In vivo joint synovial fluid disposition of a novel sustained-release formulation of diclofenac and hyaluronic acid in horses
Abstract
Intra-articular administration of sustained-release anti-inflammatory drugs is indicated in horses suffering from joint inflammation, but no such drugs are labelled for veterinary use. To obtain initial data on synovial disposition and safety of a new sustained-release formulation of diclofenac (SYN321) in the joints of horses, an experimental interventional study of elimination and side effects of intra-articular administration of SYN321 was conducted. Nine clinically sound horses were included in the study, and SYN321 was administered by the intra-articular route. Dose ranges and sampling intervals were established in a pilot study with two horses, and then applied in a main study involving seven horses treated in the fetlock joint. Diclofenac was detected above lower limit of quantification (LOQ: 0.5 ng/ml) in synovial fluid throughout the study period (14 days), and below LOQ (0.1 ng/ml) in plasma after 4 days and in urine after 14 days. No obvious clinical side effects were detected. Clinical examination and objective lameness evaluation suggested that SYN321 has potential as a local joint NSAID treatment with sustained release in horses, but further studies on synovial fluid exposure, safety and clinical efficacy are warranted.
1 INTRODUCTION
Osteoarthritis (OA) is the most common type of degenerative joint disease in horses (Goodrich & Nixon, 2006) and humans (Moskowitz, 2009). The disease is a major source of pain, disability and socio-economic cost worldwide (Woolf & Pfleger, 2003), and results in severe welfare issues and economic losses for humans and for horse-related industries.
Osteoarthritis is a complex disease that involves all parts of the joint. It may be caused by a number of different factors, ranging from chronic low-grade micro insults to acute trauma and infection. The involvement of inflammation produced by synovium and chondrocytes is central to the pathogenesis, with inflammatory cytokines, metalloproteases and other inflammatory mediators being present in synovial fluid of patients with OA (2006). The resulting joint pain manifests as lameness (Schlueter & Orth, 2004) and the joint capsule inflammation causes swelling and fibrosis, resulting in additional pain and decreased range of motion of the affected joint. No curative treatments exist for this disease, but early symptomatic treatment can reduce pain and the cartilage-damaging effects of the inflammatory products, and decrease the progression of cartilage destruction and fibrosis of the joint capsule (Goldring & Otero, 2011). In horses, a common treatment regime consists of systemic administration of non-steroidal anti-inflammatory drugs (NSAIDs; Goodrich & Nixon, 2006).
NSAIDs act by inhibiting the synthesis of prostaglandins and thromboxanes (Gunaydin & Bilge, 2018) and by inhibiting the action of cyclooxygenase enzymes (COX1 and COX2). NSAIDs also have beneficial modulating effects on matrix metalloproteinase activity (Chu et al., 2008; Grauw et al., 2009), collagen turnover (Grauw et al., 2009) and proteoglycan turnover (Rainsford et al., 1997). All these effects are believed to contribute positively to the treatment outcome (Abramson et al., 2006). However, systemic use of NSAIDs has a wide range of side effects, including on the gastrointestinal, renal and cardiovascular systems, which are main reasons for discontinuing treatment (Cryer & Feldman, 1992; MacAllister et al., 1993).
As an alternative to systemic use, intra-articular (IA) drug administration would be rational, because an effective concentration at the target site could be achieved using a minimum amount of the drug. Intra-articular administration of OA disease-modifying drugs is common in horses and involves use of corticosteroids, viscoelastic gels based on hyaluronic acid (HA; Goodrich & Nixon, 2006) or combinations of these. Hyaluronic acid is synthesized by synoviocytes of the synovial membrane and chondrocytes in the articular cartilage (Caron, 2005). While controversy still exists about the efficacy and duration in horses (Niemelä et al., 2018). HA is recommended for pain relief in human OA (Henrotin et al., 2015).
However, aqueous solutions of small molecules such as NSAIDs generally have relatively short half-life in synovial fluid and are hence assumed to be cleared rapidly from the joint (Burt et al., 2009; Larsen et al., 2006), necessitating frequent IA administration. In addition, the welfare issues and infection risk associated with IA medication are impediments to wider IA use of NSAID formulations available today. Consequently, development of IA sustained-release formulations of NSAID has gained much interest (Larsen et al., 2008), with the current focus on combinations of diclofenac and HA viscoelastic hydrogels. The HA macromolecule is well known for good joint biocompatibility and for its properties as a depot matrix in slow-release formulations (Jin et al., 2010). Intra-articular depot formulation of NSAIDs and HA derivatives has been suggested (Gerwin et al., 2006), but to our knowledge, none of these drugs is labelled for veterinary use. Labelling requires multiple data from a predetermined set of studies with the focus on quality, residues, safety and clinical efficacy (EMA & EMA,). However, prerequisites for any IA drug, before proceeding to the clinical studies required for labelling for use in horses, are that the drug is safe for use in vivo in the joints of horses and that the drug formulation actually possesses the expected delayed-release properties in vivo. Neither of these research questions can be studied in vitro.
The main aim of this study was therefore to evaluate potential clinical side effects in healthy horses treated with a novel intra-articular drug delivery system based on HA and diclofenac (SYN321). Another aim was to obtain pilot concentration-time data on diclofenac in synovial fluid) to evaluate if SYN321 extend synovial fluid exposure compared with a diclofenac solution. Potential clinical side effects of SYN321 were evaluated by clinical examinations and by sensor-based objective lameness assessment of the horses. A secondary aim was to explore systemic exposure to diclofenac and urinary excretion time and to relate those to equine medication and anti-doping regulations.
We expected that the sustained-release formulation SYN321 would significantly prolong the presence of diclofenac in the joint and that none of the horses would show clinical signs of side effects from the administration, including lameness, clinical disease or synovial pathology.
2 MATERIALS AND METHODS
2.1 Study design
Experimental interventional study of elimination and side effects of intra-articular administration of the new drug formulation SYN321. The experimental study consisted of a pilot study of two horses, followed by a main study involving seven horses and using dose and sampling interval data from the pilot study.
2.1.1 Synthesis of hyaluronic acid succinyl ester (SYN321)
SYN321 is a succinylated HA derivative with a substituent of diclofenac. The diclofenac is linked to HA through a spacer containing ester functionalities. Cleavage of the esters releases the diclofenac in vivo, probably through the action of hydrolytic enzymes. A representation of the chemical structure of the compound is shown in Figure 1. 1H-NMR analysis in deuterium oxide (D2O) was used to determine the degree of substitution (DS), by comparing the integral of the N-acetyl in the HA backbone with the aromatic protons in the diclofenac residue. The DS was found to be about 0.1, indicating that on average there was one diclofenac residue on every 10th HA disaccharide unit.
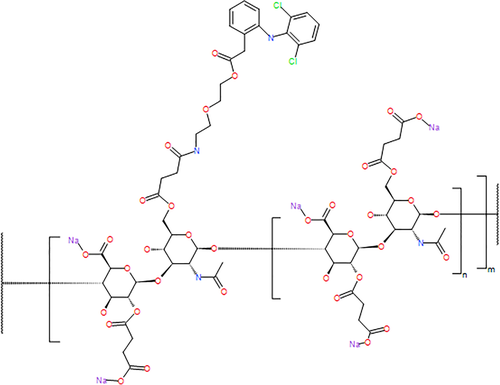
Diclofenac-substituted HA (SYN321; 255 mg) was dissolved in sterile filtered water (13 ml). Physiological NaCl phosphate buffer (13 ml) was added, followed by NaCl (55 mg). The mixture was worked in a set-up with two syringes connected tip to tip. When the solution was homogeneous, it was transferred to ampoules previously washed with 70% ethanol, and dried in a desiccator overnight. To avoid contamination, all work was performed in a glove box. A thorough description of the synthesis of SYN321 is provided in supplementary material (S1).
2.2 Horses
Nine clinically sound Standardbred trotters (eight mares and one gelding, median age 10 years (range 3–15), median weight 503 kg (range 480–601)) kept in the animal research facility at the Swedish University of Agricultural Sciences were used in the experiments. They were fed haylage and housed in individual stalls according to standard routines at the research facility and let out into a 20 m × 20 m paddock for 8 h during the day. Water was available ad libitum in both stall and paddock.
A complete clinical examination was performed before the experiment, with specific attention to the joints to be injected, and joint effusion (scale 0–4; Bertoni et al., 2019), diffuse swelling (mild, moderate, severe) or digital pulse of the distal limbs were recorded. Metacarpophalangeal (fetlock) and front knee (radiocarpal) joints were radiographed by four and three standard projections, respectively.
Exclusion criteria were as follows: clinical signs of forelimb lameness >1 degree (0–5 lameness scale), joint effusion >2 or >mild diffuse swelling of the forelimbs or radiographic signs of osteoarthritis (radiocarpal and/or fetlock joints). Two horses were euthanized after the study due to other reasons, and autopsy of the treated joints was performed.
2.3 Administration and sampling schemes
The hair over the injection site (palmar pouch of the fetlock joint of all horses and lateral to the extensor carpi radialis tendon of the radiocarpal joint for one pilot horse) was clipped, and the skin was scrubbed with chlorhexidine and alcohol to prepare for arthrocentesis. Doses and joints used for administration are presented in Table 1, and the volume for each administration was 2 ml. For the safety of horses and staff, four horses were sedated before arthrocentesis with a combination of butorphanol (0.006 mg/kg) and detomidine (0.006 mg/kg; one horse on five occasions, one horse on two occasions and two horses on one occasion). After arthrocentesis, a cover was applied over the injection site. Blood samples were taken from the jugular vein using the vacutainer technique. Synovial fluid samples (0.5–1 ml) were collected using a 21 G cannula and a 2 ml syringe, after preparation of the skin as described above. Urine samples were collected manually during voluntary urination.
| Horse no. | Drug administration | Dose of SYN321a | Dose DSb | Dose HAc | |
|---|---|---|---|---|---|
| Left FL | Right FL | (mg) | (mg) | (mg) | |
| Pilot study | |||||
| P1:1 | Saline | SYN321 | 1.0 + 20 | − | − |
| P2 | SYN321 | Saline | 1.0 + 20 | − | − |
| P1:2 | DS + HA | SYN321 | 2.4 + 40 | 2 | 8 |
| Main study | |||||
| H1 | HA | SYN321 | 1.2 + 20 | − | 20 |
| H2 | SYN321 | HA | 1.2 + 20 | − | 20 |
| H3 | HA | SYN321 | 1.2 + 20 | − | 20 |
| H4 | SYN321 | HA | 2.4 + 40 | − | 40 |
| H5 | DS + HA | SYN321 | 2.0 + 20 | 1.0 | 20 |
| H6 | DS + HA | SYN321 | 2.0 + 20 | 1.0 | 20 |
| H7 | DS + HA | SYN321 | 2.0 + 20 | 1.0 | 20 |
- Abbreviation: FL, Fetlock joint.
- a A succinylated hyaluronic acid derivative with a substituent of diclofenac.
- b Diclofenac solution for injection.
- c Hyaluronic acid.
2.3.1 Handling of samples
Synovial fluid samples were collected in EDTA tubes (4 ml), and blood samples were collected in lithium heparin tubes (4 ml). The aqueous phase of synovial fluid and of blood plasma was separated by centrifugation (1000 g), and all samples stored at −80°C until analysis.
2.3.2 Clinical examinations
Subjective lameness assessment (0–5 lameness scale) during straight-line trot on a hard surface, joint effusion and/or diffuse swelling around the treated and control joints at palpation, and increased temperature or digital pulse of the forelimbs was subjectively evaluated and recorded.
2.4 Objective motion analysis
A commercial inertial measure unit (IMU)-based gait analysis system for lameness detection was used (Lameness LocatorTM, Equinosis LLC). In brief, the horses were equipped with two uni-axial accelerometers, one to the poll with a felt head bumper and one taped to the midline between the two tubera sacrale. One uni-axial gyroscope was attached with a specially designed pastern wrap to the dorsal side of the right forelimb pastern. The sensors measured 3.2 cm × 3.0 cm × 2.0 cm and had a mass of 28 g. Measurements were digitally recorded at 200 Hz and wirelessly transmitted to a handheld computer. The data were processed with the software package for the gait analysis system. From the displacement signal, local maxima and minima were found (two per stride). Differences in minimum head height (HDmin) during left and right stance phases were computed per stride. Mean values of all strides and standard deviation (SD) were calculated per trial. Further descriptions of instrumentation and parameter calculations have been published elsewhere (Keegan, 2011). Movement asymmetry of the head, indicative of forelimb lameness, was considered to be present if absolute values of HDmin exceeded 6 mm and SD was less than the respective mean (strong evidence of lameness), as stated in the manufacturer's guidelines.
2.5 Chemical analyses
2.5.1 Analysis of diclofenac in synovial fluid
A reversed-phase high-performance liquid chromatography method with tandem mass spectrometric detection (HPLC-MS/MS) was developed for determination of total diclofenac in horse plasma and urine and for determination of both concentrations of diclofenac dissociated from HA and dissociated diclofenac + diclofenac bound to HA in synovial fluid from horses.
The procedure for determining the concentration of diclofenac in samples, prepared in 96-well format, began with an enriching step (liquid/liquid-extraction) to reach the low concentrations of diclofenac. This involved mixing 200 µl of biological matrix with 50 µl of internal standard solution (D4-diclofenac, 100 ng/ml), 200 µl buffer solution (50 mM phosphate buffer pH 3) and 1.5 ml hexane:isopropanol (90:10), and agitating thoroughly on a shaker for approximately 30 min. After centrifugation, the organic phase was transferred to a new 96-well plate and evaporated to dryness. The dry residue was resolved in 100 µl of acetonitrile:water-mixture (50:50), injected into a HPLC equipped with a X-bridge phenyl column (5 μm, 2.1 mm × 50 mm). It was eluted with a mobile phase working at room temperature and 0.4 ml/min flow rate and consisting of a mixture of 10 mM ammonium formate buffer (pH 9) and acetonitrile in a gradient starting with 98% buffer and ending with 90% acetonitrile.
The procedure for analysing the dissociated + bound to HA concentration of diclofenac in the samples comprised two additional steps to those described above: (1) Effective mixing of the samples with 50 µl 0.5 M sodium hydroxide to break the links between diclofenac and HA; and (2) neutralization of the samples with 100 µl 1 M hydrochloric acid. These steps were done before the enrichment steps with liquid/liquid extraction.
Detection of diclofenac was performed in MRM mode, using electrospray positive ionization. The ion transition monitored was as follows: m/z: 295.8 > 214.8 (diclofenac) and 299.8 > 218.8 (D4-diclofenac).
The method was found to have a lower limit of quantitation (LOQ) of 0.1 ng/ml when detecting diclofenac in plasma and urine, and 0.5 ng/ml when quantifying diclofenac in synovial fluid. The calibration range was linear within the range 0.1–50 ng/ml for plasma and urine samples and 0.5–10,000 ng/ml for synovial fluid samples. The analytical method was not further validated.
2.6 Pilot study
The purpose of the pilot study was to provide a matrix for validation of the analytical method, establish dose ranges and sampling intervals, and screen for clinical side effects. Two horses (P1, P2) were used, and SYN321 was administered in one of their fetlock joints (doses are given in Table 1). SYN321 was also administered in the radiocarpal joint 16 days later in horse P1. Synovial fluid and plasma were collected, and a clinical examination, including visual and objective lameness evaluation, was performed before treatment and 2, 6, 12, 24, 48, 96 h, 8 and 16 days post-treatment.
2.7 Main study
Seven horses (H1–H7) not included in the pilot study were used. The doses, joints and formulations used for drug administration are shown in Table 1. Before the first administration, samples were taken of blood, urine and synovial fluid from both fetlock joints, and clinical examination and objective lameness evaluation were performed. Thereafter, synovial fluid and blood sampling, clinical examination and objective lameness evaluation were performed after 12, 24, 48, 96 h, 7, 10 and 14 days post-treatment, in total nine times per horse. Urine was sampled from four horses (H1–H4) before drug administration and after 7 and 14 days post-drug administration, and from two horses (H5 and H7) before drug administration and after 24, 48, 72, 96 h, 6 and 7 days post-drug administration.
2.8 Data analyses
The individual measured synovial fluid concentrations of diclofenac after administration of SYN321 were plotted against time. In order to determine initial and final half-life in synovial fluid, the diclofenac concentration-time course in synovial fluid was analysed using a two-compartment model with a proportional error model in WinNonlin 4.0.1 (Certara).
3 RESULTS
3.1 Pilot study
3.1.1 Clinical examination
Horse P1 showed no clinical abnormalities when treated in the fetlock joint. In the radiocarpal joint treatment, the control limb showed mild increased digital pulse and heat on day 2 and mild diffuse swelling on day 3, but no joint effusion or reaction in any joint on other days.
Before treatment, horse P2 had fetlock joint effusion (degree 1) and mild diffuse swelling in both the control limb and the limb used for treatment. The same findings were present at 30 min and 2, 24 and 48 h post-treatment but at 6 and 12 h post-treatment the fetlock joint effusion was increased to degree 2 in the treated joint. After 72 h, the only findings were fetlock joint effusion in the control limb (degree 2) and in the treated limb degree (Goodrich & Nixon, 2006). Eight days post-treatment, the joint effusion remained and additional mild diffuse swelling was seen around both fetlocks. At the last examination, 16 days after treatment, there was still mild diffuse swelling around both fetlocks and fetlock joint effusion (degree 1) in the control limb. None of the treated joints showed gross pathological findings on autopsy.
3.1.2 Subjective lameness assessment
No lameness was detected in the two pilot horses at any time during the experiment.
3.1.3 Objective motion analysis (mean absolute HDmin)
Pilot horse P1, which was treated twice, showed movement asymmetry in the control limbs at 6 h (17.1, SD 7.9 mm), 12 h (14.1, SD 9.4 mm) and 24 h (15.3, SD 11.4 mm) post-treatment in the fetlock joint, and at 24 h (18.0, SD 8.6 mm), 48 h (15.7, SD 7.6 mm) and 10 days (11.4, SD 7.7 mm) post-treatment in the radiocarpal joint. Movement asymmetry was seen in the treated limb on day 3 (12.1, SD 11.3 mm) post-treatment of the radiocarpal joint.
Horse P2 showed minor head movement asymmetry (HDmin; 7.7, SD 4.9 mm) from the treated limb pre-treatment of the fetlock joint and at 6 h (11.2, SD 5.9 mm), 24 h (6.4, SD 7.9 mm) and 48 h (9.9, SD 5.7 mm) post-treatment.
3.2 Main study
3.2.1 Clinical examination
Fetlock joint distension
One horse (H4) showed no anomalies on either limb in the clinical examination during the treatment period. In six of the seven horses, consistent or intermittent joint effusion (degree 1) was observed in the control joint in horse H1, the treated joint in horse H3 and both joints in horses H2,H5, H6 and H7. Joint effusion (degree 2) was observed in the control joint at seven days post-treatment in horse H7 and at 10 days post-treatment in horse H5. No degree 3 joint effusion was noted in any horse.
Periarticular diffuse swelling of the fetlock joint region and digital pulse
On day 4, horse H6 showed mild diffuse periarticular swelling of the fetlock region of the control limb. Horse H2 showed mild diffuse periarticular swelling of the joint of the treated limb and horses H2 and H3 showed mild diffuse periarticular swelling in the joints of both limbs during some of the examinations after 3 days. None of the seven horses showed increased digital pulse at any time.
Visual lameness assessment
Two horses were found to be 0.5 degree lame (0–5 lameness scale) in the control limb, horse H6 at 96 h and horse H1 at 10 days post-treatment. Horse H2 showed 0.5 degree lameness in the treated limb on one occasion, at 7 days post-treatment.
Objective lameness evaluation
The HDmin value for horse H2 was 6.4–8.2 mm in the treated limb at day 7, 10 and 14, while HDmin for horse H6 was 12.2 mm on day 14. However, SD was larger than the mean for all these measurements, indicating inconsistent movement asymmetry. Movement asymmetries above 6 mm and with SD less than the mean were seen in the control limbs in some horses on some measurement occasions (Table 2).
| Time | Horse H1 | Horse H2 | Horse H3 | Horse H4 | Horse H5 | Horse H6 | Horse H7 |
|---|---|---|---|---|---|---|---|
| 0 | −5.2 (11.33) | −0.37 (8.47) | −2.5 (7.84) | 0.4 (8.78) | −6.9 (13.00) | −11.1 (16.84) | −2.5 (6.29) |
| 12 h | −7.5 (7.50) | 2.2 (11.26) | −3.0 (5.75) | 5.1 (11.14) | 1.5 (8.38) | −5.1 (16.75) | −5.0 (5.89) |
| 1 days | −0.0 (7.964 | 2.6 (9.95) | −2.5 (5.97) | −5.0 (6.57) | −0.3 (13.41) | −24.4 (26.00) | −8.9 (7.63) |
| 2 days | −3.7 (8.48) | 5.1 (9.54) | −1.0 (6.10) | −6.5 (13.05) | −13.4 (12.95) | 0.9 (17.03) | −9.1 (8.14) |
| 3 days | −18.8 (7.65) | 0.6 (7.06) | 4.2 (7.63) | −3.5 (12.65) | −13.2 (25.62) | −19.4 (36.60) | 0.1 (2.39) |
| 4 days | −11.0 (12.66) | −0.0 (13.78) | −0.3 (5.05) | −1.6 (9.85) | 5.5 (9.67) | −22.2 (29.71) | −16.5 (11.03) |
| 7 days | −17.2 (8.58) | 8.0 (16.50) | 2.6 (5.01) | −5.4 (9.74) | −6.0 (16.28) | −8.3 (53.20) | −11.1 (9.36) |
| 10 days | −20.5 (7.44) | 6.4 (16.45) | 2.3 (5.13) | −6.4 (11.18) | −5.1 (16.74) | −31.0 (26.94 ) | −15.2 (9.54) |
| 14 days | −21.8 (8.11) | 8.2 (9.02) | −0.1 (9.75) | −8.5 (9.31) | −0.5 (18.52) | −12.2 (41.71) | −21.5 (5.27) |
Synovial fluid exposure to diclofenac following administration of SYN321
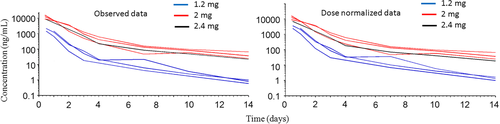
After intra-articular administration of SYN321, diclofenac dissociated from HA + diclofenac bound to HA was quantifiable in synovial fluid at concentrations exceeding LLOQ (0.5 ng/ml) in all horses except one (H 5) for 14 days (last sampling time). The highest concentration in synovial fluid was observed on the first sampling occasion (10–12 h), when the concentration was median (range) 8314 ng/ml (1455–15 900), and the dissociated diclofenac concentration was 1500 (199–2270) ng/ml. Median (range) maximum observed dissociated diclofenac + diclofenac bound to HA concentration after dose normalization to 2 mg diclofenac was 6970 ng/ml (2425–15 900). Median (range) observed dissociated concentration after dose normalization to 2 mg diclofenac was 1500 ng/ml (331–2270). Estimated median (range) half-life of the initial and final phase was 12.3 h (6.4–16.9 h) and 96.2 h (41.6–163.0 h), respectively. Diclofenac was quantifiable in synovial fluid during the entire observation period (14 days) for six horses and for 7 days in one horse. The observed synovial fluid diclofenac concentration-time courses before and after dose normalization are shown in Figures 2 and 3, respectively.
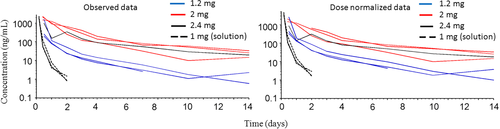
Synovial fluid exposure after administration of diclofenac solution
After intra-articular administration of 1 mg diclofenac solution to three horses, total diclofenac concentrations were quantifiable in synovial fluid for 2 days post-drug administration. The observed total concentration in synovial fluid at 4 h (first sampling time) ranged from 1030 to 2640 ng/ml (Figure 3). Estimated half-life was approximately 5–8 h.
Plasma and urine exposure to diclofenac following administration of SYN321
Total plasma and urine concentration-time courses after IA administration of diclofenac are presented in Figure 4. Diclofenac plasma concentration was below LLOQ (0.1 ng/ml) in all horses 7 days after diclofenac administration.
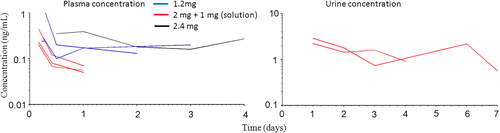
Diclofenac urine concentrations
Urine samples were obtained from six horses. For four of those horses, urine sampling was limited to day 7 and 14 and was below LOQ (0.1 ng/ml). The two remaining horses (horses H5 and H7) were more intensively sampled (Figure 4).
4 DISCUSSION
This study examined synovial fluid, plasma and urinary exposure following intra-articular administration of diclofenac linked to HA (SYN321), a formulation not previously evaluated in horses. The horses were also monitored for clinical signs that could indicate side effects of the intra-articular administration, for example joint swelling and lameness. The formulation (SYN321) was designed to provide sustained release of diclofenac, resulting in extended exposure of synovial fluid to diclofenac. This was successfully achieved, as shown by a substantial increase in diclofenac half-life in synovial fluid and by rather consistent total and free diclofenac concentrations in synovial fluid over time (Figures 2 and 3). A recent in vitro study of another diclofenac depo-injectable reported increased diclofenac half-life in horse synovial fluid (Storgaard et al., 2020). However, to the best of our knowledge, no pharmacodynamic data (potency and efficacy values) have been published previously for diclofenac in vivo in horses.
An important question is whether the data obtained also suggest clinical efficacy. In vitro studies of human chondrocytes and synovial cells suggest potency (IC50) values in the range 0.5–6 ng/ml (Henrotin et al., 1999; O’Neill & Lewis, 1989), and while studies on canine whole blood have found IC50 values of up to 1200 ng/ml (Brideau et al., 2001). In vivo, diclofenac IC50 value for PGE2 inhibition has been found to be 500 and 700 ng/ml in healthy rats and rats with induced synovitis, respectively (Zhang et al., 2012). The diclofenac exposure in synovial fluid following intra-articular administration of SYN321 in the present study might exceed the potency values for human cells at 14 days (the study period). However, the diclofenac concentration-time data reported here must be reproduced in a future study using validated analytical methods to provide sound scientific evidence. However, retention times and synovial concentrations of diclofenac are not necessarily correlated to clinical efficacy or to the duration of response in horses. The differences in potency value in previous studies could be caused by the different cell types used and/or by species differences. Species differences between horses and dogs in terms of other NSAIDs have been documented (Brideau et al., 2001). Our results therefore serve only as a point estimate with input to future efficacy studies.
As shown in Figures 2 and 3, the variation in diclofenac concentrations in synovial fluid cannot be explained solely by differences in the dose. The exact reason for the variation is unknown, but possible reasons could be the non-validated method and the simultaneous administration of 1 mg/kg aqueous diclofenac solution into the contralateral joint of horses H5–H7 treated with 2 mg diclofenac as SYN321. For other drugs, it is known that systemic exposure after intra-articular administration can cause synovial fluid exposure to the drug in other joints (Knych et al., 2014). The treatment protocol used in this study was designed for practical reasons and based on availability of horses, but future studies should take this source of error into consideration.
Following IA administration of SYN321, diclofenac was quantifiable for up to 4 days in plasma and for at least 7 days in urine (Figure 4). However, these findings are based on few individuals, a non-validated method and different doses, so it is likely that detection times may be even longer than observed here. This warrants further studies on the exposure, excretion times and pharmacodynamics of diclofenac in horses, in order to avoid doping of sport horses. Injured sport horses are allowed legitimate medication (e.g. NSAIDs), but the integrity of the sport, fair play and horse welfare demand that horses do not participate in competition under the influence of legitimate drugs or doping agents. Therefore, use of diclofenac in equine athletes is not encouraged today, based on the weak scientific data on its dynamics in horses.
No side effects were observed in this study, except for very mild signs of synovitis and local irritation of the soft tissue, mild joint effusion, mild periarticular diffuse swelling and occasional very subtle lameness. The repeated arthrocentesis may have contributed to these clinical findings. Vertical head movement asymmetry (HDmin) was used to objectively quantify asymmetry, as it is a sensitive measure of forelimb lameness (Buchner et al., 1996; Keegan et al., 2000; Knych et al., 2014; Rhodin et al., 2013). When interpreting the HDmin data, mean asymmetry for a number of strides is used. For the observations indicating lameness on the treated limb in this study, the standard deviation was larger than the mean value, which indicates that the movement asymmetry was inconsistent (Equinosis; USER MANUAL LL2017v.1.1. 2017). Consequently, it cannot be concluded from the data presented here that intra-articular administration of SYN321 induces lameness. It has been shown previously that joints injected with HA can develop significant mild-to-moderate inflammatory responses, often associated with lameness and joint effusion, compared with saline control joints (Johnston et al., 2020). This is consistent with our findings of swelling and potential lameness by subjective and objective lameness measures of the control limb.
One weakness of this study is that the analytical method was not fully validated. Thus, the reported concentration-time profiles should be interpreted with caution and the results verified in additional studies. However, the apparent extended synovial exposure, relatively limited systemic exposure and low frequency of observed side effects warrant further attention and suggest that intra-articular administration of sustained-release diclofenac formulation has potential in horses. A large proportion of horses suffer from osteoarthritis (Penell et al., 2005) and could benefit from the action of anti-inflammatory drugs. Intra-articular administration of NSAIDs with extended duration may also be indicated for joint surgeries where an exaggerated inflammatory response would have detrimental effects on joint cartilage and decrease animal welfare in both the short term (pain) and the longer term (decreased potential for recovery). No obvious side effects were detected in this study but, since only seven horses were included in the main study, it is possible that rare or discrete side effects were missed. There is a need for larger studies on side effects and on the therapeutic concentrations of diclofenac to demonstrate its efficacy in horses with spontaneous joint disease.
4.1 Conclusion
Clinical examination and objective lameness evaluation suggested that SYN321 has potential as a local joint NSAID treatment with sustained release in horses, but further studies on synovial fluid exposure, safety and clinical efficacy are warranted.
ANIMAL WELFARE AND ETHICS STATEMENT
The Ethics Committee for Animal Experiments, Uppsala, Sweden, approved the study (C12/12). [Correction added on 03 September 2022, after first online publication: The Animal Welfare and Ethics Statement was included in this current version.]
CONFLICT OF INTEREST
Marie Rhodin is a shareholder (1.2%) of Synartro AB who developed the substance (SYN321). The company has no influence on the scientific process or writing of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author upon request.




