Pharmacokinetics of cefquinome in red-eared slider turtles (Trachemys scripta elegans) after single intravenous and intramuscular injections
Abstract
The purpose of this study was to evaluate the pharmacokinetics of cefquinome (CFQ) following single intravenous (IV) or intramuscular (IM) injections of 2 mg/kg body weight in red-eared slider turtles. Plasma concentrations of CFQ were determined by high-performance liquid chromatography and analyzed using noncompartmental methods. The pharmacokinetic parameters following IV injection were as follows: elimination half-life (t1/2λz) 21.73 ± 4.95 hr, volume of distribution at steady-state (Vdss) 0.37 ± 0.11 L/kg, area under the plasma concentration–time curve (AUC0–∞) 163 ± 32 μg hr−1 ml−1, and total body clearance (ClT) 12.66 ± 2.51 ml hr−1 kg−1. The pharmacokinetic parameters after IM injection were as follows: peak plasma concentration (Cmax) 3.94 ± 0.84 μg/ml, time to peak concentration (Tmax) 3 hr, t1/2λz 26.90 ± 4.33 hr, and AUC0–∞ 145 ± 48 μg hr−1 ml−1. The bioavailability after IM injection was 88%. Data suggest that CFQ has a favorable pharmacokinetic profile with a long half-life and a high bioavailability in red-eared slider turtles. Further studies are needed to establish a multiple dosage regimen and evaluate clinical efficacy.
Red-eared slider turtles are popular pet turtles in several countries (Harris et al., 2009). They have long life spans and are exposed to bacterial infections that can lead to disease and death (McArthur, 2004). Therefore, antibacterial therapy is a basic tool in their management. Antibacterial dosage regimens for these animals are either empirical or extrapolated from mammals or other reptiles (Stamper et al., 1999, 2003). However, similar to the class Mammalia, class Reptilia is divided into diverse groups of species, each with a unique pharmacologic response to drugs (Sladky & Mans, 2012). As a result, the drug safety and efficacy differ among species (Mallo, Harms, Lewbart, & Papich, 2002), and cross-species extrapolation is unsafe (Marín et al., 2009; Stamper et al., 1999, 2003).
Cefquinome (CFQ) is an aminothiazolyl cephalosporin developed exclusively for veterinary use (Murphy, Erwin, & Jones, 1994). It differs from older cephalosporins due to the addition of a quaternary ammonium side chain, which increases binding to penicillin-binding proteins and the penetration into the periplasmatic space of gram-negative bacilli (Bryskier, 1997; Murphy et al., 1994). CFQ has high antibacterial activity against most gram-positive and negative bacteria including Streptococcus spp., Actinobacillus spp., Enterobacteriaceae, Pasteurellaceae, and S. aureus (Bottner, Schmid, & Humke, 1995; Limbert et al., 1991; Murphy et al., 1994; Thomas, Thomas, & Wilhelm, 2006). It is approved for the treatment of several infections in cattle, pigs, horses, and foals (CVMP, 1995, 1999, 2003). CFQ could potentially be used for the treatment of many turtle diseases. Pharmacokinetic studies of CFQ have been investigated in salmon (San Martín, Bataglia, Hernández, Quiroz, & Cañon, 1998), camels (Al-Taher, 2010), rabbits (Hwang et al., 2011), sheep (Tohamy, 2011; Uney, Altan, & Elmas, 2011), ducks (Yuan et al., 2011), ponies (Smiet, Haritova, Heil, Fink-Gremmels, & Wijnberg, 2012), cross-bred wild boars (Liu et al., 2012), chickens (Xie, Zhang, Wang, & Du, 2013), pigs (Zhao et al., 2013), cattle (Shan et al., 2014), tilapia (Shan et al., 2015), dogs (Zhang et al., 2014), buffalo calves (Venkatachalam & Dumka, 2015), and horses (Uney et al., 2017). These studies have reported favorable pharmacokinetic features of CFQ, such as good absorption, high bioavailability, and primary elimination via the kidney. However, there is currently no information on the pharmacokinetics of CFQ in red-eared slider turtles. Therefore, the aim of this study was to investigate the pharmacokinetics of CFQ after single intravenous (IV) and intramuscular (IM) injections in red-eared slider turtles.
The study was performed in twelve healthy red-eared slider turtles weighing between 0.340 and 0.515 kg. Turtles were obtained from a retail pet supply store and acclimated for 1 month prior to the trial. Health status was evaluated by the physical examination. Turtles were maintained in four 450-L aquariums (three turtles in each aquarium), which have custom-built mechanical and biological filtration systems. The aquarium water quality was assessed twice a week to measure pH, O2, ammonia, nitrate and nitrite levels using test kits. Turtles were kept in optimal conditions using water conditioners with respect to the pH (6.8–7.5), O2 (>6 mg/L), ammonia (<0.5 mg/L), nitrate (<10 mg/L), and nitrite (<0.5 mg/L) concentrations. The aquarium water (25% of volume) was changed once a week to maintain the water quality. The temperatures of the aquarium water and the basking area were 24°C and 30°C, respectively. The Ethics Committee of the Faculty of Veterinary Medicine, University of Selcuk, approved the use of turtles for this study and all study protocols.
In the pharmacokinetic study, a parallel design was performed. The turtles were randomly assigned to IV or IM treatment groups (six per group). CFQ was supplied by Provet® (Turkey) as vials containing a powder form of the drug, and used for animal administration and drug analysis. Each animal received a single injection of CFQ (1%, in an aqueous solution) at a dose of 2 mg/kg body weight (BW). CFQ was injected into the left jugular vein or the left deltoid muscle for IV and IM administration, respectively. Blood sampling alternated between the right and left cervical sinuses at 0 (predose sample), 0.5, 1.5, 3, 6, 9, 12, 24, 48, 72, 96, and 120 hr after drug injection. At the time of blood collection, turtles were removed from the water and fixed in the basking area during for 1 min. Approximately 0.4 ml of blood was collected from each turtle using a 1-ml insulin syringe (26-gauge, 0.5-inch needle) rinsed before use with 0.05 ml of heparin sodium solution (1.000 IU/ml) as an anticoagulant. Blood was centrifuged at 2000 g for 10 min, and plasma was harvested and frozen at −70°C in Ultra-Low Temperature Freezer until analysis. All samples were analyzed within 1 month of the experiments.
Drug concentration in plasma was quantified using the reverse phase high-performance liquid chromatography (HPLC) according to the method previously described by Uney et al. (2011). The UV detection wavelength was 268 nm. Chromatography was performed on a Gemini™ C18 column (250 mm × 4.6 mm i.d., 5 μm, Phenomenex, Torrance, CA, USA) and a linear gradient of acetonitrile in water with constant 0.1% trifluoroacetic acid at a flow rate of 0.9 ml/min. Plasma samples were extracted with methanol, and 50 μl of supernatant was injected into the chromatographic system. The selectivity of the method in turtle plasma was evaluated by comparing the chromatogram from the extraction of CFQ standard and the chromatogram from blank plasma samples from six turtles. No interference was observed with biological compounds in plasma. The calibration curve provided excellent linearity with a correlation coefficient r2 > .9998 within the range of the calibration curve from 0.01 to 10 μg/ml. The limit of detection was 0.01 μg/ml. The limit of quantification was found to be 0.02 μg/ml for CFQ in turtle plasma with the coefficient of variation less than 20%. The mean extraction recoveries of CFQ determined over the concentrations of 0.1, 1 and 10 μg/ml were 95.24 ± 4.56, 93.76 ± 2.69, and 96.14 ± 3.96%, respectively. The intraday variability calculated from three plasma QC samples sixfold injected on the same day was low, with precision ranging from 2.04% to 4.98% for the coefficient of variation and accuracy from −4.21% to 5.17% for bias. The interday evaluation of plasma QC samples gave good results with precision from 1.24% to 6.05% for the coefficient of variation and accuracy from −6.09% to 4.78% for bias.
Pharmacokinetic analyzes were performed with Phoenix WinNonlin V6.3 software (Pharsight, Certara, St. Louis, MO, USA) using noncompartmental methods. The pharmacokinetic parameters were calculated using the formulae described by Gibaldi and Perrier (1982). The variables calculated included the area under the curve from time 0 to infinity (AUC0–∞) estimated by the log-trapezoidal rule from the plasma drug concentration versus time plots, total body clearance (ClT), volume of distribution at steady-state (Vdss), elimination rate constant (λz), terminal elimination half-life (t1/2λz), and mean residence time extrapolated to infinity (MRT). The λz was determined by logarithmic regression of the time points in the terminal elimination phase. The maximum plasma concentration (Cmax) and time to reach Cmax (Tmax) after IM administration were determined directly from the plasma concentration versus time curve for each turtle. Bioavailability (F) of CFQ for IM administration was determined by dividing the mean AUC0–∞ after IM administration by the mean AUC0–∞ after IV administration.
All values are expressed as mean ± SD. Harmonic means were calculated for t1/2λz and MRT. The Mann–Whitney U test was used to identify significant differences between administration routes in t1/2λz and MRT. Other pharmacokinetic data were compared for statistical significance using the Independent t test. A value of p < .05 was considered significant. All statistical analyzes were performed with SPSS V22.0 statistical software (IBM Corp, Armonk, NY).
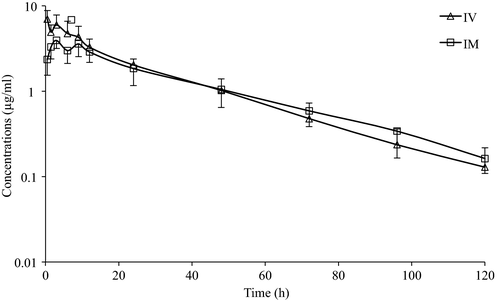
No clinical abnormalities in any of the red-eared slider turtles were detected during the study. No local signs of pain or soft tissue swelling at injection sites or systemic adverse reactions to CFQ were observed after IV or IM injection. The mean plasma concentration–time curves for CFQ in turtles following single IV and IM injection are presented in Figure 1. The pharmacokinetic values of CFQ are summarized in Table 1. The initial plasma drug concentration following IV injection was 8.42 μg/ml. A peak plasma concentration (Cmax) of 3.94 μg/ml occurred at a Tmax of 3 hr following IM injection. These data indicate that all pharmacokinetic parameters of CFQ in red-eared sliders after single IV and IM injections of CFQ at 2 mg/kg BW are similar.
| Parameter | Units | IV | IM |
|---|---|---|---|
| AUC0–∞ | hr μg/ml | 163 ± 32 | 145 ± 48 |
| AUCextrap % | % | 2.79 ± 2.33 | 4.86 ± 1.67 |
| t1/2 λz (HM) | hr | 21.73 ± 4.95 | 26.90 ± 4.33 |
| ClT | ml hr−1 kg−1 | 12.66 ± 2.51 | – |
| MRT0–∞ (HM) | hr | 27.59 ± 9.49 | 37.67 ± 3.76 |
| V dss | L/kg | 0.37 ± 0.11 | – |
| C 0 | μg/ml | 8.42 ± 2.71 | – |
| T max | hr | – | 3.00 ± 0.00 |
| C max | μg/ml | – | 3.94 ± 0.84 |
| F | % | – | 88 |
- Data represent mean ± SD. There was no statistically significant difference between IV and IM administration in the same parameter (p > .05).
- AUC0-∞, area under the plasma concentration–time curve; AUCextrap %, area under the plasma concentration–time curve extrapolated from tlast to ∞ in of the total AUC; t1/2λz, elimination half-life; ClT, total body clearance; MRT0–∞, mean residence time; Vdss, volume of distribution at steady-state; C0, plasma concentration at time 0; Tmax, time to reach maximum concentration; Cmax, maximum plasma concentration; F, bioavailability; HM, harmonic mean.
The dose of CFQ used in the present study (2 mg/kg) was chosen based on pharmacokinetic data in several mammalian (Dumka, Dinakaran, Ranjan, & Rampal, 2013; Hwang et al., 2011; Li et al., 2008; Uney et al., 2011) and avian species (Xie et al., 2013). Our results suggested that single IV or IM administration of 2 mg/kg CFQ was well tolerated and did not cause clinically adverse effects. Moreover, CFQ doses of 5–20 mg/kg caused no adverse reactions and were well tolerated at in Coho salmon and tilapia (San Martín et al., 1998; Shan et al., 2015).
CFQ in red-eared slider turtles displayed a small Vdss with the mean value of 0.37 L/kg, which was similar to the ranged of 0.36–0.49 L/kg reported in some mammalian and avian species (Dumka et al., 2013; Li et al., 2008; Uney et al., 2011; Xie et al., 2013; Yuan et al., 2011) and to a Vdss of 0.42 L/kg following ceftazidime administration in loggerhead sea turtles (Stamper et al., 1999). This limited distribution of CFQ might be due to its hydrophilic nature and low pKa values of 2.51 or 2.91 (CVMP, 1995). Because of the similarity in volumes of distribution across species, the remarkably long half-life in turtles found in this study can likely be attributed to differences in the clearance of CFQ. The mean ClT of CFQ in this study was 12.66 ml hr−1 kg−1, which is much lower than the corresponding values of 0.06–0.35 L hr−1 kg−1 reported in some mammalian and avian species for CFQ (Dumka et al., 2013; Li et al., 2008; Uney et al., 2011; Xie et al., 2013; Yuan et al., 2011). The mean t1/2λz of CFQ following IV administration in red-eared slider turtles was 21.73 hr, which is consistent with a t1/2λz of 20.59 hr reported for ceftazidime in loggerhead sea turtles (Stamper et al., 1999). The t1/2λz of CFQ following IM administration was similar to that recorded in Coho salmon (20.56 hr) following IP administration of CFQ (20 mg/kg) at 10°C (San Martín et al., 1998) and in loggerhead sea turtles (19.08 hr) following IM injection of ceftazidime (Stamper et al., 1999). Different results are reported in tilapia, for which elimination half-lives following IM and IP administrations of CFQ (10 mg/kg) at 30°C were 5.81 and 6.85 hr, respectively (Shan et al., 2015). CFQ is minimally metabolized and excreted predominantly by the kidneys in mammals (CVMP, 1995). Differences in the ClT and t1/2λz of CFQ between species are possibly the result of species differences and temperature adaptations, which affect renal clearance rates and the activity of the cardiovascular system (Kik & Mitchell, 2005; Van Ginneken, Nouws, Grondel, Driessens, & Degen, 1991).
Following IM injection, the Cmax (3.94 μg/ml) was lower than those normalized to a 2 mg/kg dose in tilapia (9.88 μg/ml and 8.88 μg/ml, respectively) following IM and IP administrations of CFQ (10 mg/kg) at 30°C (Shan et al., 2015) and substantially greater than that normalized to 2 mg/kg dose in Coho salmon (0.34 μg/ml) following IP administration of CFQ (20 mg/kg) at 10°C (San Martín et al., 1998). The Tmax of CFQ in turtles (3 hr) was longer than those in tilapia (0.14 and 0.17 hr) after IM and IP administrations of CFQ (10 mg/kg) at 30°C (Shan et al., 2015), but shorter than that in Coho salmon (12 hr) after IP administration of CFQ (20 mg/kg) at 10°C (San Martín et al., 1998). Different Tmax values may be related to differences in cardiac rate and tissue blood flow resulting from species difference and temperature adaptations (Kik & Mitchell, 2005; Van Ginneken et al., 1991). The overall MRT was longer for IM injection (31.92 hr) compared with that for IV injection (24.99 hr); this was expected because the disposition and absorption rates after IM injection affect the MRT. The MRT of CFQ was similar to that recorded in loggerhead sea turtles (31.7 hr) following IM administration of ceftazidime (Stamper et al., 1999).
Secondary peaks, which occur after administrations of various drugs by different routes in chelonians, are generally attributed to enterohepatic recirculation, altered blood circulation at rest and during swimming, and urinary reabsorption (Butler, Milsom, & Woakes, 1984; Kummrow, Tseng, Hesse, & Court, 2008; Lai et al., 2015; Stamper et al., 1999). In our trial, secondary peaks were observed following IV and IM administrations at 3 and 9 hr, respectively. CFQ is excreted unchanged in the urine (CVMP, 1995). Thus, it is unlikely that the secondary peaks are due to enterohepatic recirculation in the present case. As the composition of urine in tortoises and turtles can be altered across the urinary bladder wall, soluble drugs excreted unchanged in the urine may be passed into the blood with a concentration gradient, leading to a secondary drug peak (Lawrence, Palmer, & Needham, 1986). A secondary peak has been reported after the IM administration of ceftazidime in loggerhead sea turtles (Stamper et al., 1999) and of carbenicillin in Greek tortoises and Hermann's tortoises (Lawrence et al., 1986). In this study, urinary resorption and altered blood circulation during diving responses may explain the secondary peak of CFQ.
In this study, a limitation associated with the study protocol was taken from the dorsal cervical sinus (left and right) of blood samples in the red-eared sliders. In turtles, the sample contamination with lymph is common at most venipuncture sites other than the jugular vein. However, serial collection of blood from the jugular vein for pharmacokinetic analysis is difficult for especially small turtles. Because the dorsal cervical sinus (left and right) is a reliable site for uncontaminated blood collection with minimal restraint in turtle (Flanagan, 2014) the dorsal cervical sinus (left and right) for blood collection in this study was preferred. However, lymphatic dilution affected plasma CFQ levels cannot be overlooked.
In the present study, the AUC (145 μg hr−1 ml−1) of CFQ following IM injection was not significantly lower than the AUC of the drug following IV injection (163 μg hr−1 ml−1). The bioavailability of 88% following IM injection indicated excellent absorption of CFQ after IM injection. A high bioavailability of CFQ after IM injection, ranging from 89% to 96%, has been reported in some mammalian and avian species after IM injection (Hwang et al., 2011; Li et al., 2008; Uney et al., 2011; Xie et al., 2013; Yuan et al., 2011).
In conclusion, CFQ shows a favorable pharmacokinetic profile (a long half-life and high bioavailability) in red-eared slider turtles following a single dose of 2 mg/kg by IV and IM routes. The long elimination half-life in turtles may reduce the frequency of administration of CFQ. However, further studies are needed to evaluate the in vitro and in vivo antibacterial effects, the multiple dosage regimen, the clinical efficacy, and toxicological properties of CFQ before its application in turtles.
ACKNOWLEDGMENTS
This study was presented in abstract form as poster presentation in the 12th International Congress of the European Association for Veterinary Pharmacology and Toxicology, 8-12 July 2012, 123-124pp., Noordwijkerhout, the Netherlands.
CONFLICT OF INTEREST
The authors declare no conflict of interests.




