The Single-Dose Pharmacokinetics of a Compounded Levetiracetam Formulation and Bioequivalence to a Commercial Formulation in Healthy Dogs
Funding: This study was supported by University of Illinois Companion Animal Research Grant Program and PO-COMP tablets and funding for the IV-COMM phase of this study were provided by Epicur Pharma.
ABSTRACT
Levetiracetam (LEV) is an anti-epileptic drug used extra-label in dogs. Commercially available extended-release formulations (LEV-ER), administered twice daily, cannot be crushed or split, limiting their use in small dogs. A compounded LEV-ER formulation (PO-COMP) can purportedly be partitioned without loss of extended-release properties. The aims of this study were to establish the pharmacokinetic parameters of PO-COMP, divided at the tablet score, and determine the bioequivalence of partitioned PO-COMP to an intact commercially available Food and Drug Administration-approved human oral generic formulation of LEV-ER (PO-COMM). In a randomized crossover design, 12 healthy dogs received a single IV dose (30 mg/kg) of IV-COMM, a single oral dose (500 mg) of intact PO-COMM, or a single oral dose (500 mg) of partitioned PO-COMP and underwent serial measurement of plasma LEV concentrations over 24 h. PO-COMP was bioequivalent to PO-COMM using the 90% confidence interval method for maximum concentration (−3.2% difference [CI −7.4% to −1.1%]) and area under the curve (−14.4% difference [CI −17.8% to 10.8%]). PO-COMP may improve medication adherence and seizure control relative to immediate-release LEV, which requires three times daily dosing. Efficacy studies of PO-COMP are warranted.
1 Introduction
Levetiracetam (LEV) is an anti-epileptic drug (AED) that acts primarily through binding to synaptic vesicle protein SV2A, which plays a major role in neurotransmitter release in the central nervous system (Mastrocco et al. 2023; Feyissa 2019). Its complete mechanism of action has yet to be elucidated but is thought to be multimodal, with effects on multiple cellular transporters, neuronal excitability, and GABAA receptor stabilization (Palma et al. 2007; Lee, Chen, and Liou 2009; Surges, Volynski, and Walker 2008; Yan et al. 2013). LEV is unlikely to be a target of multi-drug efflux transporters, a suspected mechanism for the development of pharmacoresistant epilepsy in humans (Surges, Volynski, and Walker 2008). LEV gained Food and Drug Administration (FDA) approval for use in human epileptics in 1999 (Podell et al. 2016). It is used extra-label in dogs and cats (Mastrocco et al. 2023; Johnson et al. 2019). LEV has become popular in these species due to its perceived efficacy, wide safety margin, mild adverse effect profile, suitability for use in patients with hepatic dysfunction, and potential synergy with other AEDs (Mastrocco et al. 2023; De Risio and Muñana 2022).
Commercial oral LEV is available in both immediate-release (LEV-IR) and extended-release (LEV-ER) tablet formulations. Pharmacokinetic studies of both formulations have been conducted in dogs. The oral bioavailability of LEV-IR is 100%, elimination half-life is 2–3 h and maximum serum concentration occurs in 30–150 min (Boozer et al. 2015; Dewey et al. 2008; Moore et al. 2010; Patterson et al. 2008; Strolin Benedetti et al. 2004). Metabolism does not change with repeated oral dosing and therefore does not negatively impact medication administration (Moore et al. 2010). The bioavailability of LEV-ER is also 100% and the elimination half-life (4–5 h) and time to maximum concentration (3–8 h) are prolonged relative to LEV-IR (Mastrocco et al. 2023; Boozer et al. 2015; Beasley and Boothe 2015). The maximum plasma concentration is lower than with LEV-IR but remains above 5 μg/mL, the minimum therapeutic concentration extrapolated from human clinical practice, for at least 12 h (De Risio and Muñana 2022; Boozer et al. 2015). The absorption of both oral formulations is slowed with food, prolonging time to maximum serum concentration (Patterson et al. 2008; Beasley and Boothe 2015).
To maintain serum concentrations above 5 μg/mL, the short elimination half-life of LEV-IR requires dosing every 8 h, while the longer half-life and time to maximum concentration (Tmax) of LEV-ER permit twice daily dosing (Johnson et al. 2019; Boozer et al. 2015; Beasley and Boothe 2015). A pharmacokinetic comparison of branded LEV-ER and several generic LEV-ER tablets in dogs demonstrated minor differences between products, but all formulations maintained therapeutic serum concentrations for a minimum of 12 h (Boozer et al. 2015).
It is well-established in human medicine that the frequency of drug administration and dosing compliance are inversely related (Leppik and Hovinga 2013; Claxton, Cramer, and Pierce 2001; Coleman et al. 2012). Veterinary data are less clear cut but a positive impact on medication adherence has been reported with lower dosing frequencies (Wareham, Brennan, and Dean 2019; Booth et al. 2021). Accordingly, LEV-ER administration might lead to improved owner compliance and seizure control relative to LEV-IR. Commercially available LEV-ER tablets must be administered whole, as the extended-release properties of the film-coating are lost if the integrity of the tablet is compromised (Sun et al. 2016; Keppra XR [Package Insert] 2017). In one study comparing dissolution of intact, split, and crushed LEV-ER tablets, splitting and crushing resulted in loss of extended-release properties relative to the intact tablet (Sun et al. 2016). The need to administer LEV-ER intact can be problematic in smaller patients, as the tablets are physically large, and in patients that are otherwise unable to swallow intact tablets. Dosing of commercial LEV-ER in smaller patients (< 16 kg) also exceeds the current recommended starting dose for this medication of 30 mg/kg by mouth every 12 h because the minimum tablet size is 500 mg (De Risio and Muñana 2022; Beasley and Boothe 2015).
A compounded LEV-ER formulation is available in standard sizes from a veterinary 503B outsourcing facility. A major potential benefit of such a formulation in dogs is that the tablet is scored and purportedly can be partitioned without loss of extended-release properties. This would facilitate its use in dogs whose owners are unable to administer LEV-IR every 8 h, that are unable to swallow an intact LEV-ER tablet due to size or persistent chewing/crushing, or for which the smallest commercially available LEV-ER tablet results in an excessive dose. A recent study performed in cats found that a different compounded formulation of LEV-ER, supplied in capsules, maintained serum concentrations within the human therapeutic range for nearly 24 h (Johnson et al. 2019). To date, no published studies exist regarding any compounded LEV-ER formulation in dogs.
The goals of the present study were to (1) establish the pharmacokinetic parameters of a compounded formulation of LEV-ER (PO-COMP), including absolute bioavailability relative to commercially available intravenous LEV (IV-COMM), and (2) determine the bioequivalence of PO-COMP to a commercially available oral generic formulation of LEV-ER (PO-COMM) in healthy dogs. We hypothesized that pharmacokinetic parameters of PO-COMP would be similar to PO-COMM and appropriate for twice daily dosing in dogs with high absolute bioavailability, and that PO-COMP would be bioequivalent to PO-COMM.
2 Materials and Methods
2.1 Study Population
Twelve healthy dogs belonging to individuals associated with the University of Illinois College of Veterinary Medicine were enrolled in the study. Dogs were eligible if they were between 1 and 8 years of age and weighed a minimum of 15 kg. They were determined to be healthy based on a complete physical examination performed by a veterinary neurology resident under the supervision of a board-certified veterinary neurologist, in addition to the results of a complete blood count, serum biochemistry panel, and urinalysis. Dogs were excluded if they had clinically relevant abnormal physical examination findings or laboratory results or were receiving oral medications other than routine prophylactics. Each owner provided informed consent and approval for the study was granted by the University of Illinois Institutional Animal Care and Use Committee (protocol #19157).
2.2 Study Design
A randomized, three-way, balanced, crossover study was performed. Dogs were randomly assigned to groups of four by blindly drawing lots from an envelope. The study phases were likewise randomized for each dog within each group by blindly drawing lots from an envelope and comprised (i) oral administration of a single intact 500 mg tablet of PO-COMM,1 (ii) oral administration of a single partitioned 500 mg tablet of PO-COMP,2 and (iii) intravenous administration of 30 mg/kg of IV-COMM.3 PO-COMP was divided at the tablet score to simulate the treatment of dogs requiring a smaller dose than 500 mg or unable to swallow an intact tablet. The IV-COMM phase was included to allow calculation of absolute bioavailability for PO-COMP. Based on the previously reported half-lives of LEV-ER (4.47 ± 0.50 h) and IV-COMM (4.00 ± 0.82 h) in dogs, a minimum washout period of 7 days between study phases was employed in order to exceed 10 half-lives (Boozer et al. 2015; Dewey et al. 2008). This washout duration is recommended by the FDA for pharmacokinetics studies of bioequivalence (U.S. Food and Drug Administration 2006).
2.3 PO-COMP Formulation
PO-COMP was manufactured by an FDA-registered outsourcing facility operating pursuant to Section 503B of the Federal Food, Drug, and Cosmetic Act. PO-COMP is a white, ovoid tablet weighing an average of 1.194 g, with an average thickness of 7.376 mm and an average hardness of 18.47 kP. It contains the following ingredients: levetiracetam (41.67%), silicified microcrystalline cellulose (22.33%), untreated silica dioxide (0.5%), magnesium stearate (0.5%), and Methocel K100M DC2 (35%). Silicified microcrystalline cellulose and Methocel K100M DC2 were added by the manufacturer to slow drug delivery. Third-party4 analysis of the test lot of 500 mg tablets revealed 499.59 mg of levetiracetam per tablet using high-performance liquid chromatography.
2.4 Sample Collection
A single lumen, 18ga (4Fr)-25 cm, centrally inserted venous catheter (CVC)5 was placed in the jugular vein of the subject dogs utilizing the modified Seldinger technique (Beal and Hughes 2000). Dogs were sedated for CVC placement with 0.3 mg/kg butorphanol6 and 5 μg/kg dexmedetomidine7 intravenously. If further sedation was required, additional doses of these medications were administered under the direction of a board-certified veterinary anesthesiologist. While under sedation, an 18ga or 20ga cephalic intravenous catheter8 was also placed in dogs receiving IV-COMM. Following CVC placement, reversal of dexmedetomidine was achieved with intramuscular administration of 0.05 mg/kg atipamezole.9
At least 12 h following sedation, a baseline (time 0) blood sample was obtained from the CVC and placed directly into a plastic additive-free red top tube.10 The dogs were then fed a meal of dry kibble after which the applicable treatment was administered orally (PO-COMM and PO-COMP) or intravenously (IV-COMM). Following administration, 3 mL blood samples were collected via the CVC into separate plastic red top tubes (see note 10) at the following time intervals: 0.25, 0.5, 0.75, 1, 2, 4, 8, 12, and 24 h. The samples were allowed to coagulate at room temperature and, within 2 h of collection, centrifuged for 10 min at 1900 g. Serum was harvested, transferred into an additive-free glass red top tube11 or polypropylene collection tube,12 and stored at −80°C until submitted for analysis within 6 months.
For each phase, dogs were hospitalized from the time of sedation until the conclusion of sampling. Vital parameters, including temperature (°F), heart rate (beats/min), and respiration rate (breaths/min) were assessed prior to sedation and then every 6 h until discharge to monitor for potential adverse effects. All CVCs were removed prior to discharge. Adverse events that occurred during and after drug administration are reported descriptively.
2.5 Quantification of Serum Levetiracetam
Quantitation of LEV in serum was performed by the Clinical Pharmacology Laboratory at Auburn University (Mastrocco et al. 2023; Boozer et al. 2015). This assay has been previously used in other canine levetiracetam pharmacokinetic studies (Beasley and Boothe 2015). At the time of sample analysis, serum samples were thawed at room temperature and then vortexed to assure homogeneity. LEV was detected and quantitated in canine serum by an FDA human-approved immunoassay13 on a general chemistry analyzer,14 as previously described (Carnes, Axlund, and Boothe 2011). The system was validated in canine serum using pooled canine serum to which had been added known concentrations of LEV. Subsequent analysis was based on the manufacturer's LEV calibrator and control kits,15 which were designed for human serum. The package insert for the assay indicates a lack of cross-reactivity with the major metabolite (L057/PBA) (ARK 2020). Furthermore, this metabolite represents only 2%–9% of the dose (based on urinary excretion) in dogs compared to 24% of the dose in adult humans (Strolin Benedetti et al. 2004; Isoherranen et al. 2001). The upper and lower limits of quantitation are 100 and 2 μg/mL, respectively (ARK 2020). After validation in canine serum, manufacturer's controls are the basis for quality assurance, with coefficients of variation (CV) ≤ 20% for the low control and ≤ 15% for the high control (ARK 2020). The CV using the manufacturer's quality controls for the time period during which the samples were analyzed was < 14% for the low control and < 9% for the high control.
2.6 Pharmacokinetic and Statistical Analysis
Continuous data are presented as geometric mean ± standard deviation. Noncompartmental pharmacokinetic analysis of serum LEV concentration for the PO-COMM, PO-COMP, and IV-COMM phases was performed using commercially available software.16 For the purpose of these analyses, concentrations reported as below the limit of quantification (< 2 μg/mL) for the assay were set as 0 μg/mL (U.S. Food and Drug Administration 2018). Most concentrations at the final timepoint (24 h) were below the limit of quantification (32/39) and the remainder were at or near it (2.0–3.3 μg/mL). Therefore, observed area under the curve (AUC) and area under the moment curve (AUMC) are reported and used in downstream analyses and these parameters extrapolated to infinity are not included.
Bioequivalence between PO-COMM and PO-COMP was evaluated using the 90% confidence interval (CI) method using log-transformed data as advocated by the FDA for veterinary bioequivalence studies (U.S. Food and Drug Administration 2006). Both the maximum concentration (Cmax) and AUC were compared between formulations using this method. Multivariate linear regression models were developed for each pivotal parameter using formulation, sequence, period, and dog nested in sequence as explanatory variables. The mean squared error was obtained from the analysis of variance and used in the calculation of the 90% CI. Following analysis, data were back transformed and results are reported as the difference in means and % difference in means between the two formulations with upper and lower confidence bounds reported as % difference. The formulations were considered bioequivalent if the 90% CI fell within −20% and +25% for both Cmax and AUC.
3 Results
3.1 Study Population
Twelve dogs were enrolled in the study, including one intact female, one intact male, three neutered males, and seven neutered females, with a mean age of 3.3 ± 2.2 years (range 1–8 years). The following breeds were represented: mixed breed dog (n = 7), Catahoula Hound (n = 2), Labrador Retriever (n = 1), Old English Sheepdog (n = 1), and Rottweiler (n = 1). At the time of study enrollment, the weight range was 15.1–33.3 kg (23.3 ± 5.5 kg).
Free-catch urinalysis in one study participant (Dog03) revealed bacteriuria and in another study participant (Dog05) revealed bacteriuria and mild pyuria. No clinical signs of lower urinary tract infection (pollakiuria, stranguria, hematuria, or pain upon voiding) were present in these subjects (Kendall et al. 2024). The serum chemistry panel for one study participant (Dog06) showed hypoglycemia which was determined to be spurious, suspected secondary to delayed sample processing, via spot blood glucose measurement (Turchiano et al. 2013).
The mean doses of PO-COMM and PO-COMP administered were 22.58 ± 5.72 mg/kg (range 15.20–34.25 mg/kg) and 21.90 ± 5.04 mg/kg (range 15.02–32.89 mg/kg), respectively, and the precise dose of IV-COMM administered to all dogs was 30 mg/kg.
IV-COMM was inadvertently administered to five dogs (Dog01, Dog02, Dog03, Dog06, and Dog07) through the CVC line rather than peripherally. Data from these dogs were excluded from further analysis of IV-COMM. Data from one dog (Dog10) in the IV-COMM phase and one dog (Dog12) in the PO-COMM phase appeared to be out of order due to suspected sample labeling errors during processing. Therefore, data from the IV-COMM phase for Dog10 and the PO-COMM phase for Dog12 were excluded from further analyses.
3.2 Pharmacokinetics
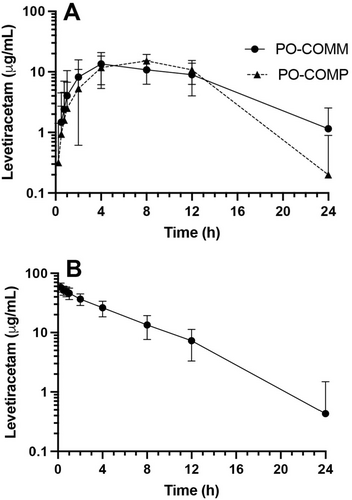
The pharmacokinetic analyses for the PO-COMM, PO-COMP, and IV-COMM phases included 11, 12, and 6 dogs, respectively. Pharmacokinetic parameters for PO-COMM and PO-COMP are presented in Table 1 and Figure 1A. Pharmacokinetic parameters for IV-COMM are presented in Table 2 and Figure 1B. Using the AUC method, the absolute bioavailability of PO-COMP was 78% ± 19% (range 44%–97%) and the absolute bioavailability of PO-COMM was 95% ± 15% (range 83%–119%). LEV serum concentrations and pharmacokinetic parameters for individual dogs are presented in Tables S1–S6.
| Parameter | Unit | PO-COMM | PO-COMP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | ||
| D | mg/kg | 22.3 | 6.1 | 15.2 | 34.2 | 21.3 | 5.3 | 15.0 | 32.9 |
| t 1/2 | h | 4.9 | 1.5 | 3.0 | 8.0 | 8.7 | 42.9 | 2.8 | 154.8 |
| T max | h | 4.9 | 3.8 | 2.0 | 12.0 | 6.2 | 2.6 | 4.0 | 12.0 |
| C max | μg/mL | 17.0 | 5.3 | 8.6 | 25.3 | 16.6 | 3.6 | 12.4 | 23.2 |
| Cmax/D | (μg/mL)/(mg/kg) | 0.76 | 0.18 | 0.51 | 1.15 | 0.78 | 0.23 | 0.45 | 1.15 |
| AUC | μg*h/mL | 154 | 41 | 93 | 213 | 133 | 33 | 85 | 201 |
| AUC/D | (μg*h/mL)/(mg/kg) | 6.88 | 3.02 | 4.04 | 14.00 | 6.22 | 1.72 | 4.12 | 9.98 |
| AUMC | μg*h2/mL | 1145 | 724 | 477 | 2432 | 963 | 395 | 570 | 2093 |
| MRT | h | 7.5 | 2.8 | 4.1 | 12.3 | 7.3 | 1.7 | 5.6 | 11.4 |
| F | % | 95 | 15 | 83 | 119 | 78 | 19 | 44 | 97 |
- Note: Geometric mean, standard deviation, minimum, and maximum value for each parameter are reported.
- Abbreviations: AUC = observed area under the curve; AUC/D = AUC normalized to dose; AUMC = observed area under the moment curve; Cmax = maximum concentration; Cmax/D = Cmax normalized to dose; D = dose; F = bioavailability; MRT = mean residence time; t1/2 = terminal half-life; Tmax = time to maximum concentration.
| Parameter | Unit | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| D | mg/kg | 30 | 0 | 30 | 30 |
| t 1/2 | h | 4.0 | 0.8 | 3.0 | 5.2 |
| C 0 | μg/mL | 64.7 | 12.4 | 48.0 | 81.2 |
| AUC | μg*h/mL | 280 | 113 | 187 | 499 |
| AUC/D | (μg*h/mL)/(mg/kg) | 9.33 | 3.75 | 6.25 | 16.65 |
| V z | mL/kg | 561 | 116 | 431 | 757 |
| Cl | mL/h/kg | 96 | 34 | 58 | 150 |
| AUMC | μg*h2/mL | 1123 | 920 | 597 | 3120 |
| MRT | h | 4.0 | 1.1 | 3.2 | 6.2 |
| V ss | mL/kg | 531 | 106 | 416 | 711 |
| F PO-COMP | — | 0.78 | 0.19 | 0.44 | 0.97 |
- Note: The absolute bioavailability of oral compounded extended-release levetiracetam (FPO-COMP) is included. Geometric mean, standard deviation, minimum, and maximum value for each parameter are reported.
- Abbreviations: AUC = observed area under the curve; AUC/D = AUC normalized to dose; AUMC = observed area under the moment curve; C0 = predicted concentration at time 0 h; Cl = clearance; D = dose; FPO-COMP = absolute bioavailability of oral compounded extended-release levetiracetam; MRT = mean residence time; t1/2 = terminal half-life; Vss = volume of distribution at steady state; Vz = volume of distribution by the area method.
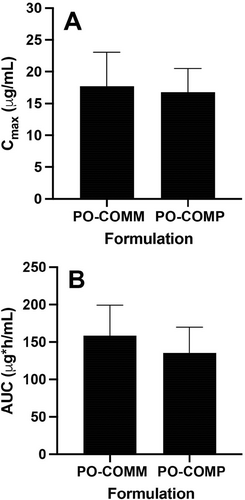
3.3 Bioequivalence
Because the data for the PO-COMM phase for one dog (Dog12) were excluded, 11 dogs were included in the bioequivalence analysis. The Cmax and AUC for the PO-COMM and PO-COMP phases are presented in Figure 2A,B. For the purposes of the analysis, PO-COMM was considered the reference treatment and PO-COMP was considered the test treatment. The geometric mean of the difference in Cmax between treatments was −0.56 μg/mL (−3.2% relative to PO-COMM) with a 90% CI of −7.4% to +1.1%. The geometric mean of the difference in AUC between treatments was −22 μg*h/mL (−14.4% relative to PO-COMM) with a 90% CI of −17.8% to −10.8%. Based on a target CI of −20% and +25% for both parameters using log-transformed data, the treatments were considered bioequivalent (U.S. Food and Drug Administration 2006).
3.4 Adverse Events
Transient inappropriate urination, comprising one to two episodes of in-house urination, was noted by the owners of two dogs (Dog06 and Dog07) within 48 h following administration of PO-COMM. The episodes were self-limiting, did not re-occur, and no diagnostic testing was performed. No similar abnormalities occurred with PO-COMP or IV-COMM. No other potential adverse effects were observed.
4 Discussion
The use of PO-COMP in patients unable to receive PO-COMM, whether due to small body size or inability to swallow intact tablets, may allow for twice-daily dosing and improve medication adherence and seizure control relative to every 8-h dosing of LEV-IR. We first hypothesized that pharmacokinetic parameters of PO-COMP would be similar to PO-COMM and appropriate for twice daily dosing in dogs, with high absolute bioavailability. Cmax of PO-COMM and PO-COMP were nearly identical. Tmax for PO-COMP (6.2 ± 2.6 h) was slightly prolonged relative to PO-COMM (4.9 ± 3.8 h). This finding may indicate that PO-COMP more slowly achieves maximal serum concentration, though a significant difference is considered unlikely given the overlap in the ranges of Tmax within and between cohorts. Terminal half-life of PO-COMM (4.9 ± 1.5 h) was similar to prior studies and prolonged in PO-COMP (8.7 ± 42.9 h). The longer average terminal half-life with PO-COMP was primarily driven by three dogs (Dog05, Dog07, and Dog09), while the terminal half-lives for the other nine dogs were similar to the average value for PO-COMM. This finding may reflect the less frequent sampling intervals in the terminal portion of the curves (12–24 h) or individual differences in the in vivo release of the PO-COMP. Larger studies would help to more thoroughly evaluate the possibility of a dog-by-formulation interaction as the reason for the greater variability observed in the t1/2 values for PO-COMP.
We next hypothesized that intact PO-COMM and partitioned PO-COMP would be bioequivalent. The absolute bioavailability of partitioned PO-COMP (0.78 ± 0.19) was lower than the absolute bioavailability of intact PO-COMM (0.95 ± 0.15). However, using the 90% CI method for both AUC and Cmax, PO-COMP and PO-COMM were bioequivalent. This suggests that the difference in absolute bioavailability, which is calculated from AUC, is within the acceptable range and unlikely to cause clinically relevant differences in drug concentrations or efficacy when PO-COMP is used. Furthermore, these values are consistent with the calculated relative bioavailability of generic LEV-ER in a prior study (Boozer et al. 2015). In that comparison study of branded LEV-IR, branded LEV-ER, and two generic formulations of LEV-ER, bioavailability of the generic formulations relative to LEV-IR ranged from 0.77 to 1.05 (Boozer et al. 2015).
While the analysis supports bioequivalence, the results for several dogs demonstrated high variability between PO-COMM and PO-COMP. This finding suggests that these formulations may not be bioequivalent in all dogs and highlights a limitation of this bioequivalence analysis. These differences are evidenced by the fact that, although the 95% CI for AUC was within the accepted bioequivalence range of −20% to +25%, both bounds fell below 100%. Thus, while AUC between formulations is statistically significantly different, that difference is not considered clinically relevant by current human standards. Additionally, interindividual variation resulted in a wide range of bioavailabilities for both formulations (PO-COMM: 83%–119%; PO-COMP: 44%–97%). The low percentage (44%) bioavailability for one dog with PO-COMP probably represents poor absorption in that individual, while the > 100% bioavailability for one dog with PO-COMM is likely an overestimation of absolute bioavailability resulting from infrequent sampling during the terminal portion of the time–concentration curve. Moreover, only two dogs receiving PO-COMP and two dogs receiving PO-COMM had LEV concentrations below 5.0 μg/mL at the end of the usual dosing interval for LEV-ER (12 h) (Tables S2 and S3). Serum LEV concentrations would likely be higher with repeated dosing due to mild accumulation. The possibility of clinical equivalence despite the lower LEV bioavailability of the PO-COMP formulation depends on whether a serum LEV concentration of 5 μg/mL, the minimum therapeutic concentration extrapolated from human studies, is an appropriate therapeutic target for dogs. Clearly, further studies are indicated.
In veterinary medicine, clinicians rely on compounding to ensure adequate doses and formulations of drugs for their canine and feline patients (Gochenauer et al. 2019). Despite the rise of compounded medications in veterinary medicine, there is limited information and data regarding safety, efficacy, and pharmacokinetic properties of these medications (Davidson 2017). Thus, we undertook the present study to further characterize a compounded product with the potential to fill a need in veterinary neuropharmacology. Based on our results, twice daily dosing of LEV-ER, including PO-COMP, may be justified.
Extended-release drug formulations are used to slow drug dissolution into the gastrointestinal tract and prolong the dosing interval for drugs with short elimination half-lives (Muñana et al. 2018). Slower release into the gastrointestinal tract generally prolongs Tmax for the extended-release products and, ideally, yields less variability in serum concentration (Muñana et al. 2018). Even where bioequivalence has been shown in human studies, use of these medications in dogs is generally extra-label and not based upon demonstrated bioequivalence or efficacy (Beasley and Boothe 2015). This can be problematic, as these products may not demonstrate the same extended-release properties in dogs due to interspecific differences (Beasley and Boothe 2015; Brown and Forrester 1991). While this study demonstrated the bioequivalence of PO-COMP and PO-COMM in a population of healthy dogs, there are no published efficacy studies of LEV-ER in dogs. Efficacy studies involving multiple oral dosing of PO-COMM and PO-COMP are indicated.
Due to the technologies employed to slow drug delivery, compounded extended-release products pose additional challenges (Johnson et al. 2019; Leppik and Hovinga 2013). PO-COMP was compounded for this study by a single outsourcing facility using Methocel K100M, a high molecular weight chemical thickening agent. Methocel is derived from cellulose and forms a viscous gel membrane when swallowed by the patient, binding the drug, and increasing absorption time from the gastrointestinal tract. Silicified microcrystalline cellulose was also utilized to decrease tablet porosity and water erosion/diffusion due to LEV's high solubility and permeability. As Methocel is evenly spread throughout each PO-COMP tablet, the viscous gel membrane should form regardless of partitioning, unlike with the film-coat utilized with PO-COMM (Sun et al. 2016). We do not expect that tablet porosity, erosion, and diffusion would change significantly enough with partitioning to result in clinical consequence. The lack of clear evidence in this area, however, provides a target for future study.
Use of these additives appeared to succeed, as bioequivalence was found between partitioned PO-COMP and intact PO-COMM. It is unknown whether LEV-ER compounded by other pharmacies utilizes these additives to achieve delayed drug delivery and, regardless, these products require testing before their use can be recommended. Because compounded drugs do not undergo consistent testing for identity, quality, strength, purity, and stability, results of research described in reports using compounded products may not be reproducible. These concerns may be mitigated, as in this study, by obtaining compounded medications from outsourcing facilities operating under Section 503B of the Federal Food, Drug, and Cosmetic Act, as they are subject to more stringent oversight than individuals or facilities operating pursuant to Section 503A thereof (Federal Food, Drug, and Cosmetic Act 1934).
Several limitations were present in this study. While the sample size in this study was adequate for the tests performed, a larger sample would have reduced the likelihood of error and potentially decreased variability in the results. The dogs that participated in this study were healthy dogs recruited from the community. As a result, they were heterogenous in terms of age, size, breed, and neuter status. Purpose-bred animals may have potentially reduced data variability, though the instant study population was likely more representative of the canine population in which PO-COMM or PO-COMP would be used. Additionally, this study evaluated single oral and intravenous dosing rather than multiple oral dosing, which has more real-life applicability. All dogs in this study were fed prior to treatment, which may slow absorption and prolong Tmax (Beasley and Boothe 2015). Thus, the formulations might not be bioequivalent in the fasted state and this should be investigated in a future study. As most canine patients receive their medications with food, however, these results are more likely to represent what will occur in the “home” setting than administering the medications to fasted patients. Finally, technical issues limited the number of dogs included in the IV-COMM analysis, and the results for one dog in the PO-COMM phase were excluded due to a suspected labeling error.
5 Conclusion
This study demonstrated that PO-COMP is bioequivalent to PO-COMM using the 90% CI method. A potential benefit of PO-COMP over PO-COMM is the ability to divide the tablet at the score without loss of extended-release properties, potentially improving medication adherence and seizure control relative to LEV-IR, which must be administered every 8 h. Therapeutic drug monitoring may be of benefit in cases where improvement is not seen with dose escalation, as serum concentrations may vary between dogs. It may also prove valuable if an untested compounded product is used, particularly if improved seizure control is not achieved. Efficacy studies involving multiple oral dosing of PO-COMM and PO-COMP are warranted.
Author Contributions
All authors have read and approved the final manuscript.
Ethics Statement
The authors declare that this study was approved by and conducted in accordance with the requirements of the University of Illinois Institutional Animal Care and Use Committee (protocol #19157). The authors confirm that they have adhered to either US or European standards for the protection of animals used for scientific purposes.
Conflicts of Interest
The authors declare no conflicts of interest.
Endnotes
Open Research
Data Availability Statement
The data that support the findings of this study are available in Appendix S1 of this article.




