Pharmacokinetics of ceftiofur sodium in equine pregnancy
Abstract
Eleven pregnant pony mares (D270-326) were administered ceftiofur sodium intramuscularly at 2.2 mg/kg (n = 6) or 4.4 mg/kg (n = 5), once daily. Plasma was obtained prior to ceftiofur administration and at 0.5, 1, 2, 4, 8, 12, and 24 hr after administration. Eight pony mares were re-enrolled in the study at least 3 days from expected foaling to ensure steady-state concentrations of drug at the time of foaling. Mares were administered ceftiofur sodium (4.4 mg/kg, IM) daily until foaling. Parturition was induced using oxytocin 1 hr after ceftiofur sodium administration. Allantoic and amniotic fluid, plasma, and colostrum samples were collected at time of foaling. Serial foal plasma samples were obtained. Placental tissues were collected. Desfuroylceftiofur acetamide (DCA) concentrations were measured in samples by high-performance liquid chromatography (HPLC). Mean (±SD) peak serum concentrations of DCA were 3.97 ± 0.50 μg/ml (low dose) and 7.45 ± 1.05 μg/ml (high dose). Terminal half-life was significantly (p = .014) shorter after administration of the low dose (2.91 ± 0.59 hr) than after administration of the high dose (4.10 ± 0.72 hr). The mean serum concentration of DCA from mares at time of foaling was 7.96 ± 1.39 μg/ml. The mean DCA concentration in colostrum was 1.39 ± 0.70 μg/ml. DCA concentrations in allantoic fluid, amniotic fluid, placental tissues, and foal plasma were below the limit of quantification (<0.1 μg/ml) and below the minimum inhibitory concentration of ceftiofur against relevant pathogens. These results infer incomplete passage of DCA across fetal membranes after administration of ceftiofur sodium to normal pony mares.
1 Introduction
Data from North America and Europe attribute approximately 30%–60% of terminated pregnancies in horses to placentitis (Giles et al., 1993; Hong et al., 1993; Laugier, Foucher, Sevin, Leon, & Tapprest, 2011). Several organisms cause placentitis including bacterial and fungal species (Hong et al., 1993). Ascending placentitis, the most common form of placentitis, results after streptococcal or enteric pathogens migrate through the caudal reproductive tract and colonize the chorioallantois at the cervix. Streptococcus equi subsp. zooepidemicus (S. zooepidemicus) is the bacterium most frequently isolated from clinical cases of placentitis (Giles et al., 1993; Laugier et al., 2011). While bacterial infection causes disease, secondary inflammation and prostaglandin production initiate premature delivery. Advances in treatment for equine placentitis have been directed at allowing time for precocious maturation of the fetus so that survival is improved if delivery is premature.
Studies in other species (Gravett, Sadowsky, Witkin, & Novy, 2003; Gravett et al., 2007) have shown that a drug combination including an antimicrobial, an anti-inflammatory or immunomodulatory agent, and progestins (to inhibit uterine contractions) delays preterm delivery after placental infection. Based on this hypothesis, similar studies in pregnant mares (Murchie, Macpherson, Leblanc, Luznar, & Vickroy, 2006; Rebello, Macpherson, Murchie, Leblanc, & Vickroy, 2006) have examined several clinically used drugs to determine their ability to penetrate fetal membranes and/or result in delivery of live foals after experimental placental infection. When infected mares were administered trimethoprim sulfamethoxazole, pentoxifylline and altrenogest, the majority of mares (10/12; 83%) delivered live foals (Bailey et al., 2007). Untreated, infected mares all aborted. These data supported the premise that combining an antimicrobial with an anti-inflammatory and tocolytic (progestin) was effective for treating mares with placentitis. However, uterine bacteria have been shown to persist even after long-term treatment with trimethoprim sulfamethoxazole (Bailey et al., 2007) suggesting limitations in efficacy of this antimicrobial for streptococcal species (Ensink, Bosch, & van Duijkeren, 2003).
Consequently, use of other antimicrobials with known efficacy for eradicating bacteria that cause placentitis, including streptococcal species, has been explored. Cephalosporins are β-lactam antimicrobials with broad-spectrum, bactericidal activity. Ceftiofur is a third generation cephalosporin that has very good activity against streptococcal organisms as well as many gram-negative aerobes and some anaerobes found in equine infections (Salmon, Watts, & Yancey, 1996; Yancey et al., 1987). When administered parenterally to horses, ceftiofur is rapidly metabolized to desfuroylceftiofur (Jaglan et al., 1994). Parenteral administration of ceftiofur to horses results in therapeutic drug concentrations in body fluids, endometrium, joints, and pulmonary sites of infection (Cervantes, Brown, Gronwall, & Merritt, 1993; Credille et al., 2012; Folz et al., 1992; Jaglan et al., 1994; Mills et al., 2000; Vanduijkeren et al., 1995; Witte, Bergwerff, Scherpenisse, Drillich, & Heuwieser, 2010). Presumably, ceftiofur would have good applicability for treating mares with placentitis. Recently, the long-acting preparation of ceftiofur crystalline free acid (CCFA; Excede®) was examined in mares with placentitis (Macpherson et al., 2013). Administration of ceftiofur crystalline free acid (CCFA), both alone and in combination with pentoxifylline and altrenogest, failed to prevent abortion in mares with induced placentitis. Importantly, concentrations of ceftiofur and desfuroylceftiofur were below therapeutic concentrations in fetal fluids, placental tissues, and foal plasma. In contrast, drug concentrations in pregnant mare plasma and colostrum were similar to concentrations measured in geldings or nonpregnant mares (McClure & Sibert, 2010). It is conceivable that intramuscular administration of ceftiofur sodium, which results in much higher plasma concentrations of ceftiofur and metabolites than ceftiofur crystalline free acid, might result in drug concentrations in fetal fluid, placental tissue, and fetal tissues sufficient to be above the MIC for bacterial pathogens associated with placentitis. Therefore, the objectives of this study were to compare the pharmacokinetics of ceftiofur sodium (in an aqueous solution) administered at two different doses to pregnant mares and to evaluate the disposition of the drug in fetal fluids, fetal membranes, colostrum, and plasma of foals. We hypothesized that ceftiofur and desfuroylceftiofur would penetrate fetal membranes and attain therapeutic concentrations in fetal/foal tissues and fluids.
2 Experimental Approach and Animals
Fourteen pregnant pony mares were used for this study in two separate experiments. In Experiment 1, 11 mares were enrolled from gestational days 270–326 for collection of allantoic fluid after administration of ceftiofur sodium. In Experiment 2, eight mares were enrolled at gestational day 300 to be monitored for impending parturition. Once mares showed signs of foaling, ceftiofur sodium was administered and parturition was induced for collection of placental, fetal, foal, and mare samples. Mares were maintained on pasture at the College of Veterinary Medicine, University of Florida and supplemented with hay and concentrate. The project was approved by the Institutional Animal Care and Use Committee of the University of Florida (IACUC Protocol #201105225).
3 Experiment 1: Collection of Allantoic Fluid
3.1 Drug administration and sampling schedule
Eleven pregnant pony mares (gestational days 270–326) were randomly assigned to one of two treatment groups and administered ceftiofur sodium (Naxcel®, Zoetis, Florham Park, NJ, USA). Five mares were administered ceftiofur at a dose of 4.4 mg/kg IM once daily, and six different mares were administered ceftiofur at a dose of 2.2 mg/kg IM, once daily. Mares were administered ceftiofur sodium at the same time of day, once daily, for 4 days. On day 1 of treatment, plasma was obtained immediately prior to ceftiofur administration and at 0.5, 1, 2, 4, 8, 12, and 24 hr after administration. Mares were administered ceftiofur sodium on days 2 and 3, but plasma was not obtained. On day 4 of drug administration, plasma and allantoic fluid were collected 2 and 24 hr after drug administration.
3.2 Plasma sampling
Blood was collected by direct venipuncture into vacuum tubes containing EDTA anticoagulant (BD Vacutainer®, Becton, Dickinson and Co, Franklin Lakes, NJ, USA). All blood samples were centrifuged at 400 g for 10 min at 25°C. Plasma samples were collected in 1 ml aliquots and stored at −80°C until analysis.
3.3 Allantoic sampling
The ventral abdomen was clipped from the xyphoid process of the sternum to the mammary gland and laterally to the level of the stifle on both sides of the abdomen. The mare was administered detomidine hydrochloride (0.02 mg/kg, IV; Dormosedan®, Zoetis, Florham Park, NJ, USA) and butorphanol tartrate (0.02 mg/kg, IV; Butorphanol®, Zoetis, Florham Park, NJ, USA) for sedation of the mare and the fetus prior to allantocentesis. The abdomen was imaged using a curvilinear sector 3.5 MHz ultrasound transducer (Aloka 900®, Aloka CO, Ltd, Tokyo, Japan) to locate an allantoic fluid pocket that was free of the fetus and amniotic membrane. The area of skin adjacent to the allantoic pocket was aseptically prepared. Three milliliter of 2% lidocaine hydrochloride was administered subcutaneously, and a stab incision was made through the skin and external body wall using a #15 blade. An 18 g, 20-cm echogenic tip needle (Chiba Needle G17897; Cook Medical, Bloomington, IN, USA) was passed through the body wall and into the peritoneal space at the incision site. Needle positioning was verified using ultrasonography. The needle was inserted through the uterine wall and into the allantoic space with a sharp jab. Ten milliliter of allantoic fluid was collected by free catch. Allantoic fluid was confirmed by the characteristic amber color (vs. straw-colored peritoneal fluid or opaque, white-colored amniotic fluid) and glucose concentration below 20 mg/dl (vs. peritoneal fluid glucose > 80 mg/dl; Paccamonti, Swiderski, Marx, Gaunt, & Blouin, 1995). The needle was immediately withdrawn and the site monitored for signs of hemorrhage, swelling, or infection over a 24-hr period. Allantoic samples were centrifuged at 400 g for 10 min to remove cellular sediment. Allantoic fluid samples were stored in 1 ml aliquots at −80°C until analysis. Mares were monitored for signs of systemic illness or preterm delivery for 7 days after allantocentesis procedures were performed.
4 Experiment 2: Induction of Parturition
4.1 Monitoring mares for impending parturition
Beginning at Day 300 gestation, nine mares were monitored twice daily for evidence of impending foaling (mammary gland enlargement, presence of mammary secretions, vulvar softening, laxity of pubic tendons or vulva). Once adequate mammary secretions were available, they were tested using a stall side test measuring calcium according to manufacturer directions (FoalWatch™; Chemetrics, Calverton, VA, USA). Administration of ceftiofur sodium (4.4 mg/kg, IM, once daily) began once mammary secretion calcium was ≥100 ppm.
4.2 Induction of parturition
Mammary secretion calcium was monitored twice daily once ceftiofur sodium administration began. When mammary calcium concentrations were ≥175 ppm, parturition was induced using oxytocin. The value of 175 ppm Ca++ was selected to optimize conditions for fetal survival as well as to ensure controlled sample collection (rather than unattended spontaneous delivery). Parturition was induced 1.5 hr after administration of ceftiofur sodium to allow for delivery as close to 2 hr after drug administration as possible. The sampling time of 2 hr postadministration of ceftiofur was selected based on peak concentrations in the body fluids of mares after IM administration in a prior study (Cervantes et al., 1993). Mares were unrestrained in a small grassy paddock. Immediately prior to induction of parturition, the mare's tail was wrapped and her perineal area washed. A vaginal examination was performed to assess external cervical diameter and softening. Parturition was induced by administration of five international units of oxytocin (oxytocin injection, 20 units/ml; Bimedia Inc., Le Sueur, MN, USA) first by intramuscular injection and subsequently (at 25 min intervals) by intravenous injection until rupture of the chorioallantois (Runcan et al., 2014). Allantoic fluid was collected by free catch. Allantoic samples were centrifuged at 400 g for 10 min to remove cellular sediment. Amniotic fluid was collected by needle puncture into the amnion using an 18-g needle attached to a 35-ml syringe. Allantoic and amniotic samples were stored at −80°C until analysis. Placental samples were collected from the chorioallantois (cervical star region, gravid and nongravid horns) and umbilicus (at the fetus and at amnion) and stored at −80°C until analysis.
Blood samples for plasma collection were obtained by direct venipuncture from both the mare and foal. Samples were obtained from the mare immediately prior to drug administration (T = 0 hr), at delivery, and at 2, 4, 8, and 24 hr after drug administration. Samples were obtained from the foal within 20 min of delivery as well as 1, 2, 4, 8, and 24 hr after delivery. Blood samples were centrifuged at 400 g for 10 min, and plasma was collected. Presuckle colostrum was collected. Plasma and colostrum samples were stored at −80°C until analysis.
4.3 High-performance liquid chromatography
Plasma and fluid samples were analyzed using high-performance liquid chromatography (HPLC) for ceftiofur- and desfuroylceftiofur-related metabolites in accordance with the regulatory method approved by the Center for Veterinary Medicine in the Food and Drug Administration (Jaglan et al., 1990). Briefly, ceftiofur- and desfuroylceftiofur-related metabolites were extracted from samples as desfuroylceftiofur following addition of dithioerythritol solution. Desfuroylceftiofur was captured by use of a C-18 solid phase extraction cartridge (Varian, Inc.; Walnut Creek, CA, USA) and desfuroylceftiofur converted to desfuroylceftiofur acetamide (DCA) by derivatization with iodoacetamide as previously described (Benson, Tell, Young, Wetzlich, & Craigmill, 2003). Therefore, DCA concentrations represent the sum of ceftiofur and desfuroylceftiofur related metabolites. Additional cleanup was carried out on a strong cation exchange column (Varian, Inc.). The HPLC analyses were carried out isocratically (the mobile phase was 7% acetonitrile, 1% acetic acid, with 90 mg heptane sulfonic acid/liter, and pH = 4.0) on a C18, 4 μm, 3.9 × 150 mm column (Nova-pak column, Waters Corporation; Milford, MA, USA) with UV detection at 240 nm. The sample sizes were 1.0 ml, the flow rate was 0.8 ml/min, and sample injection volume was 25 μl. The reference standard used was ceftiofur (Sigma-Aldrich; St. Louis, MO, USA). The stock solution and dilutions were prepared in 0.1 m ammonium acetate. The standard curve was spiked into buffer and extracted along with the foal samples. The standard curve was within the range of 0.1–10.0 μg/ml (n = 6 numbers of points on the standard curve), and standards with a back calculated percent difference more than 15% were rejected. Samples with a concentration greater than the highest standard were diluted and re-injected. Quality control samples were spiked at 0, 0.2, 1.0, and 5.0 μg/ml into blank foal plasma and run with each set of samples and standard curve. Quality control data were used for determining recovery and precision. Concentrations of DCA were expressed as μg/ml of plasma. Tissue samples were analyzed using the method as described by Beconi-Barker et al. (Beconi-Barker et al., 1995). Three phases of extraction occurred using a C18 extraction cartridge, strong anion exchange, and strong cation exchange. Initial extraction started with 2 g of tissue, with a final extract amount equal to 1 g. High-performance liquid chromatography was performed by gradient elution with an injection volume of 500 μl and a detection wavelength of 266 nm. The standard curve ranged from 0.05 to 12.5 μg/g, and quality control samples were 0, 0.1, 1.0, and 10.0 μg/g. The analytical method had a limit of quantification of 0.1 μg/ml.
4.4 Pharmacokinetic analysis
For each horse, plasma concentration versus time was analyzed based on noncompartmental pharmacokinetics using computer software (PK Solutions 2.0, Summit Research Services; Montrose, CO, USA). The rate constant of the terminal phase (λz) was determined by linear regression of the terminal phase of the logarithmic plasma concentration versus time curve using a minimum of 3 data points. Half-life of the terminal phase (t½λz) was calculated as ln 2 divided by λz. The area under the concentration–time curve (AUC) and the area under the first moment of the concentration–time curve (AUMC) were calculated using the trapezoidal rule, with extrapolation to infinity using Cmin/λz, where Cmin was the final measurable DCA concentration. Mean residence time (MRT) was calculated as: AUMC/AUC.
4.5 Data analysis and statistics
Normality and equality of variance of the data were assessed by use of the Shapiro–Wilk's and Levene's tests, respectively. Pharmacokinetic variables were compared between the two groups (2.2 vs. 4.4 mg/kg) using the Student t-test for normally distributed data or the Mann–Whitney U-test for data that was not normally distributed. A value of p < .05 was considered significant.
5 Results
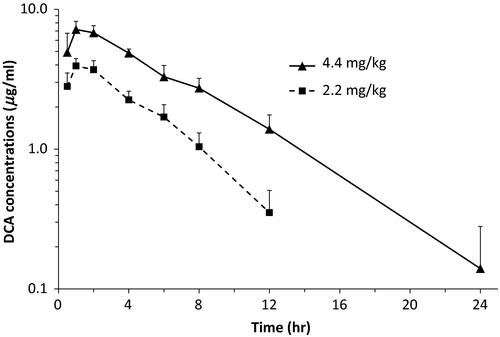
Pharmacokinetic data after administration of ceftiofur sodium to pregnant mares are presented in Table 1. Mean (±SD) peak concentrations of DCA, achieved 1–2 hr after administration, were 3.97 ± 0.50 μg/ml and 7.45 ± 1.05 μg/ml for the low dose and high dose, respectively. Terminal half-life was significantly (p = .014) shorter after administration of the low dose (2.91 ± 0.59 hr) than after administration of the high dose (4.10 ± 0.72 hr; Figure 1). DCA concentrations in allantoic fluid after administration of four doses of ceftiofur sodium at 2.2 mg/kg (n = 6) or 4.4 mg/kg (n = 5) were below the lower limit of quantification of the assay (<0.1 μg/ml) and below the MIC that inhibits at least 90% of isolates (MIC90) of S. zooepidemicus (0.12 μg/ml; Bade et al., 2009).
| Variable | Dose (mg/kg) | p-value | |
|---|---|---|---|
| 2.2 | 4.4 | ||
| First dose | |||
| λz (1/hr) | 0.247 ± 0.049 | 0.173 ± 0.030 | .018 |
| t1/2λz (hr) | 2.91 ± 0.59 | 4.10 ± 0.72 | .014 |
| AUC0-t (μg·hr/ml) | 21.63 ± 3.26 | 53.42 ± 7.40 | <.001 |
| AUC0-∞ (μg·hr/ml) | 23.21 ± 4.06 | 55.28 ± 6.34 | <.001 |
| MRT (hr) | 4.79 ± 0.64 | 6.38 ± 1.00 | .011 |
| Tmax (hr) | 1 (1–2)a | 1 (1–2)a | .931 |
| Cmax (μg/ml) | 3.97 ± 0.50 | 7.45 ± 1.05 | <.001 |
| C24 hr (μg/ml) | <0.1 (<0.1)a | 0.15 (<0.1–0.31)a | .126 |
| Last dose | |||
| C74 hr (μg/ml) | 3.01 ± 0.88 | 6.25 ± 1.34 | <.001 |
| C96 hr (μg/ml) | <0.1 (<0.1)a | 0.25 (<0.1–0.49)a | .030 |
- AUC0-t, Area under the plasma concentration versus time curve from time 0 to the last measurable plasma concentration; AUC0-∞, Area under the plasma concentration versus time curve extrapolated to infinity; Cmax, Maximum plasma concentration of DCA; C24 hr, Plasma concentration of DCA 24 hr after administration; Tmax, Time to maximum plasma concentrations; λz, rate constant of the terminal phase; t1/2λz, half-life of the terminal phase; MRT, Mean residence time. C74 hr, Concentration of DCA 2 hr after the fourth dose. C96 hr, Concentration of DCA 24 hr after the last dose.
- a Median and range.
In Experiment 2, mares received a median of seven doses (range 4–24 doses) of ceftiofur sodium at a dose of 4.4 mg/kg q 24 hr prior to parturition. The median time from the last dose of ceftiofur to foaling was 150 min (range 109–176 min). The mean plasma concentration of DCA in mares at time of foaling was 7.76 ± 1.39 μg/ml, whereas concurrent DCA concentration in colostrum was 1.39 ± 0.70 μg/ml. DCA concentrations in foal plasma, amniotic fluid, allantoic fluid, and placental tissues were below the lower limit of quantification of the assay (<0.1 μg/ml).
6 Discussion
These data illustrate the inability of ceftiofur and metabolites to effectively penetrate equine fetal membranes when administered to mares. Concentrations of DCA were below detectable concentrations in all fetal fluids and tissues, as well as foal plasma. Failure to achieve therapeutic concentrations in fetal fluids and placenta was not due to decreased absorption or altered drug disposition during pregnancy because plasma concentrations of DCA identified in the pregnant mares used in this study were similar to those reported in nonpregnant adult horses after IM administration of ceftiofur sodium (Jaglan et al., 1994). In a recent study, administration of CCFA to pregnant pony mares resulted in very low DCA concentrations (median = 0.03 μg/ml) in placental and fetal tissues (Macpherson et al., 2013). It was hypothesized that intramuscular administration of ceftiofur sodium, which results in much higher plasma concentrations of DCA than CCFA, might result in concentrations of DCA in fetal fluid, placental tissue, and fetal tissues sufficient to be above the minimal inhibitory concentration (MIC) of ceftiofur against bacterial pathogens associated with placentitis. The optimal dosing of an antimicrobial agent is determined by both the pharmacokinetics and pharmacodynamics of the drug. Currently, most pharmacokinetic/pharmacodynamic models rely on plasma concentrations and MIC. The most important factor determining the efficacy of β-lactam antimicrobials such as ceftiofur is the duration of time that plasma concentrations of the drug exceed the MIC of a given pathogen (Auckenthaler, 2002; Liu et al., 2002). The MIC90 of ceftiofur against S. zooepidemicus is 0.12 μg/ml, and DCA concentrations in fetal fluids and placenta in the present study were all <0.1 μg/ml indicating that ceftiofur is unlikely to be effective for the treatment of placentitis in mares.
There is a paucity of information regarding drug metabolism and transport in equine pregnancy, and it is likely that the process is complex. Most of the data available have been obtained in rodent or non-human primate species. Placentation varies between species thus limiting extrapolation of information about drug transport between species. In general, transport of substances across the placenta is thought to occur by passive diffusion (Syme, Paxton, & Keelan, 2004). Several properties of substances affect passive placental diffusion including molecular weight and polarity, lipid solubility, and plasma protein binding. Low molecular weight, highly lipid soluble, and unionized drugs are favored in passive placental diffusion.
The molecular weight of desfuroylceftiofur does not provide a clear explanation for poor placental diffusion in horses. Molecules <500 g/mol are thought to diffuse across placental membranes, while molecules >1,000 g/mol are too big for placental passage (Ward, 1993). Molecules in the 500–1,000 g/mol range exhibit partial placental diffusion. Desfuroylceftiofur has a molecular weight of 429 g/mol. (http://pubchem.ncbi.nlm.nih.gov/compound/Ceftiofur_sodium) While partial obstruction of these mid-sized molecules across the equine placenta is possible, one would expect detection of low to moderate concentrations of the drugs in fetal compartments. Concentrations of DCA were undetectable or well below therapeutic concentrations in fetal fluids and tissues, which is in disagreement with the weight profile of the molecules. One must assume that molecular size, alone, is not the reason for poor placental drug passage in this study.
Protein binding affinity can also affect trans-placental passage of drugs. The unbound drug fraction diffuses across the membrane gradient to establish equilibrium. While many drugs are highly protein bound, including ceftiofur, the concentration of unbound drug in circulation is often high enough to facilitate passage of the drug over a membrane gradient, such as the placenta. Given that detectable concentrations of ceftiofur and metabolites have been identified in other equine target tissues, such as the lung and joint (Jaglan et al., 1994; Mills et al., 2000), protein binding does not seem like a plausible explanation for poor placental penetration of the molecules.
Lipid solubility and pH of the drug also play a role in placental diffusion of drugs. Ceftiofur formulated as a sodium salt (NADA 40338) is found in solutions at pH < 3.2 with a solubility of 0.25 mg/ml. The partition coefficient (n-octanol/water—cLog P) is reported to be 0.3 when adjusted to a pH of 5. This coefficient is low suggesting that ceftiofur is hydrophilic or lipophobic. Hydrophilic compounds are less able to passively diffuse through a lipid dense membrane like the placenta. It is possible that poor lipid solubility of desfuroylceftiofur prevents passage of the drug across placental membranes. Interestingly, penicillin, which is also has poor lipid solubility, readily penetrated the equine placenta (Rebello et al., 2006) in normal mares and mares with placentitis. Both penicillin and trimethoprim sulfamethoxazole achieved concentrations in allantoic fluid of mares with induced placentitis that were above minimal inhibitory concentrations (MIC) against S. zooepidemicus (Murchie, Macpherson, Leblanc, Luznar, & Vickroy, 2003).
More recently, active transport mechanisms have been identified in drug transport across placental membranes. Specifically, metabolic enzymes and membrane transporters have been shown to provide an active mechanism for drug passage across the placenta. Several enzyme systems, most notably those in the cytochrome P450 (CYP) and UDP glucuronosyltransferase (UGT) series, have been identified in the human placenta (Isoherranen & Thummel, 2013). Probe studies using marker drugs relevant to a specific enzyme pathway have demonstrated differences in drug clearance across placental membranes, in vitro. For instance, drug metabolism via the cytochrome P450 (CYP) pathway was evaluated using caffeine and dextromethorphan as markers for CYP1A2 and CYP2D6 enzymatic pathways (Tracy, Venkataramanan, Glover, & Caritis, 2005). Caffeine clearance (CYP1A2 activity) was increased, and dextromethorphan clearance (CYP2D6 activity) was decreased, at all stages of pregnancy. These data demonstrated that changes in enzymatic pathways along a common system (CYP) could have dramatically different outcomes for drug metabolism. The cytochrome P450 enzymatic pathway is one of many that can affect trans-placental transport of drugs. Active transport of drugs across the placenta is poorly understood, yet the implications that drug transporters have for metabolic effects, and proper dosing, of drugs in pregnancy is staggering.
In summary, data from the present study suggest negligible transport of ceftiofur and desfuroylceftiofur across the equine placenta. The mechanism inhibiting transport of ceftiofur and desfuroylceftiofur from crossing the equine placenta is not known. These data confirm how essential pharmacokinetic profiling in a given species or body system is prior to formulating therapeutic plans. Furthermore, a better understanding of placental transport mechanisms could have a profound effect on drug selection and dosing for a given condition.
Acknowledgements
This work was funded by the Grayson-Jockey Club Research Foundation and the University of Florida Equine Endowment. Drugs were provided by Zoetis/Pfizer Animal Health (ceftiofur sodium) and Merck Animal Health (altrenogest). The authors gratefully acknowledge the intellectual contributions of Dr. Michelle LeBlanc (now deceased) to the design of this study.




