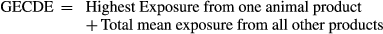Dietary exposure assessment of cyadox based on tissue depletion of cyadox and its major metabolites in pigs, chickens, and carp
Abstract
The tissue kinetics of cyadox, an antibacterial agent used in food animals, and its major metabolites in pigs, chickens, and carp were investigated followed by a complete dietary exposure assessment to evaluate the food safety of cyadox. Cyadox and its major metabolites, bisdeoxycyadox (Cy1), 4-desoxycyadox (Cy2), N-(quinoxaline-2-methyl)-cyanide acetyl hydrazine (Cy4), quinoxaline-2-carboxylic acid (Cy6), and 2-hydromethyl-3-hydroxy-quinoxaline (Cy12), were simultaneously quantitated with a high-performance liquid chromatography−ultraviolet (HPLC-UV) method. Pigs, chickens, and carp were fed with 150 mg/kg cyadox in feed for consecutive 60, 40, and 30 days, respectively. The residue amount of cyadox and its major metabolites in liver, kidney, muscle, and fat (skin) tissues was determined. Cy2 was below the limit of quantitation even at the withdrawal time of 6 hr, cyadox, Cy4, Cy6, and Cy12 could be detected at 6–24 hr with low level less than 50 μg/kg. By contrast, Cy1 persisted for 3 days in the kidney of pigs and chickens, and in the liver of carp. Based on these residue depletion data and previous toxicology results, the global estimated chronic dietary exposure assessment of cyadox for general population was conducted, indicating a zero withdrawal time (WDT) may be appropriate for cyadox in food animals when used in feed for prolonged administration. These results provide analytical techniques and safety standards suitable for residue monitoring of cyadox in food animals.
1 INTRODUCTION
Cyadox (CYA, Figure 1), 2-formylquinoxaline-1,4-dioxide cyano acetyl hydrazone (CAS No.65884-46-0, C12H9N5O3), is an antibacterial agent that belongs to the quinoxaline-1,4-dioxide family. It is effective against a number of the pathogenic bacteria in food-producing animals (Fan, Yuan, & Wang, 1999; Huang et al., 2002), and could promote the average daily gain and feed conversion ratio, and prevent Escherichia coli infection in pigs and chickens (Ding, Yuan, Wang, Zhu, & Fan, 2006; Huang & Yuan, 2005; Wang, Yuan, Zhu, Ding, & Fan, 2005). Toxicology studies showed that CYA was much safer than olaquindox (OLA) and carbadox (CBX), well-known members of the quinoxaline family but banned or strictly limited in food-producing animals due to their potential toxicities(He et al., 2006; Huang et al., 2010; Ihsan et al., 2013; Wang, Fang, et al., 2011; Wang, He, et al., 2011). The food safety of CYA in animal products needs to be investigated to assure the health of the consumers. Residue depletion and dietary exposure assessment are the essential data for the food safety evaluation of veterinary drugs. However, all these data remain unclear for CYA up to now.
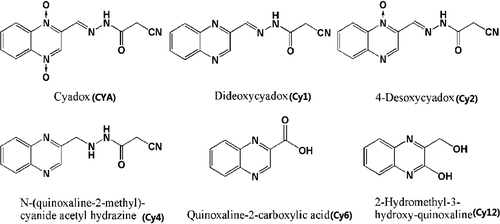
In vitro metabolism of CYA has shown that CYA can be metabolized and converted into a variety of metabolites (Liu et al., 2009; Wu et al., 2012; Xu et al., 2011). Five main metabolites (Figure 1), bisdeoxycyadox (Cy1), 4-desoxycyadox (Cy2), N-(quinoxaline-2-methyl)-cyanide acetyl hydrazine (Cy4), quinoxaline-2-carboxylic acid (Cy6), and 2-hydromethyl-3-hydroxy-quinoxaline (Cy12), had been designated in the recent report on isotopic tracing distribution and metabolism of CYA in food animals (Huang et al., 2015). These metabolites are closely associated with the food safety of CYA, and should be an important component of residue monitoring of CYA in food animals. It is indispensable to develop a sensitive and accurate bioanalytical method to quantitate CYA and these five major metabolites in edible tissues of food-producing animals. Several papers have reported the quantitative methods for their determination of CYA, Cy1, and Cy6 in pig and chicken tissues (Wu et al., 2007; Yan et al., 2012). Recent papers reported the determination of CYA, Cy1, Cy2, and Cy6 in fish and pigs, and described the residual depletion of CYA and these three metabolites (Cheng et al., 2012; He et al., 2011; Li et al., 2013). These studies mainly focused on the determination of two or three known metabolites (Cy1, Cy2, and Cy6), new and major metabolites (Cy4 and Cy12) have not been investigated yet. Further complete studies need to be conducted to scientifically evaluate the food safety of CYA in food animals.
Exposure assessment of veterinary drug to the human is a vital step for the food safety evaluation. It describes the entrance pathway of the drug residue into the human body and estimates the possible harmful effects of drug residues for different population. Based on the amount of the drug residues in diet and the food consumption of the population, the global dietary intake of drug residues could be calculated. By comparing the global dietary intake of drug residues with the acceptable daily intake (ADI) of the drug, a reasonable withdrawal time (WDT) of the drug could be set to ensure the food safety of the drug residues (FAO/WHO, 2011).
In this study, a sensitive and accurate HPLC-UV method was firstly developed to simultaneously and quantitatively determine CYA and its five main metabolites in edible tissues of pigs, chickens, and carp. Then, residue depletion of CYA in pigs, chickens, and carp was investigated to characterize the kinetics of CYA and its five major metabolites in the muscle, liver, kidney, and fat (skin) tissues. Subsequently, the withdrawal time (WDT) of CYA in food-producing animals was calculated based on the residue depletion data and dietary exposure assessment. These results provided a scientific food safety standard for the clinical application of CYA, and proposed an efficient method for the residual monitoring of CYA in animal products.
2 MATERIALS AND METHODS
2.1 Chemicals and reagents
The analytical standards of CYA, Cy1, Cy2, Cy4, Cy6, and Cy12 (≥98.5% purity) were obtained from the Institute of Veterinary Pharmaceuticals (Huazhong Agricultural University, Wuhan, PR China). Individual stock standard solutions (1,000 μg/ml) of all analytes were prepared by dissolving CYA, Cy1, Cy2, Cy4, and Cy12 pure standard in dimethylsulfoxide (DMSO) and Cy6 in methanol (MEOH). A mixed standard fortification solution (10 μg/ml [20 μg/ml for Cy4]) was prepared by combining 1.0 ml (2.0 ml for Cy4) of each stock standard and diluting with methanol (MeOH) to obtain a final volume of 100 ml. The stock solutions were stored in amber vials at −20°C and were tested weekly to investigate the stability. The stock solutions were shown to be stable for 3 months with a slight change of 1.34% in content. The mixed fortification solution was also stored in an amber vial at −20°C and then stabilized for 1 month. Distilled water was further purified by passing it through a Milli-Q Plus apparatus (Millipore, Bedford, MA, USA). HPLC-grade MeOH and acetonitrile (MeCN) were purchased from Tedia (Fairfield, OH). Other chemicals, including formic acid, metaphosphoric acid, and zinc acetate, were of analytical reagent grade.
2.2 Animals and sampling
Thirty healthy castrated crossbred (duroc × large white × landrace) pigs (28–32 kg) were purchased from the Breeding Pig Testing Center (Wuhan, PR China); these pigs were housed in six 8 × 10 m pigpens, which were cleaned daily. Thirty-six-one-day-old healthy Cobb500 chickens were purchased from Charoen Pokphand Group (Wuhan, PR China); these chickens were kept in stainless steel cages (six chickens/cage). Sixty healthy Xiangyun carp (400–500 g) were purchased from the Wuhan Fish Breeding Farm (Wuhan, PR China); these carp were placed in six fish tanks (10 carps/tank) with circulating water, and the water temperature of the fish tank was maintained to be 22–25°C. The animal houses were maintained at 25 ± 2°C room temperature with 45%–65% relative humidity. All of the animals were allowed a 7-day acclimation period before our experiments were conducted; a standard ration based on corn and soybean was fed twice a day, and tap water was available ad libitum. The animals were randomly divided into a control group (n = 5, 6, and 10 for pigs, chickens, and carp, respectively) and a test group (n = 25, 30, and 50 for pigs, chickens, and carp, respectively). The control groups were fed with standard ration without quinoxaline compounds. The test groups were provided with medicated feed for 60, 40, and 30 consecutive days for pigs, chickens, and carp, respectively. The medicated feed contained a standard ration premixed with CYA at a level of 150 mg/kg diet. The concentration of cyadox in the feed was confirmed to be 150 ± 1.24 mg/kg by the method of Wu et al. (2006). The study was performed in accordance with the guidelines of the Committee on the Care and Use of Laboratory Animals of China (permit SYXK 2007-0044) and was approved by Animal Ethic Committee of Huazhong Agricultural University.
At different time points (6 hr, 1, 3, 7, and 14 days [6 hr, 1, 3, 5, and 7 days for chickens and carp]), one control and five medicated pigs (six medicated chickens, and ten medicated carp) were anesthetized and killed. The pigs and chickens were slaughtered using captive bolt stunning equipment and exsanguinated in accordance with the guidelines provided by the American Veterinary Medical Association for euthanasia (AVMA Panel on Euthanasia, 2001), the carp were euthanized in a MS-222/water (1:10,000) bath. Liver, kidney, muscle, and fat (skin) specimens were collected. All of the samples were placed in labeled plastic bags in an ice bath, immediately homogenized, and frozen at −20°C until these samples were analyzed within 1 week.
2.3 Sample analysis
2.3.1 Sample preparation
Samples (2 ± 0.1 g) were transferred into a 50-ml polypropylene centrifuge tube, and 6 ml of 1% metaphosphoric acid in MeOH/MeCN/water (50:10:40, v/v/v) was added to the sample. The mixtures were shaken using a vortex system for 5 min, and centrifuged at 4,000 g for 10 min. The supernatant was collected into a 50-ml centrifuge tube. The same extraction procedure was repeated. The two supernatants were combined, and 1 ml of 10% aqueous zinc acetate was added to the extracts. These extracts were mixed by vortexing for 2 min and centrifuged at 10,000 g for 10 min; afterward, the supernatant was transferred, defatted with 12 ml of n-hexane twice, and diluted with 1% aqueous metaphosphoric acid to obtain a final volume of 48 ml, which was ready for the clean-up procedure.
The HLB cartridge (60 mg, 3 ml) (Waters Corp., Milford, MA, USA) was preconditioned with 3 ml of MeOH and 3 ml of water. Flow rates for conditioning and washing were set at 3 ml/min. The entire extracts were loaded onto the SPE column at flow rate of 1 ml/min. The column was washed with 3 ml of water and 3 ml of 10% MeOH and dried by purging air at a rate of 10 ml/min for 5 min. The analytes were eluted with 3 ml of MeOH at a flow rate of 1.0 ml/min into a 50 ml tube and diluted by 3 ml of 2% aqueous metaphosphoric acid. Six milliliters of chloroform was added, and the mixtures were shaken for 2 min. The under layer was transferred into a 15-ml tube. The back extraction procedure was repeated. Two hundred microliters of 10% aqueous sodium hydroxide was added into the eluant, and the third back extraction aqueous was repeated. The three back extracts were combined and evaporated to dryness under a stream of nitrogen at 45°C. The dry residue was dissolved in 1 mL of 15% MeCN, vortexed for 1 min, and centrifuged at 16,600 g for 10 min. The resulting solution was then filtered using a 0.22 μm nylon Millipore chromatographic filter, and a 20 μl of aliquot was analyzed by HPLC. The other tested cartridge was Oasis MAX (60 mg, 3 ml) (Waters Corp., Milford, MA, USA).
2.3.2 HPLC analysis
HPLC analysis was performed using a Waters 2695 HPLC system coupled with a UV detector. Chromatographic separation was conducted using a ZORBAX SB-C18 column (250 mm × 4.6 mm i.d., 5 μm; Agilent Technology, USA) coupled with a 2 mm C18 guard column at a flow rate of 1.0 ml/min at 30°C in a column oven. Mobile phase component A was 0.5% formic acid and component B was MeCN. The mobile phase gradient profile was as follows: 0−4 min, 85% A; 15 min, 70% A; 15.1 min, 85% A; 23 min, 85%A. The UV detector was set at a wavelength of 320 nm for all of the compounds.
2.3.3 Specificity
Method specificity was verified by analyzing blank samples and observing residue-interfering peaks. The results were evaluated by the presence of interfering substances at the specified retention time.
2.3.4 Calibration curve and Linearity
The standard mixture calibration curves were generated on five different days at seven concentration levels from 10 to 200 μg/L for CYA, Cy1, Cy2, Cy6, and Cy12 and from 20 to 400 μg/L for Cy4. The analyses were performed in triplicate. The method was further tested by matrix-matched calibration curves, which were obtained by fortification with the six compounds at each of the six concentrations from 10 to 100 μg/kg. The calibration curves constructed on five separate days were analyzed to evaluate the linearity of each curve. Slope, intercept, and correlation coefficient were calculated for each standard curve. Unknown concentrations were calculated from the equation of the calibration curve.
2.3.5 Limits of detection (LOD) and Limits of quantification (LOQ)
The LOD is described as the lowest concentration that an analyte can be reliable detected, and the LOQ is defined as the lowest drug concentration in food samples that can be measured with a desired level of accuracy and precision. Five sets, each of five, of blank samples were spiked with a series level of working solution. When analyzed as the methods described above, the signal-to-noise (S/N) ≧ 3could be considered as LOD. The S/N ≧ 10 could be considered as LOQ.
2.3.6 Accuracy and precision
Blank samples were fortified at three different levels (1, 2, and 4 times of LOQ). For the intraday experiment, six sets of each concentration were run. Six replicates of the three concentrations were analyzed in five different days for the interday experiment. Accuracy was defined by the mean absolute recovery, which was calculated by comparing the peak area of the extracted sample with that of the standard working solution. Precision was defined by relative standard deviation (RSD).
2.3.7 Stability
Stability experiments were conducted to investigate the stability of CYA and its metabolites in incurred samples stored at −20°C within 6 months. The incurred samples were extracted, thawed, and analyzed every week. The measured values were compared with those in freshly incurred samples in triplicate. When the concentration of the measured value in stored samples maintained to be over 95% of the freshly incurred samples, the samples were considered to be stable. The results indicated that CYA and its metabolites in stored samples were 96.5%–99.6% of the fresh samples during the first 3 months, but decreased to be 92.5%–95.2% in the next 3 months, indicating the incurred samples in the incurred samples during storage at −20°C.
2.4 Dietary exposure assessment procedures
The chronic dietary exposure assessment to CYA from the diet undertaken by combining food consumption data with residue data was conducted following Joint FAO/WHO Expert Meeting on Dietary Exposure Assessment Methodologies for Residues of Veterinary Drugs (FAO/WHO, 2011). Chronic dietary exposure estimates cover food consumption over the long term and are used for comparison with ADI, a health-based guidance value based on chronic toxicity.
Firstly, ADI was calculated based on the previous toxicology study (Fang et al., 2006). The following assumptions were used in dietary exposure estimates: residues are found in pork, poultry, and fish; residues are found in muscle, liver and kidney, skin and fat; all poultry offal is assumed to be kidney (worst-case scenario).
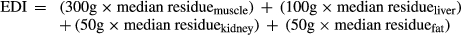 (1)
(1)2.5 Data analysis
The concentrations of CYA and its metabolites were analyzed using a linear regression model of natural log-transformed average concentration of CYA and its metabolites (lnC) against time. The last three time-point data were fit to the first-order rate equation C=C0e−Kt, where C is the concentration of cyadox and its metabolites on day t, C0 is the initial concentration, elimination rate constant (K) is the slope of the linear regression equation for the log-transformed residue concentration (lnC) against time, and the half-life of elimination (t1/2K) is calculated from the equation t1/2K = ln2/K for each tissue.
The withdrawal time (WDT) based on the acceptable daily intake (ADI), the estimated daily intake (EDI), and the global estimated chronic dietary exposure (GECDE) was estimated by the Statistical Tool for Data Analysis of Draft Maximum Residue Limits for Veterinary Drugs (MRLVDs) in Edible Tissues (Arnold, 2003). An iterative procedure is used to calculate for different time points on the depletion curve the intake of residues of concern in the food basket. The calculated intake of residues is compared with the acceptable daily intake (ADI), and the time point of depletion below the ADI is selected to determine the WDTs.
3 RESULTS
3.1 HPLC method validation
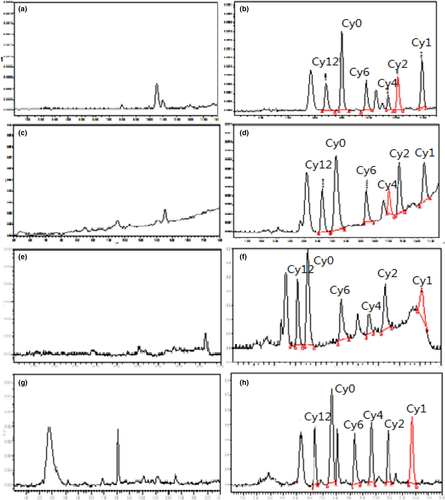
Based on the comparison of background noise with various matrices, the current method showed excellent specificity as no interference peak was detected at the retention time of CYA and its metabolites (Figure 2). The correlation coefficients (r) of standard curves for all of the six compounds were >.9999. The correlation coefficient values of matrix-matched calibration standard curves within the range from 10 to 100 μg/kg were >0.99. On the basis of signal-to-noise ratios of >3 and 10, the LODs of CYA, Cy1, Cy2, Cy6, and Cy12 were 10 μg/kg, but 20 μg/kg for Cy4 in all the tested tissues. The LOQs of CYA, Cy1, Cy2, Cy6, and Cy12 were 20 μg/kg, but 40 μg//kg for Cy4 all the tested tissues. The recoveries and relative standard deviation (RSD) of six compounds in tested tissues at the specified concentration range on five separate days were presented in Tables 1–3. The mean recoveries ranged 64.9%−79.5%, and the interday RSD values were <9.6%, indicating that the proposed method is accurate and precise enough for the quantitation detection of CYA and its metabolites in animal tissues.
| Compounds | Added level (μg/kg) | Liver | Kidney | Muscle | Fat/Skin | ||||
|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | Interday RSD (%) | Recovery (%) | Interday RSD (%) | Recovery (%) | Interday RSD (%) | Recovery (%) | Interday RSD (%) | ||
| Cy0 | 20 | 68.9 ± 6.3 | 9.1 | 76.5 ± 6.7 | 8.7 | 77.4 ± 4.8 | 6.2 | 69.3 ± 4.6 | 6.6 |
| 40 | 74.8 ± 5.9 | 7.9 | 74.6 ± 4.5 | 6.0 | 69.5 ± 5.4 | 7.8 | 68.9 ± 6.3 | 9.1 | |
| 80 | 75.7 ± 6.5 | 8.6 | 77.6 ± 6.3 | 8.1 | 79.6 ± 7.4 | 9.3 | 75.4 ± 5.9 | 7.8 | |
| Cy1 | 20 | 68.7 ± 5.4 | 8.7 | 71.3 ± 4.6 | 6.5 | 68.7 ± 5.4 | 7.9 | 76.1 ± 4.8 | 6.3 |
| 40 | 76.6 ± 7.4 | 9.7 | 72.4 ± 5.4 | 7.5 | 78.3 ± 4.6 | 5.9 | 73.4 ± 5.4 | 7.4 | |
| 80 | 73.4 ± 5.5 | 7.5 | 72.8 ± 5.2 | 7.1 | 78.3 ± 5.8 | 7.4 | 71.5 ± 6.5 | 9.1 | |
| Cy2 | 20 | 78.5 ± 4.5 | 5.7 | 72.3 ± 5.9 | 8.2 | 74.3 ± 3.8 | 5.1 | 72.1 ± 5.9 | 8.2 |
| 40 | 75.6 ± 5.6 | 7.4 | 79.4 ± 6.5 | 8.2 | 74.5 ± 6.7 | 9.0 | 79.4 ± 6.6 | 8.3 | |
| 80 | 77.6 ± 6.5 | 8.4 | 75.4 ± 5.7 | 7.6 | 72.3 ± 4.7 | 6.5 | 71.5 ± 4.1 | 5.7 | |
| Cy4 | 40 | 76.7 ± 4.7 | 6.1 | 78.4 ± 6.2 | 7.9 | 75.4 ± 5.5 | 7.3 | 77.0 ± 6.4 | 8.3 |
| 80 | 69.5 ± 5.4 | 7.8 | 72.5 ± 5.5 | 7.6 | 69.6 ± 6.7 | 9.6 | 78.1 ± 5.0 | 6.4 | |
| 160 | 77.5 ± 4.8 | 6.2 | 74.6 ± 6.7 | 9.0 | 78.4 ± 8.3 | 10.5 | 79.5 ± 5.3 | 6.7 | |
| Cy6 | 20 | 79.5 ± 6.6 | 8.3 | 69.4 ± 3.4 | 4.9 | 71.3 ± 6.0 | 8.4 | 69.8 ± 4.4 | 6.3 |
| 40 | 76.4 ± 6.5 | 8.5 | 77.3 ± 6.2 | 8.0 | 79.4 ± 4.3 | 5.4 | 74.1 ± 5.0 | 6.7 | |
| 80 | 72.5 ± 6.0 | 8.3 | 79.3 ± 3.6 | 4.5 | 72.5 ± 7.0 | 9.7 | 74.6 ± 6.6 | 8.8 | |
| Cy12 | 20 | 73.5 ± 4.8 | 6.5 | 69.7 ± 6.5 | 9.3 | 74.5 ± 7.3 | 9.8 | 74.7 ± 7.2 | 9.6 |
| 40 | 75.7 ± 6.1 | 8.1 | 77.8 ± 7.0 | 9.0 | 69.6 ± 5.4 | 7.7 | 74.3 ± 6.1 | 8.2 | |
| 80 | 68.7 ± 6.4 | 9.3 | 74.8 ± 4.8 | 6.4 | 78.5 ± 6.6 | 8.4 | 78.3 ± 5.2 | 6.6 | |
| Compounds | Added level (μg/kg) | Liver | Kidney | Muscle | Fat/Skin | ||||
|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | Interday RSD (%) | Recovery (%) | Interday RSD (%) | Recovery (%) | Interday RSD (%) | Recovery (%) | Interday RSD (%) | ||
| Cy0 | 20 | 73.1 ± 5.2 | 7.0 | 67.8 ± 3.2 | 4.6 | 70.4 ± 4.5 | 5.7 | 77.4 ± 6.4 | 8.3 |
| 40 | 75.9 ± 4.9 | 6.4 | 70.4 ± 4.6 | 6.5 | 71.6 ± 6.3 | 8.8 | 74.3 ± 5.8 | 7.8 | |
| 80 | 77.2 ± 4.3 | 5.6 | 73.8 ± 5.3 | 7.0 | 79.4 ± 5.9 | 7.4 | 75.4 ± 6.3 | 8.4 | |
| Cy1 | 20 | 70.2 ± 7.0 | 9.1 | 73.1 ± 4.8 | 6.5 | 76.5 ± 4.9 | 6.4 | 77.4 ± 6.3 | 8.1 |
| 40 | 72.1 ± 6.0 | 8.3 | 68.5 ± 3.7 | 5.5 | 77.3 ± 6.2 | 8.0 | 70.3 ± 5.5 | 7.8 | |
| 80 | 74.3 ± 5.1 | 7.0 | 73.0 ± 5.0 | 6.8 | 77.0 ± 6.2 | 8.1 | 72.5 ± 6.4 | 8.8 | |
| Cy2 | 20 | 75.1 ± 5.4 | 7.2 | 68.0 ± 4.4 | 6.5 | 75.4 ± 7.1 | 9.4 | 75.7 ± 4.9 | 6.5 |
| 40 | 77.1 ± 5.9 | 7.7 | 76.5 ± 6.4 | 8.4 | 79.4 ± 5.4 | 6.8 | 78.4 ± 6.6 | 8.4 | |
| 80 | 75.1 ± 4.6 | 6.1 | 78.2 ± 5.8 | 7.5 | 76.3 ± 7.2 | 9.4 | 79.0 ± 6.3 | 8.0 | |
| Cy4 | 40 | 65.5 ± 3.6 | 5.5 | 64.9 ± 4.1 | 6.3 | 70.4 ± 6.1 | 8.7 | 69.5 ± 7.4 | 1.1 |
| 80 | 72.5 ± 5.1 | 7.2 | 74.6 ± 6.9 | 9.3 | 71.5 ± 6.0 | 8.4 | 73.6 ± 5.4 | 7.3 | |
| 160 | 75.1 ± 5.9 | 7.8 | 75.5 ± 6.1 | 8.3 | 79.4 ± 6.3 | 7.9 | 74.5 ± 6.6 | 8.9 | |
| Cy6 | 20 | 67.0 ± 4.4 | 6.8 | 66.3 ± 3.9 | 5.8 | 69.5 ± 3.5 | 5.0 | 68.7 ± 4.8 | 7.0 |
| 40 | 75.1 ± 6.6 | 8.8 | 73.5 ± 5.1 | 6.7 | 72.6 ± 5.3 | 7.3 | 79.4 ± 6.4 | 8.1 | |
| 80 | 75.4 ± 7.0 | 9.3 | 75.9 ± 5.4 | 7.7 | 74.6 ± 6.2 | 8.3 | 71.7 ± 5.4 | 7.0 | |
| Cy12 | 20 | 72.5 ± 6.2 | 8.5 | 69.3 ± 4.9 | 7.2 | 74.3 ± 5.9 | 7.9 | 76.7 ± 5.7 | 7.4 |
| 40 | 76.3 ± 3.7 | 4.8 | 73.6 ± 6.3 | 8.7 | 70.3 ± 6.2 | 8.8 | 75.3 ± 6.6 | 8.8 | |
| 80 | 75.2 ± 5.5 | 7.3 | 73.0 ± 5.9 | 8.1 | 74.3 ± 6.6 | 8.9 | 69.4 ± 5.1 | 7.3 | |
| Compounds | Added level (µg/kg) | Liver | Kidney | Muscle | Fat/Skin | ||||
|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | Interday RSD (%) | Recovery (%) | Interday RSD (%) | Recovery (%) | Interday RSD (%) | Recovery (%) | Interday RSD (%) | ||
| Cy0 | 20 | 74.1 ± 5.3 | 7.2 | 69.8 ± 3.5 | 5.0 | 77.4 ± 5.3 | 6.8 | 78.2 ± 6.4 | 8.2 |
| 40 | 74.9 ± 4.8 | 6.4 | 71.4 ± 4.6 | 6.4 | 71.3 ± 5.6 | 7.4 | 76.3 ± 5.1 | 6.7 | |
| 80 | 78.2 ± 4.5 | 5.8 | 74.8 ± 5.5 | 7.4 | 73.1 ± 6.3 | 8.6 | 76.7 ± 4.9 | 6.4 | |
| Cy1 | 20 | 76.2 ± 7.1 | 9.3 | 74.1 ± 4.0 | 5.4 | 75.3 ± 5.5 | 7.3 | 77.0 ± 5.7 | 7.4 |
| 40 | 73.1 ± 6.9 | 9.4 | 69.5 ± 3.8 | 5.5 | 75.4 ± 6.9 | 9.2 | 77.4 ± 6.1 | 7.9 | |
| 80 | 75.3 ± 5.4 | 7.1 | 72.0 ± 5.1 | 7.1 | 74.3 ± 6.3 | 8.5 | 78.4 ± 7.1 | 9.1 | |
| Cy2 | 20 | 73.1 ± 5.3 | 7.3 | 69.0 ± 4.0 | 5.8 | 76.3 ± 6.7 | 8.8 | 70.5 ± 6.9 | 9.8 |
| 40 | 76.1 ± 5.8 | 7.6 | 75.5 ± 6.6 | 8.7 | 76.1 ± 5.0 | 6.6 | 74.0 ± 6.0 | 8.1 | |
| 80 | 73.1 ± 4.8 | 2.4 | 76.2 ± 5.5 | 7.2 | 79.3 ± 5.1 | 6.4 | 70.5 ± 4.1 | 5.8 | |
| Cy4 | 40 | 69.5 ± 3.9 | 5.6 | 68.9 ± 4.6 | 6.7 | 69.0 ± 6.6 | 9.6 | 68.9 ± 7.0 | 10.2 |
| 80 | 73.5 ± 5.3 | 7.2 | 78.6 ± 6.0 | 7.6 | 72.3 ± 4.9 | 6.8 | 70.5 ± 6.2 | 8.8 | |
| 160 | 70.1 ± 5.4 | 7.7 | 79.5 ± 6.3 | 7.9 | 75.2 ± 6.6 | 8.8 | 78.4 ± 5.4 | 6.9 | |
| Cy6 | 20 | 68.0 ± 4.6 | 6.8 | 69.3 ± 3.5 | 5.1 | 68.5 ± 6.2 | 9.1 | 69.6 ± 5.5 | 7.9 |
| 40 | 76.1 ± 6.7 | 8.8 | 77.5 ± 5.8 | 7.5 | 75.8 ± 4.9 | 6.5 | 76.4 ± 6.3 | 8.2 | |
| 80 | 78.4 ± 7.1 | 9.0 | 78.9 ± 5.9 | 7.5 | 75.8 ± 3.9 | 5.1 | 76.5 ± 7.0 | 9.2 | |
| Cy12 | 20 | 71.5 ± 6.0 | 8.3 | 70.3 ± 4.2 | 6.0 | 78.4 ± 5.7 | 8.5 | 77.4 ± 7.3 | 9.4 |
| 40 | 75.3 ± 3.9 | 5.2 | 74.6 ± 6.0 | 8.0 | 77.2 ± 6.0 | 4.8 | 75.1 ± 6.4 | 8.5 | |
| 80 | 74.2 ± 5.6 | 7.5 | 78.0 ± 5.8 | 7.4 | 71.9 ± 6.2 | 7.3 | 77.5 ± 5.5 | 7.1 | |
3.2 Tissue depletion of CYA and its metabolites in food animals
The average concentrations of CYA and its metabolites in pigs, chickens, and carp edible tissues for each time point were listed in Table 4. The highest concentrations of all of the related compounds in all of the edible tissues were obtained at 6 hr after the last dosing. At all time points, the highest concentrations were detected in the kidney or liver, and the lowest concentrations were measured in the muscle or fat (skin).
| Animal | Tissue | Time (day) | Compound(μg/kg) | ||||
|---|---|---|---|---|---|---|---|
| Cyx | Cy1 | Cy4 | Cy6 | Cy12 | |||
| Pigs | Liver | 0.25 | 46.5 ± 9.0 | 55.0 ± 8.7 | NDa | 33.6 ± 5.9 | ND |
| 1 | ND | 20.8 ± 2.5 | ND | ND | ND | ||
| Kidney | 0.25 | 27.5 ± 9.0 | 59.5 ± 10.7 | ND | 46.1 ± 6.3 | ND | |
| 1 | ND | 45.1 ± 4.9 | ND | 38.2 ± 8.2 | ND | ||
| 3 | ND | 21.8 ± 2.7 | ND | ND | ND | ||
| Muscle | 0.25 | 27.5 ± 10.3 | ND | ND | 37.6 ± 9.9 | ND | |
| 1 | ND | ND | ND | 22.8 ± 6.4 | ND | ||
| Fat | 0.25 | 22.2 ± 5.7 | ND | 44.6 ± 9.9 | ND | ND | |
| Chickens | Liver | 0.25 | 24.7 ± 6.7 | 41.7 ± 9.3 | ND | 49.7 ± 10.7 | 42.8 ± 13.3 |
| 1 | ND | 25.5 ± 3.8 | ND | 22.0 ± 3.5 | ND | ||
| Kidney | 0.25 | 32.5 ± 7.0 | 53.6 ± 8.9 | 42.2 ± 3.8 | 45.7 ± 8.6 | 34.8 ± 7.2 | |
| 1 | ND | 34.9 ± 7.6 | ND | 20.5 ± 2.2 | ND | ||
| 3 | ND | 20.9 ± 1.7 | ND | ND | ND | ||
| Muscle | 0.25 | ND | ND | ND | ND | 24.5 ± 4.3 | |
| Fat | 0.25 | ND | ND | ND | ND | 24.2 ± 4.8 | |
| Carp | Liver | 0.25 | 38.2 ± 8.3 | 43.2 ± 6.5 | 43.2 ± 6.1 | 28.2 ± 4.5 | ND |
| 1 | 20.2 ± 7.7 | 37.8 ± 4.1 | ND | 21.3 ± 3.9 | ND | ||
| 3 | ND | 21.0 ± 4.3 | ND | ND | ND | ||
| Kidney | 0.25 | 40.9 ± 6.3 | 41.3 ± 4.7 | 40.3 ± 5.4 | 25.8 ± 6.1 | ND | |
| 1 | 27.1 ± 3.2 | 25.8 ± 3.8 | ND | 21.6 ± 3.2 | ND | ||
| Muscle | 0.25 | 22.5 ± 3.1 | 31.4 ± 5.2 | 42.8 ± 8.4 | 22.6 ± 5.6 | ND | |
| Skin | 0.25 | 28.3 ± 5.6 | 44.3 ± 6.4 | 41.2 ± 5.3 | 27.4 ± 6.8 | ND | |
| 1 | ND | 23.5 ± 5.7 | ND | 20.3 ± 2.8 | ND | ||
- a Samples were below the LOQ of analysis method.
- The concentrations of cyadox and its metabolites at the other time point (not list in this table) were “ND”.
In pigs, CYA, Cy1, and Cy6 were detected in the liver and kidney at 6 hr postmedication (Figure 3), and the residual concentrations ranged between 27.5 and 55.0 μg/kg after CYA was consecutively administered for 2 months. Low amounts of Cy6 were detected in the kidney at the withdrawal time of 1 day. Cy1 was detected in the liver after 1 day and in the kidney after 3 day. CYA and Cy6 were detected in the muscle at the withdrawal time of 6 hr, and the residual concentrations were 27.5 and 37.6 μg/kg, respectively, and Cy6 was found that values approximately equal to the LOQ of the method after a withdrawal time of 1 day. CYA and Cy4 were detected in the fat at the withdrawal time of 6 hr, and the residual concentrations were 22.2 and 44.6 μg/kg, respectively. The linear regression analysis result between the natural log of the concentration of Cy1 and the withdrawal time in the kidney was Ckidney-Cy1 = 64.7e−0.36t. Hence, t1/2Ke value of Cy1 in the kidney of pigs was 1.93 days.
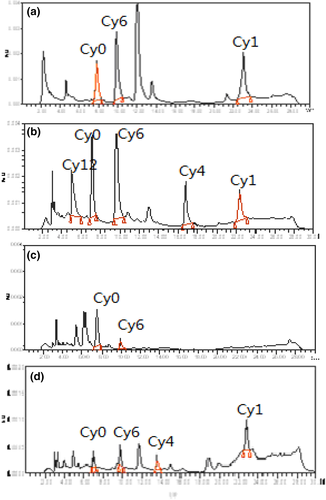
In chickens, CYA, Cy1, Cy6, and Cy12 were detected in the liver at the withdrawal time of 6 hr, and the residual concentrations ranged between 24.7 and 49.7 μg/kg. CYA, Cy1, Cy4, Cy6, and Cy12 were detected in the kidney at the withdrawal time of 6 h (Figure 4), and the residual concentrations were between 32.5 and 53.6 μg/kg. Cy6 was detected in the liver and kidney after 1 days. Cy1 was detected in the liver for 1 days and in the kidney for 3 days. CYA was detected in the muscle and fat at 6 hr at very low concentrations of 24.5 and 24.2 μg/kg, respectively, slightly higher than the LOQ of the method. The elimination equation of Cy1 in the kidney of broiler was calculated using the regression curve, which was Ckidney-Cy1 = 53.5e−0.33t. Hence, the t1/2Ke of Cy1 in the kidney of the broiler was 2.10 days.
In carp, CYA, Cy1, Cy4, and Cy6 were detected in the liver, kidney, muscle, and skin at the withdrawal times of 6 hr, and the residual concentrations ranged between 22.5 and 44.3 μg/kg. CYA, Cy1, and Cy6 were detected in the liver and kidney at the withdrawal time of 1 days, and the residual concentrations ranged between 20.2 and 37.8 μg/kg. Cy1 and Cy6 were detected in the skin at the withdrawal time of 1 days (Figure 3). Cy1 was detected in the kidney for 3 days. The linear regression analysis result between the natural log of the concentration of Cy1 and the withdrawal time in the liver was Cliver-Cy1 = 47.5e−0.27t. The elimination half-live of Cy1 in the liver of carp calculated from the elimination equation was 2.57 days.
3.3 Chronic dietary exposure assessment
3.3.1 ADI
To identify ADI, a series of toxicology study was performed to determine the No Observed Effect Level (NOEL). Both the subchronic oral toxicity study and two generation reproduction and teratogenicity studies in rats showed a NOEL of 15 mg/kg bw/day (Fang et al., 2006; Wang, Fang, et al., 2011). The subchronic oral toxicity of cyadox in Beagle dogs also showed a NOEL of 15 mg/kg bw/day (Wang et al., 2015). A chronic toxicity study of cyadox in Wistar rats and a 2-year dietary carcinogenicity study of cyadox in Sprague-Dawley rats showed a NOEL of 40 mg/kg bw/day and 132 mg/kg bw/day, respectively(Liu et al., 2017; Wang, He, et al., 2011). Based on these toxicology results, the minimum NOEL of cyadox was identified to be 15 mg/kg bw/day. The safety factor usually chosen is 100 in the situation where a NOEL is derived from a long-term animal study on the assumption that humans are ten-times as sensitive as the test animals used in such studies and that ten-fold range of sensitivity within the human population may exist. Then the ADI is 150 μg/kg bw/day.
3.3.2 EDI
Based on the established model diet, the exposure to CYA expressed as the EDI, and calculated from the M:T and analytical method recovery adjusted median residue was 82 μg/person/day. Dietary exposure was estimated to be 1.0% of the ADI (Table 5). Results below the LOQ are assigned a value of one half of the LOQ when calculating the median residue concentration.
| Food categories | Food consumptiona (g/day) | Median residueb (μg/kg) | M:Tc | Analytical method recoveryd (%) | Estimates of dietary intake (EDI)e (μg/day) | Exposure from each foode (μg/day) | Acceptable daily intake (ADI) (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Model diet | Mean chronic | High chronic | High consumers | Mean total population | ||||||
| Pork muscle | 300 | 114 | 415 | 10 | 0.30 | 68.7 | 14.6 | 20.1 | 5.5 | |
| Mammalian trimmed fat, skin and added fat | 50 | 14 | 125 | 21 | 0.41 | 76.1 | 3.4 | 8.4 | 0.9 | |
| Mammalian liver | 100 | 2 | 237 | 55 | 0.32 | 68.7 | 25.0 | 59.3 | 0.5 | |
| Mammalian kidney | 50 | 0.5 | 166 | 59 | 0.34 | 71.3 | 12.2 | 40.4 | 0.1 | |
| Poultry muscle | 300 | 118 | 352 | 10 | 0.64 | 76.5 | 6.1 | 7.2 | 2.4 | |
| Poultry fat and skin | 50 | 1 | 23 | 10 | 0.60 | 77.4 | 1.1 | 0.5 | 0.02 | |
| Poultry offal (kidney) | 150 | 5 | 188 | 53 | 0.59 | 73.1 | 18.4 | 23.1 | 0.6 | |
| Fish | 300 | 27 | 655 | 30 | 0.29 | 75.3 | 41.2 | 90.0 | 3.7 | |
| EDI | 82f | 1.0 | ||||||||
| Mean exposure from all foods(μg/day) | 14 | |||||||||
| Mean exposure for adult of 60 kg body weight (μg/kg bw/day) | 0.2 | 0.2 | ||||||||
| GECDE(μg/day) | 100g | |||||||||
| GECDE for adult of 60 kg body weight(μg/kg bw/day) | 1.7 | 1.0 | ||||||||
- a Refers to Annex 5 in final report of Joint FAO/WHO Expert Meeting on Dietary Exposure Assessment Methodologies for Residues of Veterinary Drugs (FAO/WHO, 2011).
- b Calculated from the residue amount of Cy1at the withdrawal time of 0.25 day in specific species.
- c Refers to Huang et al., 2015.
- d Refers to the recovery ratio of Cy1in Table 1–3.
- e Presented as Total residue which equals to marker residue/ratio(M:T)/analytical method recovery.
- f For each food commodity, the highest species-specific median residue value (italics values) is used in the calculation of EDI.
- g The global estimated chronic dietary exposure (GECDE) is the sum of the bold values for high chronic exposure and mean chronic exposure.
3.3.3 GECDE
For the general population group, fish was the major contributor to estimated exposure from CYA residue (Table 5). Mammalian kidney and poultry fat and skin only contributed negligible amounts to overall exposure estimates. Using the median residue as input, GECDE was 1.7 μg/kg bw/day, 1.0% of the ADI of 150 μg/kg bw/day (Table 5), and did not exceed the ADI. The results showed that GECDE exposure estimates were below the ADI for the general population, and a little higher than the EDI. Fish and mammalian offal were the major contributor to chronic exposure estimates. In summary, none of the chronic exposure estimates resulted in exceeding the ADI.
3.4 Withdrawal time (WDT)
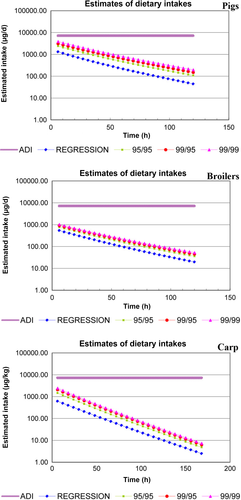
The EDI versus time charts in pigs, chickens, and carp were presented in Figure 4. The results showed that the EDI was below the ADI even at zero withdrawal intervals in pigs, chickens, and carp (Figure 4). Therefore, it is recommended that the WDT of CYA is 0 days in pigs, chickens, and carp when the animals were fed CYA in feed at 150 mg/kg for over 30 days.
4 DISCUSSION
Several papers had reported the disposition of quinoxalines in animals in recent years. Most of these papers focused on the metabolism and pharmacokinetics of quinoxalines in animals (Li, Huang, Pan, et al., 2014; Liu et al., 2009; Xu et al., 2011; Zhao et al., 2013). Limited information was reported about the residue depletion of quinoxalines in animals. Li, Huang, Wang, et al. (2014) investigated the depletion of quinocetone(QCT) and its main metabolites in food animals, and found that QCT and its four metabolites could be depleted to less than the LOQ within 24 hr in all the tissues, whereas di-deoxyquinocetone (Q2) persisted for 7 days in the liver after the animals were fed with 100 mg kg−1 QCT for consecutive more than 2 months. A recent paper reported the residue depletion of cyadox and its three metabolites in pigs, indicating that CYA and Cy2 were depleted to be less than LOQ within 72 hr, whereas Cy1 and Cy6 could persist for 7–14 days in kidney of pigs (Li et al., 2013).
In this study, depletion of CYA and its five metabolites in pigs, chickens, and carp were investigated. The results showed that CYA was eliminated rapidly in pigs, chickens, and carp, and no metabolites could be detected after the withdrawal time of 3 days. Cy1 persisted for the longest time in kidney of pigs and chickens. The depletion of CYA and most metabolites in pigs were consistent with that of Li et al. However, Cy6 showed obvious difference from those in previous studies, suggesting that Cy6 is the marker residue (Li et al., 2013). It had been proved that Cy6 likely conjugates with glycine in vivo(Sestakova & Kopanica, 1988). In Li et al.'s studies, hydrochloric acid was applied to hydrolyze the samples, resulting that Cy6 glycine conjugates were hydrolyzed to release Cy6. Consequently, Cy6 persists longer time than the other metabolites. In our study, a method previously reported (Wu et al., 2007) was modified using 1% metaphosphoric acid in MeOH/MeCN/water (50:10:40, v/v/v) to ensure the stability of the compounds in tissues. In this case, the real concentration of Cy6 (only free Cy6) in animal tissues was obtained.
The depletion rates of CYA and its metabolites in muscle and fat were more rapid than that in liver and kidney of pigs, chickens, and carp. This result indicated that the kidney and liver are the key organs of cyadox residues. The intact parent drug could be detected only in the first sampling time (6 hr), and Cy2 could not be detected in all the edible tissues in three species; Cy12 could be detected only in broiler tissues at 6 hr after the last dosing; Cy4 could be detected in several tissues of chickens and carp; Whereas, Cy6 and Cy1 could be detected in edible tissues of three species at 24 hr and 72 hr, respectively. Hence, the order of elimination rate of the six compounds in the kidney and liver was in Cy2 > Cy12 > Cy4 > CYA > Cy6 > Cy1. This result indicated that Cy1 is the main compound that produces residues in pigs, chickens, and carp for the longest time when CYA was administered consecutively these animals, which was consistent with the results of radioactive tracer studies (Huang et al., 2015). Therefore, Cy1 could be the residue marker of cyadox in the three animals compared with other compounds.
Although Cy1 was the common residue marker of CYA in three species, certain differences were found in the residue characteristics of different animals. Cy12 could be detected only in chicken tissues. Cy4 was mainly detected in carp tissues. Moreover, Cy1 persist the longest time in kidney of pigs and chickens, but in liver of carp. In addition, t1/2Ke-Cy1 in the kidney (liver) of food animals was found in the following order: carp > chickens > pigs. Hence, cyadox-related residues depleted slowest in carp due to the slower metabolism than livestock and poultry.
Based on the chronic dietary exposure assessment, zero withdrawal time was recommended for the application of CYA in pigs, chickens, and carp for longtime administration in feed. This result indicated that CYA could cause little hazard on animal products when fed to food animals in livestock breeding with the recommended dosage. However, Cy1, the marker residue of CYA in food animals, was the N-oxide reduction metabolite of CYA by reducing oxygen atoms on both N1 and N4 sites on the benzene. As N-oxide reduction is driven by single-electron reductases (Liu et al., 2009), single-electron reduction in quinoxaline-N, N-dioxides leads to DNA damage by radical intermediate production (Badham & Winn, 2010; Huang et al., 2010). Moreover, it had been proved that Bisdeoxycarbadox, the bisdeoxy metabolite of carbadox, was positive in the Ames test and cell transformation test, its tumorigenic potential was apparently greater than that of the parent drug (FAO/WHO, 1990). However, there was no evidence for genotoxic activity of Cy1 in the bacterial reverse mutation test, mouse bone marrow micronucleus assay, or an in vitro assay for clastogenicity (Huang et al., 2016), indicating cyadox may not share the safety concerns of carbadox.
Cyadox is a new animal drug with antimicrobial activity, both a microbiological ADI and toxicological ADI should be assigned to evaluate the human food safety. The lower of the toxicological and microbiological ADIs should be considered to be the final ADI (FDA, 2016). The microbiological safety of cyadox on human intestinal flora had been investigated, and a mADI of 1552.03 μg/kg day was proposed based on four microbiological endpoints (Hao et al., 2013). While a number of toxicological studies suggested that the toxicological NOEL was 15 mg/kg.b.w./day with a derived toxicological ADI of 15 μg/kg day (Fang et al., 2006; Wang, Fang, et al., 2011; Wang et al., 2015), the mADI of cyadox is much higher than the toxicology ADI. Hence, the toxicology ADI was adopted to be final ADI of cyadox.
As a new antibacterial, the resistance to cyadox in pathogenic bacteria needs to be considered. It had been found that high concentrations of cyadox(32 and 128 L μg/ml) could change bacterial population and increase the proportion of resistant E.coliand Enterococcus. More than 26% (12/46) of cyadox-resistant E. coli strains contained oqxAB gene, a horizontally transmissible resistance determinants, while all the resistant Enterococcus were negative to oqxAB gene. However, one E. coli strain (7.08-4-1) obtained prior to the administration of cyadox also contained oqxAB genes (Hao et al., 2013). Another research reported that none of the laboratory-induced cyadox-resistant E. coli strains contained oqxAB genes, but more than 80% of pig-originated E. coli isolates carried oqxAB gene (Guo et al., 2012). It seemed that there was no direct relationship between administration of cyadox and acquisition of oqxAB-resistant gene. Hence, although oqxAB gene indicates a risk of horizontally transmissible resistance, the relationship between the occurrence of oqxAB gene and cyadox exposure needs to be further investigated.
In summary, this study provided a complete residue profile of cyadox and its five metabolites in edible tissues of pigs, chickens, and carp compared with the previous study. Based on these residue data in edible tissues, the pig and chicken kidney and carp liver are recommended as the target tissue, and Cy1 is the residual marker of CYA in food animals. A withdrawal period of 0 d was estimated for CYA in pigs, chickens, and carp. In addition, an HPLC-UV method was established to simultaneously and quantitatively determine CYA and its five major metabolites in edible tissues of pigs, chickens, and carp. These results provided scientific evidence for the food safety evaluation of CYA and a convenient and fast detection technique for the routine residue monitoring of CYA in food-producing animals.
ACKNOWLEDGMENTS
The authors thank the financial supporting from National Risk Assessment Program of Quality and Safety of Livestock and Poultry Products (GJFP2014007), and the fundamental research funds for the Central Universities(2662017PY081).