation amongst the skulls from Suwannee, Apalachicola and Pontchartrain/Mississippi drainages. Yellow/Conecuh specimens group together and as independent in morphospace as the Apalachicola, Suwannee and Pontchartrain/Mississippi are to each other. Other Gulf Coast drainages appear intermediately scattered in morphospace with some overlap with Yellow/Conecuh (Fig. 6b). Procrustes anova recovers significant variation in ventral cranial shape amongst drainages (p = <0.0001). Centroid size was not significantly different amongst drainages (p = 0.1268).
Discussion
Cranial shape variation across the M. temminckii distribution is predominantly evident in seven characters: length of supraoccipital relative to head length, length of prefrontal relative to head length, width of the cranium relative to length, width and height of parietal relative to head width and height, length of basisphenoid relative to width and angle of maxilla relative to head width. These characteristics change along an east–west axis based on the Suwannee, Apalachicola and Mississippi/Pontchartrain drainage specimens that dominated our sample. Regarding general shape, the Suwannee specimens represent an extreme characterized by robust skulls that are nearly as wide as they are long. This is most strongly reflected in the parietal bones, which are also nearly as wide as they are long. At the opposite extreme are Mississippi/Pontchartrain specimens that possess skulls that are notably elongate and gracile, for an alligator snapping turtle. These features are most easily seen in parietals and basisphenoids, both of which are noticeably longer than they are wide. An intermediate condition is exhibited by specimens from the Apalachicola River despite this drainage being geographically closer to the Suwannee River than it is to the Mississippi River. However, specimens from the Apalachicola River were also more variable in skull shape than were specimens from the other two drainages, a feature that caused PCA plots for Apalachicola River specimens to overlap one or both of the other two localities in all three aspects of the skull (top, lateral and ventral).
Were these the only specimens included, our results would have been consistent with two separate morpho-groups, a Suwannee River population possessing a robust skull from the Apalachicola River and a Mississippi/Pontchartrain group possessing skulls that are progressively more gracile. Such a scenario would be consistent with the proposed relationships of Roman et al. (1999). However, the specimens from other drainages complicate a simple concordance with this hypothesis of evolutionary history. In both PCA and CVA plots, the specimen from Callaway Creek in Florida clusters most closely with specimens from the Apalachicola River and the specimen from the Mobile River clusters most closely with specimens from the Mississippi/Pontchartrain drainages. These features are consistent with molecular data documenting a central lineage centred on the Apalachicola River and a western clade centred on the Mississippi River (Roman et al. 1999). The three specimens from the Conecuh/Yellow River drainages cluster most closely with the Mississippi/Pontchartrain specimens in PCA plots, but display unique positioning in CVAs. The Conecuh/Yellow drainage is unique in morphospace when using ventral landmarks, clusters with Mississippi/Pontchartrain specimens in lateral view and groups with both Apalachicola and Mississippi/Pontchartrain specimens in dorsal view. An even more complicated pattern is observed in our Perdido River specimen, which tends to occupy unique morphological positions in both PCA and CVA analyses. These observations, despite low sample size, suggest drainage-specific skull morphologies, features corroborating the difficulty that Echelle et al. (2010) had in finding molecular evidence that the central (Apalachicola/Choctawhatchee/Pea Rivers) and western (Yellow through Mississippi Rivers) clades described by Roman et al. (1999) are reciprocally monophyletic.
Pritchard (1989) observed variation in head shape in M. temminckii, noting differences in head width, snout length, length of jaw hooks and expansion of jaw surface. He interpreted this variation to result from ontogenetic plasticity or dietary habits. Only head width was considered to vary geographically. Additionally, Pritchard (1989: p 44) elucidated the potential for independent evolutionary trajectories across the M. temminckii range, stating that the distribution appears as a ‘series of disjunct populations each isolated within a river system…’ Our morphological analyses appear to validate these observations. In particular, the Perdido River specimen is notably divergent from other regions. This small river is the only one along the Gulf Coastal Plain that lacks a member of the genus Graptemys, an occurrence attributed to a lack of mollusks as a food source. Our description of skull shape in M. temminckii from this river may provide a second line of evidence, suggesting an altered prey base for the Perdido River relative to the other rivers of this region.
Our morphological data corroborate Roman et al.'s (1999) and Echelle et al.'s (2010) discovery of an ‘eastern’ clade restricted to the Suwannee and Santa Fe Rivers. These independent lines of evidence are sufficient to diagnose this lineage as a divergent population, which currently is being described (Krysko pers. comm.). The ‘central’ and ‘western’ genetic clusters of Roman et al. (1999) are variable from both the molecular and morphological perspectives. Although we describe evidence of independent evolutionary trajectories, we find no morphological basis for further taxonomic splitting. However, biogeographic studies of other vertebrates of the Gulf Coast suggest that further splitting may eventually be warranted. For example, the genus Graptemys exhibits drainage-specific lineages along the Gulf Coast (Lovich and McCoy 1992; Ennen et al. 2010), a pattern consistent with the morphological diversity that we observed amongst M. temminckii populations. Additionally, freshwater mussels of the genus Lampsilis (Roe and Hartfield 2005) and the fish species Cyprinella venusta (Gibbs 1957 and Kristmundsdóttir and Gold 1996) maintain phylogeographic patterns consistent with skull morphology of M. temminckii. In particular, the morphological clustering of the Calloway Creek specimen with specimens from the Apalachicola River and clustering of the Mobile River specimen with specimens from the Mississippi/Pontchartrain rivers are consistent with historical geological association of these pairs of rivers. This relationship is responsible for creating independent evolutionary histories of drainages from the Mobile River westward relative to drainages from the Perdido River eastward. The potential for M. temminckii to exhibit complex biogeographic patterns is a reasonable hypothesis considering this follows what has been observed in other aquatic Gulf Coast herpetofauna (Soltis et al. 2006).
Acknowledgements
We thank Kenneth L. Krysko and Justin Mann for assistance in accessing specimens and Kyle Piller for analytical support.
Appendix 1: Specimens examined
AUM, Auburn University Museum; TU, Tulane University; UF-Herpetology, Florida Museum of Natural History.
Material Examined: Alabama—AUM 755-56, AUM 2013; Florida–AUM 735-36, AUM 738, AUM 749, AUM 762, AUM 772, AUM 774, AUM 952, AUM 2263, AUM 3747, AUM 3753, UF 12694-95, UF 22267, UF 25047, UF 48437, UF 49967, UF 57967-68, UF 89890-91, UF 115413, UF 117204, UF 140993, UF 152479, UF 166146; Georgia—AUM 2323, AUM 2325-29, AUM 2331-32, AUM 2335-36, AUM 2339, AUM 2340-42, AUM 2344-50, AUM 2359, AUM 3748 (or Florida), AUM 2334, AUM 3127-28; Louisiana—AUM 734, AUM 739-40, AUM 742-43, AUM 746-47, AUM 752-53, AUM 760, AUM 763, AUM 769, AUM 771, TU 15206, TU 15208.2, TU 16154, TU 18072, TU 18077-78, TU 18080-81, TU 18083, TU 18086-87, TU 18090-96, TU 18099, TU 18106, TU 18110-12, TU 18114, TU 18116, TU 18118-19, TU 18121, TU 18123, TU 18126, TU 18132-33, TU 18139, TU 18140-41, TU 18144-45, TU 18147, TU 18149-51, TU 18153-54, TU 18156-58, TU 18164-65; Texas—AUM 745.
Appendix 2: Landmarks descriptions
Dorsal—(1) anterior tip of suture between left and right prefrontals, (2) lateral-most depression of left prefrontal associated with orbit, (3) lateral-most depression of right prefrontal associated with orbit, (4) mid-sagittal junction of prefrontals and frontals, (5) junction of left frontal, prefrontal and postorbital, (6) mid-sagittal junction of frontal and parietal, (7) junction of right frontal, prefrontal and postorbital, (8) posterior-most junction of left postorbital and parietal, (9) posterior-most junction of right postorbital and parietal, (10) condyle of left quadrate, (11) condyle of right quadrate, (12) posterior-most point along suture between left supraoccipital and opisthotic, (13) posterior-most point along suture between right supraoccipital and opisthotic, (14) intersection of left quadrate, opisthotic and squamosal, (15) intersection of right quadrate, opisthotic and squamosal, (16) posterior tip of left squamosal (17) posterior tip of right squamosal, (18) posterior tip of supraoccipital, (19) posterior-most point along suture between left opisthotic and squamosal and (20) posterior-most point along suture between right opisthotic and squamosal. Lateral—(1) ventral tip of prefrontal, (2) anterior point along suture between prefrontal and maxilla, (3) antero-dorsal tip of prefrontal, (4) anterior-most point of orbit, (5) junction of prefrontal and frontal, (6) junction of orbit, prefrontal and postorbital, (7) junction of maxilla, orbit and jugal, (8) junction of postorbital, jugal and quadratojugal, (9) posterior-most point along parietal-postorbital suture, (10) ventral-most junction of squamosal and postorbital, (11) dorsal-most junction of squamosal and postorbital, (12) junction of quadrate, quadratojugal and ventral-most part of tympanic cavity, (13) ventral-most point along quadrate, (14) anterior-most junction of quadratojugal and quadrate, (15) ventral tip of squamosal, (16) dorsal junction of parietal and supraoccipital, (17) posterior tip of supraoccipital, (18) posterior tip of squamosal, (19) posterior junction of maxilla and jugal and (20) posterior tip of maxilla. Ventral—(1) anterior-most point along suture between right and left prefrontals, (2) anterior-most point along suture between right and left vomers, (3) start of left maxillary widening, (4) start of right maxillary widening, (5) posterior-most point along suture between right and left vomers at level of secondary palate, (6) posterior tip of right infraorbital fenestrae, (7) posterior tip of left infraorbital fenestrae, (8) posterior-most point along right palatine-vomer suture, (9) posterior-most point along left palatine/vomer suture, (10) anterior margin of right medial tympanic cavity, (11) anterior margin of left medial tympanic cavity, (12) junction of right palatine, maxilla and pterygoid, (13) junction of left palatine, maxilla and pterygoid, (14) lateral-most point along suture between right maxilla and pterygoid, (15) lateral-most point along suture between left maxilla and pterygoid, (16) posterior-most point along right maxillary cutting surface, (17) posterior-most point along left maxillary cutting surface, (18) tip of right pterygoid posterior projection, (19) tip of left pterygoid posterior projection, (20) anterior junction of basisphenoid and interpterygoid suture, (21) anterior vertex of right basisphenoid, (22) anterior vertex of left basisphenoid, (23) posterior vertex of right basisphenoid, (24) posterior vertex of left basisphenoid, (25) posterior-most point of basisphenoid along mid-sagittal plane, (26) lateral-most point along right pterygoid-exoccipital suture, (27) lateral-most point along left pterygoid-exoccipital suture, (28) lateral-most point of right pterygoid, (29) lateral-most point of left pterygoid, (30) medial-most point of right quadrate articulatory surface, (31) medial-most point of left quadrate articulatory surface, (32) lateral-most point of right quadrate articulatory surface, (33) lateral-most point of left quadrate articulatory surface, (34) lateral-most point on post-tympanic surface of right squamosal, (35) lateral-most point on post-tympanic surface of left squamosal, (36) distal-most point from landmark 26 along right squamosal, (37) distal-most point from landmark 27 along left squamosal, (38) lateral-most point along suture between right opisthotic and squamosal, (39) lateral-most point along suture between left opisthotic and squamosal, (40) posterior tip of occipital condyle and (41) posterior tip of supraoccipital. Ventral (in ordination statistics)—(1) anterior-most point along suture between right and left prefrontals, (2) anterior-most point along suture between right and left vomers, (3) start of left maxillary widening, (4) start of right maxillary widening, (5) posterior-most point along suture between right and left vomers at level of secondary palate, (6) posterior tip of right infraorbital fenestrae, (7) posterior tip of left infraorbital fenestrae, (8) posterior-most point along right palatine-vomer suture, (9) posterior-most point along left palatine/vomer suture, (10) lateral-most point along suture between right maxilla and pterygoid, (11) lateral-most point along suture between left maxilla and pterygoid, (12) posterior-most point along right maxillary cutting surface, (13) posterior-most point along left maxillary cutting surface, (14) tip of right pterygoid posterior projection, (15) tip of left pterygoid posterior projection, (16) anterior junction of basisphenoid and interpterygoid suture, (17) anterior vertex of right basisphenoid, (18) anterior vertex of left basisphenoid, (19) posterior vertex of right basisphenoid, (20) posterior vertex of left basisphenoid, (21) posterior-most point of basisphenoid along mid-sagittal plane, (22) medial-most point of right quadrate articulatory surface, (23) medial-most point of left quadrate articulatory surface, (24) distal-most point along right squamosal, (25) distal-most point along left squamosal, (26) posterior tip of occipital condyle and (27) posterior tip of supraoccipital.




