Effect of dasatinib in a xenograft mouse model of canine histiocytic sarcoma and in vitro expression status of its potential target EPHA2
Abstract
Canine histiocytic sarcoma (HS) is an aggressive and highly metastatic tumor. Previously, the kinase inhibitor dasatinib was shown to have potent growth inhibitory activity against HS cells in vitro, possibly via targeting the EPHA2 receptor. Here, the in vivo effect of dasatinib in HS cells was investigated using a xenograft mouse model. Moreover, the expression status of EPHA2 was examined in six HS cell lines, ranging from insensitive to highly sensitive to dasatinib. In the HS xenograft mouse model, dasatinib significantly suppressed tumor growth, as illustrated by a decrease in mitotic and Ki67 indices and an increase in apoptotic index in tumor tissues. On Western blot analysis, EPHA2 was only weakly detected in all HS cell lines, regardless of sensitivity to dasatinib. Dasatinib likely results in the inhibition of xenograft tumor growth via a mechanism other than targeting EPHA2. The findings of this study suggest that dasatinib is a targeted therapy drug worthy of further exploration for the treatment of canine HS.
Canine histiocytic sarcoma (HS) is an aggressive and highly metastatic tumor. Thus, multidisciplinary therapy involving surgery, radiotherapy, and chemotherapy is often applied for the treatment of this tumor. However, even with the use of these therapeutic approaches, effective treatment of HS remains challenging, and long-term prognosis is generally poor (Fidel et al., 2006; Klahn, Kitchell, & Dervisis, 2011); therefore, improved therapies are needed. To develop a foundation for new therapeutic approaches to HS, we previously screened 171 chemical compounds potentially applicable for targeted therapy using HS cell lines. As a result, we found that the kinase inhibitor dasatinib exhibited potent growth inhibitory activity in certain cell lines of HS in vitro, possibly via targeting EPHA2, a member of the large family of ephrin receptor tyrosine kinases (Ito, Kuroki, et al., 2013).
Dasatinib targets tyrosine kinases including SRC family kinases (SRC, LCK, YES, and FYN), BCR-ABL, KIT, PDGFRβ, and EPHA2 (Steinberg, 2007). Further, it has been demonstrated to have potent therapeutic activity toward chronic myelogenous leukemia and acute lymphoblastic leukemia expressing BCR-ABL in humans (Talpaz et al., 2006). In addition, the therapeutic potential of dasatinib has been suggested in dogs with osteosarcoma, although the target was not identified (Marley, Gullaba, Seguin, Gelberg, & Helfand, 2015). However, no in vivo evidence of its efficacy against HS has been reported.
In this study, in vivo effects of dasatinib on the growth of HS were examined using a xenograft mouse model. Moreover, the expression status of the potential target EPHA2 was examined in six HS cell lines.
The HS cell line CHS-1 (Azakami et al., 2006) was used for the xenograft mouse model experiment because this cell line exhibited the lowest IC50 against dasatinib among six HS cell lines examined in a previous study (Ito, Kuroki, et al., 2013). Dasatinib was purchased from LC Laboratories (Woburn, MA). CHS-1 cells maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (cDMEM) were trypsinized, washed in PBS, and suspended in PBS. A 200-μl aliquot of PBS containing 1 × 107 CHS-1 cells was injected subcutaneously into one flank of 3-week-old female BALB/c nu/nu mice (n = 12; CLEA Japan, Tokyo, Japan). Tumor size was measured, and the tumor volume was estimated using the following formula: V = (L × W2)/2 (V: volume, L: length, W: width). After palpable subcutaneous tumors reached 100–200 mm3, mice were randomized to either the dasatinib-treatment (n = 7) or control (n = 5) group. For the dasatinib-treatment group, dasatinib was orally administered at a dose of 10 mg/kg in 100 μl of 80 mm citrate buffer (pH 3.1) containing 5% DMSO daily. In the previous nude mice studies, Cmax of dasatinib at oral dose of 5 mg/kg has been reported as 0.104 μm (Kamath, Wang, Lee, & Marathe, 2008) and 0.119 μm (Luo et al., 2006) and that of 15 mg/kg has been reported as 0.32 μm (Kamath et al., 2008). In this study, to bring Cmax of dasatinib in the mice close to that in human patients with CML treated with clinically maximum dose of dasatinib (Cmax, around 0.174 μm at 140 mg/person; Luo et al., 2006), we use a dose of 10 mg/kg. The control group was administered 100 μl of the same buffer but without dasatinib in the same manner. The first day of administration was set at Day 0, and daily treatment was continued until Day 28, at which time the xenograft tumors were excised. The mice then underwent necropsy and pathological examination of vital organs.
Using sections prepared from the excised tumor xenografts, the mitotic, Ki67, and apoptotic indices were calculated as described previously (Ito, Kobayashi, et al., 2013). Briefly, sections were stained with hematoxylin and eosin (HE), Ki-67, and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL), and counted as morphologically identifiable mitotic cells, Ki67-positive cells, and TUNEL-positive cells, respectively. The indices were expressed as a percentage of positive cells/total number of cells in the same field (three noncontiguous fields per section at ×400 magnification).
All experiments in this study were conducted following approval from the Committee for Animal Experimentation of Nippon Veterinary and Life Science University (approval number 13-19).
As shown in Figure 1a, dasatinib significantly suppressed the growth of tumors on Day 4 and thereafter (dasatinib vs. control: Days 4–24, p < .01; Days 26 and 28, p < .005). During the treatment, no visible toxic effects, including loss of bodyweight, were observed in either dasatinib-treated or control mice. At necropsy and upon subsequent pathological evaluation of vital organs, no metastasis of tumor cells was observed in both dasatinib-treated and control mice. No possible drug-related toxicity was identified. Tumors from the dasatinib-treated mice showed decreases in both mitotic and Ki67-positive cells (Figure 1b), with significantly lower mitotic and Ki67 indices compared with those from controls (p < .05 and p < .01, respectively) (Figure 1c). The TUNEL-positive cells increased in the tumors from the dasatinib-treated mice (Figure 1b), with significantly higher apoptotic index than that of controls (p < .01) (Figure 1c).
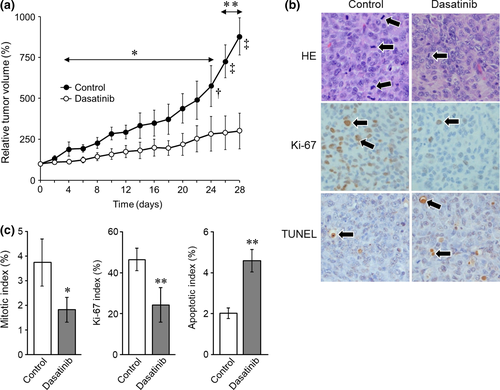
Results of the xenograft mouse model experiment confirmed our previous in vitro findings of the growth inhibitory activity of dasatinib against HS cells (Ito, Kuroki, et al., 2013) and suggested the therapeutic potential of dasatinib against HS in dogs. Moreover, it was found that dasatinib exerts both cytostatic (decrease in mitotic and Ki67 indices) and cytotoxic (increase in apoptotic index) effect against HS cells. However, in spite of its cytotoxic activity, no mouse showed shrinkage of tumors with dasatinib treatment. One possible explanation for the lack of tumor regression is that dasatinib may not have had sufficient apoptosis-inducing activity at the dose employed in this study. Alternatively, as opposed to our previous study (Ito, Kuroki, et al., 2013), the survival of HS cells did not appear to be strongly dependent on kinase(s) targeted by dasatinib, as a xenograft mouse harboring the human myelogenous leukemia cell line K562, which is strongly dependent on Bcr-Abl for survival, showed remarkable tumor regression following treatment with 5 mg/kg dasatinib, a dose lower than our dose of 10 mg/kg (Lombardo et al., 2004). As such, the effects of dasatinib could be described as cytostatic rather than cytotoxic in HS cells in vivo.
Encouraged by the finding that dasatinib exhibits a potent growth inhibitory effect on HS cells in vivo, we attempted to identify the target of dasatinib in HS cells. Although dasatinib targets several kinases as described above, SRC family kinases, BCR-ABL, KIT, and PDGFRβ are not likely to be the therapeutic targets in CHS-1 cells, as we observed that compounds targeting BCR-ABL (four compounds), SRC family members (SRC, LCK, YES, FYN) (five compounds), KIT (five compounds), and PDGFR (nine compounds) showed no or minimal growth inhibitory effect on CHS-1 cells in a previous study (Ito, Kuroki, et al., 2013). Therefore, it was hypothesized that dasatinib resulted in inhibition of tumor growth in the xenograft mouse model by inhibiting another target, EPHA2. Therefore, we focused on EPHA2 in subsequent experiments.
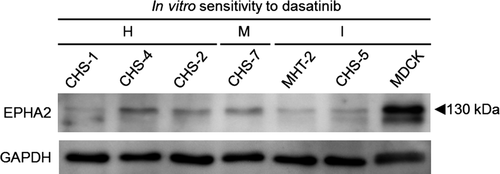
Overexpression of EPHA2 has been shown to be an oncogenic driver of human cancers (Tandon, Vemula, & Mittal, 2011). We thus examined the expression status of EPHA2 in CHS-1 cells and other HS cell lines that exhibit different sensitivities against dasatinib in vitro (Ito, Kuroki, et al., 2013). These cell lines included the following: highly sensitive, CHS-1 (IC50, 5.4 nm), CHS-2 (IC50, 8.3 nm), and CHS-4 (IC50, 7.5 nm); moderately sensitive, CHS-7 (IC50, 54.5 nm); and insensitive, CHS-5 (IC50, >1,000 nm) and MHT-2 (IC50, 968.1 nm). The non-HS dasatinib-insensitive canine cell line MDCK (Madin-Darby canine kidney) was also used. Cell lines were subjected to Western blot analysis using polyclonal rabbit anti-mouse EPHA2 (Santa Cruz Biotechnology, Santa Cruz, CA) or polyclonal goat anti-human GAPDH antibody (Santa Cruz Biotechnology) combined with biotin-conjugated goat anti-rabbit immunoglobulin (Thermo Fisher Scientific, Rockford, IL) or biotin-conjugated rabbit anti-goat immunoglobulin (Thermo Fisher Scientific). Immunoreactive proteins were visualized using peroxidase-conjugated streptavidin and an enhanced chemiluminescence system (GE Healthcare, Chalfont, UK).
As shown in Figure 2, an immunoreactive band for EPHA2 was detected in all cell lines at the size of 130 kDa, which corresponds to the size of human EPHA2 (Duxbury, Ito, Zinner, Ashley, & Whang, 2004). Regardless of the level of sensitivity against dasatinib, all HS cell lines, especially CHS-1, MHT-2, and CHS-5, weakly expressed EPHA2, whereas only MDCK showed clear expression. No relationship between the expression level of EPHA2 and sensitivity to dasatinib was observed. From these findings, it is likely that EPHA2 is not crucially involved in the growth of either dasatinib-insensitive or dasatinib-sensitive HS cells. Moreover, in addition to the Western blot analysis, we examined the nucleotide sequence of EPHA2 in CHS-1 cells, as a mutation in EPHA2 has been shown to cause constitutive kinase activation in human lung squamous cell carcinoma and contribute to its development (Faoro et al., 2010). Using spleen cells collected from a normal dog, we identified the canine counterpart of human EPHA2 gene (registered as KY793106 in NCBI), and the complete nucleotide sequence of EPHA2 was examined using CHS-1 cells. However, no mutation altering the amino acid sequence was identified (data not shown). Therefore, these findings suggest that dasatinib inhibits xenograft tumor growth via a mechanism other than targeting EPHA2. Suppression of the phosphorylation of undefined target(s) by dasatinib could underlie the growth inhibition of the xenograft tumor. To identify the target(s), future comprehensive kinome analysis using canine HS cells will be needed.
In the present study, dasatinib was found to have potent growth inhibitory activity against tumors in the xenograft mouse model of HS. This finding suggests that kinase inhibition therapy using dasatinib is a strategy worthy of further exploration for the treatment of canine HS. However, we were unable to identify the target of dasatinib in HS cells. As there are differences in sensitivity to dasatinib among HS cell lines, it was suggested that HS cells possess different growth drivers among patients. Therefore, it is postulated that dasatinib has benefits only for a certain subset of HS. Identification of the target of dasatinib in HS and subsequent establishment of an individualized approach to treatment are important before moving forward to the clinical study of dasatinib.
ACKNOWLEDGMENTS
This research was partially supported by a Grant-in-Aid for Scientific Research (No. 15H04601) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
CONFLICT OF INTEREST
None of the authors of this article has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of this paper.




