Pharmacokinetic evaluation of marbofloxacin after intravenous administration at different ages in llama crias, and pharmacokinetic/pharmacodynamic analysis by Monte Carlo simulation
Abstract
In llama crias (tekes), Escherichia coli and Staphylococcus aureus are major pathogens, and marbofloxacin could be a suitable choice. The objectives of this study were (a) to evaluate the serum pharmacokinetics of marbofloxacin (5 mg/kg) after intravenous administration in tekes and simulate a multidose regimen; (b) to emulate pharmacokinetic profiles after single dose and steady-state conditions by Monte Carlo simulation (c) to determine the MIC of regional strains of Escherichia coli and Staphylococcus aureus; (d) to perform a PK/PD analysis by Monte Carlo simulation. Pharmacokinetics of marbofloxacin was evaluated in six animals at 3, 10, 24, 50, and 80 days after birth. Marbofloxacin were determined by HPLC. A steady-state multi-dose simulation was carried out, and concentration-time profiles were generated by Monte Carlo simulation. MIC of marbofloxacin against regional E. coli and S. aureus strains were also determined. Finally, a PK/PD analysis was conducted by Monte Carlo simulation. After pharmacokinetic analysis, clearance showed a trend to increase (0.14 and 0.18 L kg−1 hr−1), and AUC (36.74 and 15.21 μg hr−1 ml−1) and Vss (3.06 and 3.37 L/kg) trended to decrease at 3 and 80 days-old, respectively, showing accumulation ~50% in animals with 3 days. All strains tested of E. coli (MIC90 = 0.06 μg/ml) and S. aureus (MIC90 = 0.25 μg/ml) were susceptible to marbofloxacin. PK/PD analysis suggests that the therapeutic regimen of marbofloxacin could be effective for infections caused by E. coli strains in animals between 3 and 80 days, with a CFR for Cmax/MIC > 10 of 100% and for AUC24/MIC > 125 of 99.99%; and for infections produced by S. aureus in animals between 3 and 24 days old, with a CFR for Cmax/MIC > 10 of 93.08% and for AUC24/MIC > 60 of 97.01%, but a higher dose should be used in older animals, because PK/PD endpoints were not met.
1 INTRODUCTION
Llama production constitutes a source of sustainable development in areas of the planet where other animal species would not adapt properly. In llama crias (tekes), Escherichia coli and Staphylococcus aureus are major pathogens associated with diarrhea, sepsis and wound or skin infections (Cebra & Cebra, 2013; Dolente, Lindborg, Palmer, & Wilkins, 2007; Frank, Couetil, & Clarke, 1998; Sharpe, Lord, Wittum, & Anderson, 2009; Whitehead, 2009; Whitehead & Anderson, 2006). Management and nutrition measures contribute to reduce the prevalence of infectious diseases, but when preventive measures fail, antimicrobial treatment is needed.
There are few studies assessing the pharmacokinetics of fluoroquinolones in camelids. These include enrofloxacin, danofloxacin, marbofloxacin, and moxifloxacin in camels (Abd El-Aty et al., 2007; Aliabadi, Ali, Landoni, & Lees, 2003; Gavrielli, Yagil, Ziv, Creveld, & Glickman, 1995; Laraje et al., 2006), enrofloxacin in alpacas (Gandolf, Papich, Bringardner, & Atkinson, 2005) and enrofloxacin and marbofloxacin in llamas (Christensen, Smith, Murdane, & Hollingshead, 1996; Rubio-Langre et al., 2012). In these studies, some authors reported pharmacokinetic differences between camelids and other ruminant species, such as cattle, sheep, or goats, and were attributed to a variable permanence of the drug in the organism. This evidence the risk of extrapolating dose regimens between different species. Extrapolation of dose regimens may lead to therapeutic failure, promote the emergence of resistance or increased toxicity and antimicrobial residues in animal products. Therefore, pharmacokinetic studies in targeted species are needed to optimize posologies.
Age is a major physiological factor influencing the pharmacokinetic behavior of antimicrobials. Pharmacokinetic changes in young animals could be related to increased total body water content, resulting in higher volume of distribution and relative hypoalbuminemia, leading to lower plasma protein binding. Immature metabolic and excretory functions results in decreased body elimination of toxins may also play a role (Asín-Prieto, Rodríguez-Gascón, & Isla, 2015; Baggot & Guigère, 2013; Nouws, 1992). Hence, more pharmacokinetic studies are needed for dose optimization.
Marbofloxacin (MFX) is a third generation fluoroquinolone for veterinary use, approved for the treatment of pyoderma, otitis and digestive, respiratory, urinary, reproductive and soft tissue infections (EMEA, 2000; USP, 2007). MFX exerts a bactericidal concentration-dependent antimicrobial effect. To the best of our knowledge, no pharmacokinetic studies of MFX are currently available in tekes, so the suitability of this drug for the treatment of E. coli and S. aureus infections in these animals remains to be evaluated.
Fluoroquinolone use in pediatric patients is contraindicated. However, two different studies in children showed no evidence of arthropathy during fluoroquinolone therapy. The authors concluded that restriction of fluoroquinolone use in children may no longer be justified (Ball, Mandell, Niki, & Tillotson, 1999; Jick, 1997). These data highlight the need of more evidence on fluoroquinolone toxicity in different animal species. Additionally, the potential benefit of using this group of antimicrobials in young animals may overcome the risk of adverse reactions.
Antimicrobials are invaluable compounds that provide great benefits to society if used rationally. In order to maintain its utility over time, it is necessary to minimize the occurrence of bacterial resistance, by its prudent and rational use (Asín-Prieto et al., 2015; Boucher et al., 2017; EFSA, 2009; Holmes et al., 2016; Ungemach, Muller-Bahrdt, & Abraham, 2006). The massive and indiscriminate use of antimicrobials produces an intense selection pressure of resistant mutants in the global bacterial population, resulting in a more rapid and generalized dissemination of bacterial resistance and generating reservoirs of resistance in animal and human population and the environment (Holmes et al., 2016; Papich, 2014; Sharma, Johnson, Cizmas, McDonald, & Kim, 2016; Singh & Tam, 2011).
Pharmacokinetic/pharmacodynamic (PK/PD) analysis is an invaluable tool to optimize the efficacy of antimicrobial dose regimens, while minimizing resistant mutant selection and toxicity for patients (Asín-Prieto et al., 2015; Boucher et al., 2017; Drusano, Louie, MacGowan, & Hope, 2016; Papich, 2014). Since fluoroquinolones are concentration-dependent antimicrobials, evidence suggests that Cmax/MIC and AUC24/MIC ratios are the best predictors of clinical outcome and microbiological cure (Asín-Prieto et al., 2015; Cao et al., 2015; Drusano et al., 2016; Papich, 2014).
Monte Carlo simulation (MCS) is a powerful statistical tool that helps PK/PD analysis by considering the population variability of PK and PD parameters, and expanding the sample size by mathematical simulation. MCS estimates the probability of achieve a determined PK/PD target for a particular dose regimen, and it is useful when large-scale studies involving high numbers of individuals are difficult to conduct. The main advantage of using MCS in PK/PD analysis is that the conditions to achieve a PK/PD endpoint are more demanding (Asín-Prieto et al., 2015).
The main objectives of this study were (a) to evaluate the serum pharmacokinetics of marbofloxacin (5 mg/kg) after intravenous administration in tekes and to simulate a multidose regimen on steady-state conditions; (b) to model pharmacokinetic profiles after single dose and steady-state conditions by Monte Carlo simulation (c) to determine the MIC of regional strains of Escherichia coli and Staphylococcus aureus isolated from llamas; and (d) to carry out a PK/PD analysis by Monte Carlo simulation based on the steady-state pharmacokinetic parameters of marbofloxacin and pharmacodynamic parameters determined from regional strains of Escherichia coli and Staphylococcus aureus in order to assess the antimicrobial efficacy of the proposed dose regimen in tekes.
2 MATERIALS AND METHODS
2.1 Animals, treatments and samples
This study was approved by the Commission of Bioethics and Animal Welfare of the Faculty of Agricultural Sciences of Catholic University of Córdoba (reference no CBBA.10.2014.UCC), and carried out with six healthy llama crias (four females and two males) of the “Chaku” variety. The animals were studied at days 3, 10, 24, 50, and 80 after birth. Mean weights at these periods were 11.30 ± 0.81, 14.60 ± 0.76, 19.20 ± 0.92, 25.20 ± 1.16, and 29.60 ± 1.99, respectively. Only animals born with eutocic parturition, weighing equal to or greater than 8 kg and with no evidence of abnormalities during clinical examination at birth were included in the study. According to their age, the crias (tekes) were allowed breastfeeding or combined breastfeeding and alfalfa fed, ad libitum. Animals developing clinical complications or evidencing any adverse reaction to the treatment would be withdrawn from the study and receive the appropriate treatment.
Blood samples were taken from each teke before intravenous administration of MFX (5 mg/kg) in a longitudinal design at days 3, 10, 24, 50, and 80 after birth. Blood samples were taken at 5, 10, 15, 30, and 45 min, and 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 48, and 72 hr after MFX administration.
2.2 Marbofloxacin determination
Serum concentrations of MFX were determined by high-performance liquid chromatography with ultraviolet detection (HPLC/uv), as described by Schneider, Thomas, Boisrame, and Deleforge (1996) with some modifications (Lorenzutti et al., 2017). Marbofloxacin and ofloxacin (used as internal standard), were purchased by Fluka Aldrich and Sigma Chemical, respectably.
For the validation of the chromatographic method, two calibration curves were calculated by linear regression for low (0.025–1 μg/ml; R2: 0.9910) and high (1–15 μg/ml; R2: 0.9897) concentration ranges. The lower limit of quantification (LLOQ) for MFX in serum of llamas was estimated at 0.025 μg/ml, with precision and accuracy values of 6.55% ± 5.12% and 119% ± 1.22%, respectively. The intra and inter-assay reproducibility of the HPLC method was 5.71% ± 3.90% and 5.97% ± 1.41%, respectively. The mean recovery value of MFX by the HPLC method was 91.73% ± 4.57%.
2.3 Pharmacokinetic analysis
Serum concentration-time profiles of MFX after IV administration to tekes at consecutive periods after birth were analyzed with a noncompartmental pharmacokinetic model. Additionally, a multidose regimen considering an administration interval of 24 hr was modeled to evaluate whether an accumulative effect of MFX occurred. Serum concentration-time profiles at steady-state conditions were simulated and a noncompartmental pharmacokinetic analysis was used to determine the steady-state pharmacokinetic parameters of all animals at each stage of the study. Noncompartmental analysis was carried out using the pharmacokinetic software PK Solutions© 2.0 (Summit Research Services, Ashland, OH, USA).
The single dose and steady-state pharmacokinetic parameters were used to perform MCS to model concentration-time profiles of animals under 24 days of life (NEO) and animals between 24 and 80 days of life (YNG), based on pharmacokinetic differences observed between both age groups.
2.4 Simulation of pharmacokinetic profiles
Two sets of pharmacokinetic concentration-time profiles of MFX administered by IV route in tekes (NEO and YNG) were emuled under single dose and steady-state conditions by MCS, based on pharmacokinetic statistical differences. A total of 400 concentration-time profiles were simulated, 200 for NEO group (100 at single dose and 100 at steady-state conditions) and 200 for YNG group (100 at single dose and 100 at steady-state conditions). Monte Carlo simulations were conducted using Oracle Crystal Ball V.11.1.1.0.00 software (Oracle Corporation, Redwood Shores, CA, USA).
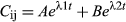
where Cij is the serum concentration of MFX of the ith animal at jth time, A and B are intercepts for absorption and elimination phase, λ1 and λ2 are distribution and elimination slopes and t is a given time. All A, B, λ1, and λ2 parameters were assumed to follow a log-normal distribution.
Each pharmacokinetic profile was analyzed using a noncompartmental model, and individual Cmax and AUC24 were determined. Then, a nonparametric bootstrap analysis was used to estimate the distribution parameters that will be used to perform the PK/PD analysis by MCS.
2.5 Determination of MICs
MICs of MFX were determined from regional strains of E. coli (n = 28) and S. aureus (n = 35), isolated from gut and soft tissue infections in llamas from Córdoba, Argentina, by the agar microdilution method, recommended by Clinical and Laboratory Standards Institute (CLSI, 2013). MIC50 and MIC90 were calculated by cumulative frequencies corresponding to 50% and 90% percentiles of the studied bacterial sub-population. Moreover, MIC90/MIC50 ratios were also included in the analysis.
2.6 PK/PD analysis
Cmax and AUC24 data in NEO and YNG groups under single dose or steady-state conditions, and MIC data from regional E. coli and S. aureus strains isolated from llamas were used to perform a 10,000-subject MCS. All parameters were assumed to follow a log-normal distribution. PK/PD endpoints for Cmax/MIC > 8, Cmax/MIC > 10, AUC24/MIC > 60 and AUC24/MIC > 125 were determined from cutoff values for gram-positive and gram-negative bacteria as described elsewhere (Papich, 2014). The probability of a dose regimen to achieve a given PK/PD endpoint for each MIC value (probability of target attainment; PTA) and the probability of a dose regimen to achieve a determined PK/PD endpoint taking into account the entire MIC distribution of the tested bacterial population (cumulative fraction of response; CFR) were calculated. PTA and CFR values >90% were generally considered adequate (Asín-Prieto et al., 2015).
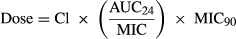
where Cl is the total body clearance, AUC24/MIC is the selected PK/PD endpoint. In this equation, absolute bioavailability and unbound fraction of MFX were not considered, because our objective was to calculate the daily dose necessary to achieve the total AUC24/MIC PK/PD endpoint (not the free-AUC24/MIC), and in consequence, total MFX concentrations should be used to calculate the daily dose. Since F = 100% after IV administration, bioavailability was also not included.
For daily dose calculation, a 10 000-subject Monte Carlo simulation was performed, and the dose of MFX (mg/kg) necessary to achieve the selected PK/PD endpoint in 90% of the simulated population was determined. For this purpose, Cl was assumed to follow a log-normal distribution (Lacey, Keene, Pritchard, & Bye, 1997).
2.7 Statistical analysis
Data were analyzed using IBM SPSS 22© software (IBM Corp©, Armonk, NY, USA). Pharmacokinetic parameters of MFX in serum at different ages in tekes were analyzed using a nonparametric ANOVA Friedman test for paired samples. Cmax and AUC24 data included in MCS and each set of PK/PD parameters were analyzed to determine differences between NEO vs. YNG groups, and single dose vs. steady-state conditions with a nonparametric ANOVA Kruskal–Wallis test, according to the log-normal distribution of the data. A significance level of p < 0.05 was used in all tests.
3 RESULTS
3.1 Pharmacokinetic analysis
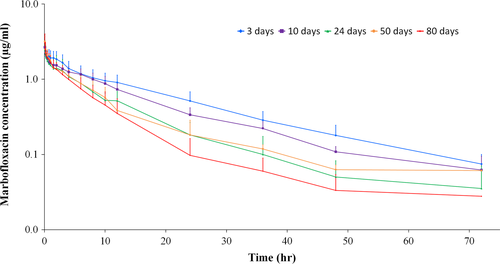
Serum MFX concentration-time profiles after IV administration in tekes at different ages are presented in Figure 1 and pharmacokinetic parameters are shown in Table 1.
| Parameter | Age (days) | ||||
|---|---|---|---|---|---|
| 3 | 10 | 24 | 50 | 80 | |
| Intercept d (μg/ml) | 1.18 ± 0.27a | 1.33 ± 0.41a | 1.22 ± 0.78a | 1.62 ± 0.43ab | 1.73 ± 0.96ab |
| λd (/hr) | 1.54 ± 2.94ab | 2.35 ± 2.01abc | 1.35 ± 0.73a | 3.32 ± 2.17c | 3.08 ± 2.80abc |
| Intercept e (μg/ml) | 1.31 ± 0.31ab | 1.41 ± 0.44ab | 1.20 ± 0.30a | 1.62 ± 0.30b | 1.53 ± 0.27ab |
| λe (/hr) | 0.04 ± 0.00a | 0.05 ± 0.01ab | 0.07 ± 0.02c | 0.10 ± 0.04d | 0.13 ± 0.06d |
| C0 (μg/ml) | 2.49 ± 0.52a | 2.74 ± 0.69a | 2.42 ± 0.91a | 3.25 ± 0.54a | 3.26 ± 1.01a |
| AUC0–t (μg hr−1 ml−1) | 34.18 ± 8.14a | 27.35 ± 5.02b | 18.79 ± 6.96 cd | 16.10 ± 5.56de | 14.98 ± 6.10e |
| AUC∞ (μg hr−1 ml−1) | 36.74 ± 8.71a | 29.20 ± 5.36b | 19.40 ± 7.40cde | 17.43 ± 5.84de | 15.21 ± 6.22e |
| Vd (L/kg) | 3.46 ± 0.74a | 3.57 ± 0.86ab | 4.07 ± 1.07b | 3.10 ± 0.52ab | 3.17 ± 0.54ab |
| Vss (L/kg) | 3.06 ± 0.62abc | 3.37 ± 0.62c | 3.61 ± 0.97abc | 2.91 ± 0.45a | 2.97 ± 0.52ab |
| t1/2λe (hr) | 17.00 ± 2.02a | 13.96 ± 2.01ab | 10.40 ± 3.05b | 7.40 ± 2.48 cd | 6.63 ± 2.93d |
| MRT∞ (hr) | 21.64 ± 2.01a | 19.13 ± 1.53a | 13.15 ± 2.93bc | 10.04 ± 3.35 cd | 8.97 ± 3.82d |
| Cl (L kg−1 hr−1) | 0.14 ± 0.03a | 0.18 ± 0.03b | 0.29 ± 0.09c | 0.32 ± 0.12c | 0.40 ± 0.23c |
Note
- Data are presented as mean ± SD. Variables sharing the same superscript letter indicate absence of statistically significant differences between them.
The main findings of this study were that AUC0–t, AUC∞, t1/2λe, and MRT∞ trend to decrease, and Cl to increase over time. Clearance values at 3 and 10 days of life were significantly lower (~50%) compared with older animals, in which no differences were detected at 24, 50, and 80 days. Similar findings were observed with AUC∞ values at different ages. Moreover, AUC∞/Dose ratios were 7.35, 5.84, 3.88, 3.49, and 3.04 for 3, 10, 24, 50, and 80-days-old animals. In the same way, MRT values were 1.45–2.41 times higher at 3 and 10 days respecting to the other ages, and similar results were observed for t1/2λe. On the other hand, similar values of Vd and Vss were obtained among different ages, and no clear trend to increase or decrease were detected in this study.
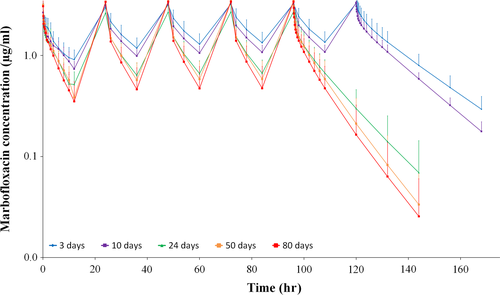
After pharmacokinetic multi-dose simulation on steady-state conditions, MFX showed an accumulative effect for the proposed dose regimen, with a significant decreasing trend with age, being ≈50% in animals with 3 days old and decreasing to ≈10% at 80 days of life, corresponding with a loading dose of ≈8 and ≈5.5 mg/kg, respectively (Table 2 and Figure 2). Consequently, AUC∞ss was proportionally higher in younger animals, as reflected by AUC∞ss/AUC∞ ratios of 1.52, 1.42, 1.30, 1.22, and 1.19 for 3, 10, 24, 50, and 80 days of life, respectively.
| Parameter | Age (days) | ||||
|---|---|---|---|---|---|
| 3 | 10 | 24 | 50 | 80 | |
| Cmax (μg/ml) | 2.49 ± 0.52 | 2.74 ± 0.69 | 2.42 ± 0.91 | 3.25 ± 0.54 | 3.26 ± 1.01 |
| Cmin (μg/ml) | 0.51 ± 0.14 | 0.41 ± 0.07 | 0.23 ± 0.09 | 0.18 ± 0.13 | 0.14 ± 0.13 |
| Cave (μg/ml) | 1.00 ± 0.23 | 0.87 ± 0.17 | 0.66 ± 0.20 | 0.65 ± 0.20 | 0.57 ± 0.22 |
| Cssmax (μg/ml) | 3.29 ± 0.64 | 3.33 ± 0.70 | 2.72 ± 1.00 | 3.46 ± 0.65 | 3.42 ± 1.00 |
| Cssmin (μg/ml) | 0.80 ± 0.23 | 0.59 ± 0.09 | 0.30 ± 0.16 | 0.21 ± 0.16 | 0.16 ± 0.16 |
| Cssave (μg/ml) | 1.53 ± 0.36 | 1.22 ± 0.22 | 0.81 ± 0.31 | 0.73 ± 0.24 | 0.63 ± 0.26 |
| R | 1.50 ± 0.07a | 1.40 ± 0.08ab | 1.22 ± 0.09abc | 1.12 ± 0.09bc | 1.10 ± 0.10c |
| Time to 99% Cssave (hr) | 112.87 ± 13.43a | 92.72 ± 13.31ab | 69.04 ± 20.23b | 49.17 ± 16.48cd | 44.04 ± 19.48d |
| Loading dose (mg/kg) | 8.02 ± 0.56a | 7.19 ± 0.53ab | 6.29 ± 0.78abc | 5.65 ± 0.48bc | 5.54 ± 0.51c |
| AUC0–tss (μg hr−1 ml−1) | 48.63 ± 12.64a | 37.78 ± 5.75a | 23.72 ± 10.09b | 20.87 ± 8.79b | 17.76 ± 8.96b |
| AUC∞ss (μg hr−1 ml−1) | 55.73 ± 15.25a | 41.39 ± 6.16a | 25.02 ± 11.86b | 21.33 ± 9.31b | 18.11 ± 9.45b |
Notes
- AUC0–tss and AUC∞ss: area under the concentration-time curve on steady-state conditions until 24 hr or extrapolated to infinity; Cmax, Cmin and Cave: maximum, minimum and average concentration of MFX at single dose; Cssmax, Cssmin and Cssave: maximum, minimum and average MFX concentration at steady-state conditions; R: accumulation factor.
- Data are presented as mean ± SD. Variables sharing the same superscript letter indicate absence of statistically significant differences between them.
3.2 Simulation of pharmacokinetic profiles
The simulated pharmacokinetic profiles of MFX (5 mg/kg) after intravenous administration in NEO and YNG groups by MCS after single dose or under steady-state conditions were within the 95% confidence interval of individually observed data. Cmax and AUC24 parameters calculated by noncompartmental pharmacokinetic analysis and bootstrap results are exposed in Table 3. At steady-state conditions, AUC24 was approximately 83% higher in NEO group compared with YNG group, but Cmax was similar.
| NEO SD | YNG SD | NEO SS | YNG SS | |
|---|---|---|---|---|
| C max | ||||
| Simulated PK profiles | ||||
| Mean | 2.59a | 2.92b | 3.28c | 3.15c |
| SD | 0.47 | 0.97 | 0.65 | 0.76 |
| CV | 18.32 | 33.35 | 19.83 | 24.20 |
| CI 95% | 2.00–3.75 | 1.84–5.56 | 2.50–5.43 | 2.01–4.54 |
| Bootstrap | ||||
| Mean | 2.60 | 2.94 | 3.30 | 3.15 |
| SD | 0.47 | 0.97 | 0.67 | 0.74 |
| CV | 17.95 | 33.20 | 20.40 | 23.56 |
| CI 95% | 1.98–3.65 | 1.86–5.13 | 2.50–4.99 | 2.03–4.66 |
| AUC 24 | ||||
| Simulated PK profiles | ||||
| Mean | 21.37a | 14.81b | 31.50c | 17.18d |
| SD | 5.36 | 3.94 | 7.56 | 4.72 |
| CV | 25.08 | 26.58 | 24.01 | 27.45 |
| CI 95% | 15.51–39.12 | 6.20–23.05 | 23.01–62.64 | 8.11–27.47 |
| Bootstrap | ||||
| Mean | 21.46 | 14.71 | 31.47 | 17.09 |
| SD | 5.46 | 3.95 | 7.42 | 4.62 |
| CV | 25.43 | 26.83 | 23.59 | 27.05 |
| CI 95% | 15.33–35.85 | 6.47–22.03 | 23.29–55.27 | 7.46–26.14 |
Notes
- CI 95%: confidence interval of 95%; CV: coefficient of variability; SD: single dose; SS: steady-state conditions.
- Variables sharing the same superscript letter indicate statistically significant differences between them.
3.3 Determination of MICs
Marbofloxacin MIC distributions for regional E. coli and S. aureus strains are represented in Table 4. Both bacterial sub-populations tested showed a unimodal distribution. The evaluated strains presented a MIC90 value of 0.06 and 0.25 μg/ml, and MIC90/MIC50 ratios were approximately 4 and 2 for E. coli and S. aureus, respectively.
| MIC distribution of Escherichia coli strains (MIC90 = 0.060 μg/ml) | ||||||
|---|---|---|---|---|---|---|
| MIC (μg/ml) | 0.015 | 0.030 | 0.060 | 0.125 | 0.250 | 0.500 |
| Absolute frequency | 20 | 1 | 7 | - | - | - |
| Relative frequency (%) | 71.40 | 3.60 | 25.00 | - | - | - |
| Cumulative frequency (%) | 71.40 | 75.00 | 100.00 | - | - | - |
| MIC distribution of Staphylococcus aureus strains (MIC90 = 0.250 μg/ml) | ||||||
|---|---|---|---|---|---|---|
| MIC (μg/ml) | 0.015 | 0.030 | 0.060 | 0.125 | 0.250 | 0.500 |
| Absolute frequency | - | - | 1 | 7 | 25 | 2 |
| Relative frequency (%) | - | - | 2.90 | 20.00 | 71.40 | 5.70 |
| Cumulative frequency (%) | - | - | 2.90 | 22.90 | 94.30 | 100.00 |
3.4 PK/PD analysis
Probability of target attainment values for Cmax/MIC and AUC24/MIC endpoints for single dose administration and steady-state conditions of MFX in NEO and YNG groups are presented in Figures 3 and 4, respectively. CFR values at different PK/PD endpoints against E. coli and S. aureus strains for the proposed dose regimen of MFX in NEO and YNG groups are shown in Table 5.
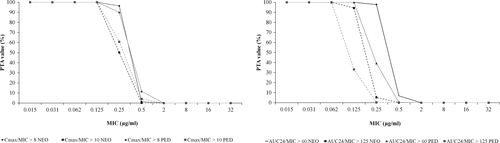
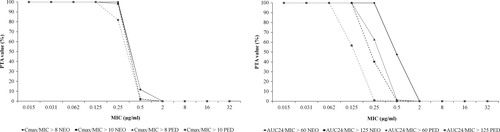
| PK/PD endpoint | Escherichia coli | Staphylococcus aureus | ||
|---|---|---|---|---|
| SD | SS | SD | SS | |
| NEO | ||||
| Cmax/MIC > 8 | 100.00 | 100.00 | 91.81 | 94.97 |
| Cmax/MIC > 10 | 100.00 | 100.00 | 58.78 | 93.08 |
| AUC24/MIC > 60 | - | - | 93.14 | 97.01 |
| AUC24/MIC > 125 | 100.00 | 100.00 | - | - |
| YNG | ||||
| Cmax/MIC > 8 | 100.00 | 100.00 | 87.83 | 93.64 |
| Cmax/MIC > 10 | 100.00 | 100.00 | 66.52 | 81.43 |
| AUC24/MIC > 60 | - | - | 50.87 | 67.78 |
| AUC24/MIC > 125 | 99.96 | 99.99 | - | - |
Note
- Values are expressed as percentage.
Since the PK/PD endpoints did not achieve adequate CFR values against S. aureus infections in the YNG group, the daily dose of MFX necessary to achieve a AUC24/MIC = 60 in the 90% of the population determined by MCS was 7.49 mg/kg.
4 DISCUSSION
4.1 Pharmacokinetic analysis
Generally, the observed volumes of distribution of MFX in tekes were higher than unity, in concordance with previously reported data of fluoroquinolones in different animal species (Guigère & Dowling, 2013). Volumes of distribution values appeared to be similar among different ages evaluated in this study. Moreover, in all cases Vd and Vss were apparently higher than reported Vss in adult llamas of 0.75 ± 0.21 L/kg (Rubio-Langre et al., 2012). These differences could be related with higher total body water and lower plasmatic albumin content in young animals. After birth, extracellular water content decreases and intracellular water content increases with age. This fact, together with a higher free-unbound fraction of the antimicrobial, could result in higher volume of distribution values (Baggot & Guigère, 2013; Nouws, 1992). Similar results have been reported for MFX and cefquinome in goat kids (Litterio, 2012; Waxman et al., 2004).
AUC0–t and AUC∞ showed a decreasing trend with age, being 56% and 59% lower at 80 days of life, compared with 3-days-old animals. This data were in concordance with the observed total body clearance values. Moreover, no significant differences in total body clearance were observed at 24, 50, and 80 days after birth. Taking into account that the renal excretion mechanisms (glomerular filtration and tubular secretion) are not completely developed until 1–2 weeks in ruminant species, and that MFX is eliminated from the body mainly by renal excretion, the higher values of Cl observed at 3 and 10 days after birth may be explained in terms of lower renal excretion of MFX (Baggot & Guigère, 2013; Nouws, 1992).
Additionally, changes in feeding habits determine variations in urinary pH. Since tubular reabsorption of fluoroquinolones is considered pH-dependent, the renal excretion of MFX could be affected by urinary pH modifications (Sörgel & Kinzig, 1993). Tekes begin feeding on grass at 14 days of life, although they continue breastfeeding until about 6 months. This marked dietary change modifies their urinary pH value from 7.0 in tekes to 8.5 in adult llamas. The relative alkalinization of the urine pH could cause a reduction in the passive tubular reabsorption rate of MFX, favoring the elimination of the antimicrobial (Fowler, 1989).
It is important to note that total body clearance observed at 80 days of life was apparently higher than that reported by Rubio-Langre et al. (2012) in adult llamas (0.09 ± 0.03 L kg−1 hr−1). It would be expected to find clearance values in adult llamas greater or similar to those observed in animals of 80 days of age. These differences could be due to different experimental conditions of the animals. In this study, tekes had ad libitum access to their mother's breastfeeding during the course of the whole study, a fact that could determine a higher daily fluid intake derived from higher water content in the diet. The adult llamas included in the study by Rubio-Langre et al. (2012) had access to solid diet with less water content and probably with some degree of water deprivation conditions, leading to lower values of total body clearance.
Regarding to t1/2λe and MRT∞, both parameters tend to decrease over time, in concordance with Cl values, from 17.00 ± 2.02 hr and 21.64 ± 2.01 hr to 6.63 ± 2.93 hr and 8.97 ± 3.82 hr, respectively. Observed t1/2λe and MRT∞ values at 80 days were similar to those reported by Rubio-Langre et al. (2012) in adult llamas (9.16 ± 1.08 and 7.85 ± 1.15 hr). Similar results with MFX were reported by Díaz (2012) in calves and Waxman et al. (2004) in goat kids.
Finally, IV administration of MFX (5 mg/kg) in tekes showed lower Cl (with longer persistence in the body), and higher AUCs values in animals at days 3 and 10 after birth, compared with older animals. These findings are in agreement with the physiological changes observed in young ruminants in the first 2 weeks of life (Nouws, 1992).
4.2 Simulation of pharmacokinetic profiles
Multi-dose simulation of the proposed dose regimen showed that MFX presented an accumulative effect about 50% when steady-state was reached in 3-day-old animals, decreasing to about 10% in animals of 80 days of age, according with t1/2λe values. It is important to note that steady-state conditions could take up to 4–5 days to be reached in animals at 3–10 days after birth, or 2–4 days in animals at 24 days after birth, so a useful option would be to use a loading dose of about 8 mg/kg in animals ≤10-days-old, and 6–7 mg/kg in animals of 24 days of age. In this study, a dose of MFX greater than 5 mg/kg was not evaluated, so the proposed loading doses should be studied for adverse reactions in tekes.
After MCS of pharmacokinetic profiles of MFX in NEO and YNG groups under single dose and steady-state conditions (n = 400), all emulated concentration-time profiles were within the 95% confidence interval of individually observed data, indicating that the simulated profiles were representative of the data sub-population from which they originated. Cmax in YNG group was higher than that of NEO group after single dose of MFX, but these differences were not observed when steady-state conditions were evaluated. Moreover, AUC24 values were higher in the NEO group under single dose and steady-state conditions, according to the pharmacokinetic differences between both sub-populations. Additionally, AUC24 under steady-state conditions were always higher than observed under single dose, in concordance with the accumulative effect of MFX observed for this dose regimen in tekes.
The bootstrap data (n = 1,000) generated from 4 sets of 100 Cmax and AUC24 data in NEO and YNG groups under single dose or steady-state conditions of MFX were used to increase the robustness of the pharmacokinetic parameters, being subsequently included in the PK/PD analysis of the proposed dose regimen of MFX by MCS.
4.3 Determination of MICs
MIC90 values for regional E. coli and S. aureus strains were estimated at 0.06 and 0.25 μg/ml, respectively. All strains tested presented MIC values below the breakpoint of 1 μg/ml, following the CLSI guidelines, and were therefore, considered susceptible to MFX (CLSI, 2013). Moreover, MIC90/MIC50 ratios were relatively low and both sub-populations presented an apparent unimodal distribution of MICs. Taking this into account, our data suggests no evidence of emergence of bacterial resistance to MFX in the evaluated strains.
Meunier, Acar, Martel, Kroemer, and Valle (2004) reported MIC90 values for E. coli strains isolated from bovine gut infections of 5.10 μg/ml and from bovine mastitis of 0.023 μg/ml. Authors attributed the susceptibility variations observed between the E. coli strains to different ecological environments, and to the fact that the intestinal ecosystem promotes a fast development and horizontal and vertical transmission of resistance genes (mainly plasmidic) among the whole intestinal microbiota (Holmes et al., 2016). Additionally, Kroemer, Galland, Guérin-Faublée, Giboin, and Woehrlé-Fontaine (2012), reported similar MIC90 value for MFX of 0.03 μg/ml, in concordance with the results observed in llamas (MIC90 = 0.06 μg/ml). The relatively lower MIC90 values from E. coli strains isolated from gut infections of llamas compared with those of bovine origin could be related to a lower use of fluoroquinolones in llamas, and to a lower antimicrobial selective pressure.
Regarding S. aureus with MIC90 = 0.25 μg/ml, similar results were reported by Meunier et al. (2004) and by Kroemer et al. (2012), with a MIC90 values for MFX of 0.284 μg/ml and 0.25 μg/ml, respectively.
4.4 PK/PD analysis
For PK/PD analysis we selected the same endpoint values of Cmax/MIC for E. coli and S. aureus (Cmax/MIC > 8 and Cmax/MIC > 10), but different for AUC24/MIC (AUC24/MIC > 125 for E. coli and AUC24/MIC > 60 for S. aureus) based on recommended PK/PD endpoints for fluoroquinolones (Drusano, 2007; Papich, 2014).
Since Meunier et al. (2004) and Kroemer et al. (2012) reported less susceptible sub-populations of E. coli and S. aureus strains ranging from 2–32 μg/ml, we decided to include this range of values into the PK/PD analysis, in order to determine the PTA values for pathogens with these MIC values.
The proposed therapeutic regimen of MFX (5 mg/kg) after IV administration after single dose and steady-state conditions in NEO group showed adequate PTA values for most exigent Cmax/MIC and AUC24/MIC endpoints against pathogens with MIC values ≤ 0.125 μg/ml, while the YNG group did so for microorganisms with MIC values of 0.06 μg/ml, respectively.
Cumulative fraction of response values of MFX for Cmax/MIC and AUC24/MIC endpoints against E. coli strains indicated that the proposed dose regimen could be effective against the studied bacterial population in both NEO and YNG groups after single dose or on steady-state conditions. On the other hand, for S. aureus only NEO group achieved the CFR endpoints after single dose and on steady-state conditions, but YNG group did not reach the efficacy criteria for most exigent endpoints. Therefore, a higher MFX dose of ≈ 7.5 mg/kg should be used in animals with ≥24 days old for the treatment of infections caused by this specific pathogen.
In conclusion, PK/PD analysis suggest that the proposed therapeutic regimen of MFX (5 mg/kg) after IV administration, could be effective for the treatment of infections caused by E. coli strains in animals between 3 and 80 days old, and for infections produced by S. aureus in animals between 3 and 24 days of life. Additionally, a higher dose should be used for the treatment of infections caused by the latter pathogen in animals older than 24 days, and further studies should be conducted to support the clinical utility of marbofloxacin in llamas.
ACKNOWLEDGMENTS
This study was funded by Universidad Complutense de Madrid and Universidad Católica de Córdoba. The authors would like to thank to Dr. Martín Alejandro Himelfarb and Dr. María del Pilar Zarazaga for their invaluable help provided in the experimental phase, and to Mr. Mariano Diaz-Flores for the important technical assistance.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
All authors have read and approved the final manuscript. S. R. L.: Design, acquisition, analysis and interpretation of data. S. A. S.: Acquisition, analysis and interpretation of data. A. M. L.: Analysis, interpretation of data and drafting the manuscript. M. I. S. A.: Design and critical revision of the manuscript for important intellectual content. J. J. D. L.: Acquisition, analysis of data and critical revision of the manuscript for important intellectual content. N. J. L.: Design and critical revision of the manuscript for important intellectual content.




