Pharmacokinetics and pharmacodynamics of the injectable formulation of methadone hydrochloride and methadone in lipid nanocarriers administered orally to horses
Abstract
We investigated the thermal, electrical and mechanical antinociceptive and physiological effects (heart rate, respiratory rate, arterial blood pressure, head height and abdominal auscultation score), and pharmacokinetics, of 0.5 mg/kg of the injectable formulation (ORAL) or nanoparticulated methadone (NANO) given orally, in six adult mares, using a crossover, blind and prospective design. Repeated-measure models were used to compare parametric data between and within treatments, followed by Tukey's test. Nonparametric data were analysed with Wilcoxon signed-rank, adjusted by Bonferroni tests. Blood samples were also collected up to 6 h after dosing for plasma drug quantification by LC-MS/MS. Methadone pharmacokinetic parameters were determined by noncompartmental and compartmental approaches. There were no differences in pharmacodynamic parameters. No statistical differences were observed in the pharmacokinetic parameters from noncompartmental analysis for both groups, except a significant decrease in peak plasma concentration, increase in apparent volume of distribution per fraction absorbed (Vdss/F) and increased mean residence time (MRT) for NANO. One-compartment open model with first order elimination best described the pharmacokinetic profiles for both groups. Neither ORAL nor NANO administered orally to horses produced antinociception. The nanoencapsulated formulation of methadone given orally to horses did not improve methadone pharmacokinetic parameters or increased systemic body exposure to methadone.
Introduction
Opioids are classical analgesics; nevertheless, in horses, they may cause potential adverse effects such as excitement, increased locomotor activity, intestinal hypomotility and cardiovascular stimulation (Clutton, 2010). The aforementioned effects are dependent on the opioid, the route of administration and the dose used (Carregaro et al., 2007; Figueiredo et al., 2012).
Methadone is a μ-opioid agonist; however, this drug produces antagonism of N-methyl-D-aspartate receptors and inhibition of serotonin and norepinephrine reuptake in the central nervous system (CNS). These activities provide analgesic characteristics that distinguish methadone from other classical opioids (Santiago-Palma et al., 2001; Inturrisi, 2005; Shaiova, 2005; Trescot et al., 2008; Oliveira et al., 2013).
Oral administration of drugs may be a good alternative to other routes as it is noninvasive, easy to administer and may produce less pronounced adverse effects (Kestenbaum et al., 2014). Previous studies reported administration of lipid-soluble and water-soluble opioids in horses, such as sublingual buprenorphine (Messenger et al., 2011), oral methadone (Linardi et al., 2009, 2012) and oral tramadol (Shilo et al., 2008; Cox et al., 2010; Guedes et al., 2013), but there is no information about possible antinociceptive effects using oral or buccal opioids in such species.
Reports in the literature indicate that the absorption of a parenteral formulation of methadone orally administered to horses was rapid and serum concentrations were sufficient to those necessary to produce analgesia in people (Linardi et al., 2009, 2012). Intravenous (i.v.) administration of methadone produces short-acting antinociception, but with clinically severe adverse effects indicating that obtaining optimal analgesia with little adverse effects is a challenge (Oliveira et al., 2013).
The use of drug delivery systems for analgesics can optimize pain treatment, reducing undesirable adverse effects. Nanoparticles have attracted interest in recent years because these systems act as carriers providing controlled and sustained drug delivery and increasing the drug's bioavailability and mean residence time (Sukhorukov et al., 2005). The pharmacodynamics and pharmacokinetics of nanoparticulated opioids administered by several routes, including orally, have been investigated in numerous species (Mystakidou et al., 2007; Paul-Murphy et al., 2009; Wang et al., 2009; Krugner-Higby et al., 2011; Hoekman et al., 2014; Han et al., 2015; Vazzana et al., 2015). However, to our knowledge, there are no studies reporting the possible antinociceptive effect of methadone administered orally to horses, as free drug or as nanoencapsulated formulation.
This work was conducted based on the hypothesis that oral administration of parenteral formulation of methadone could produce an antinociceptive effect in horses with little or no adverse effects and that nanoparticulated methadone may improve the drug oral absorption and mean residence time, potentiating and prolonging the antinociceptive effect. In this context, the aims of this study were to investigate the sedative, antinociceptive, cardiorespiratory and gastrointestinal effects of oral dosing of parenteral formulation and nanostructured lipid carriers of methadone to horses.
Materials and Methods
Lipid nanocarrier preparation
To prepare the lipid nanocarriers, methadone was obtained from the commercially available hydrochloride methadone formulation (Cristália®, Brazil) by precipitation with excess of sodium carbonate in ultrapure water under magnetic stirring for 1 h. The suspension obtained was extracted with ethyl acetate, in a dropping funnel. The resultant solution was evaporated at reduced pressure. The resulting white crystal product was measured by infrared spectrometry (FT-IR SPECTRUM BX® PerkinElmer, Boston, MA, USA), and the extraction efficiency was 80%.
Nanocarriers were prepared according to Müller et al. (2000). Coconut (Cocos nucifera) butter 5 g (Cosmetrate) and Cupuaçu (Theobroma grandiflorum) butter 5 g (Cosmetrate), Span 80 3 g (Sigma®) and methadone base 0.6 g were dissolved in water bath at 37 °C. The mixture was dispersed by high agitation (5 min) using an Ultra-Turrax (T25, Ika®) in aqueous solution (ultrapure MilliQ® water, 200 mL), containing Tween-80 (1.54 g.; Sigma®) and maintained at 37 °C. This pre-emulsion was homogenized with 5 cycles under high pressure (Panda 2K NS1001L, Niro Saovi®) and 300 bars. Finally, 0.5% carboxymethyl cellulose was added to the aqueous dispersion of methadone lipid nanocarriers. The average diameter and particle size distribution (Span) were determined by laser diffraction.
Pharmacokinetics investigation
This study was approved by the Institutional Ethical Committee of Animal Research under the protocol number 55/2011. The sample size was calculated based on methadone pharmacokinetics variability reported by Linardi et al. (2009), with a power of 80% and 5% of significance level. Six university-owned research mixed breed mares between 2 and 4 years of age and weighing 354 ± 34 kg (range 310–395 kg) were used. The health status of the animals was assessed by clinical and laboratory examinations (blood count and biochemical profile – urea, creatinine, AST and GGT). The animals were trained for a month to habituate to the surroundings, researchers and equipment used for nociceptive stimulation.
The study was conducted with a complete crossover and randomized design with a 1-week washout period between treatments. Horses received two drug treatments: ORAL, where 0.5 mg/kg of the hydrochloride methadone injectable formulation (Cristália®, Brazil) was mixed in 0.5% carboxymethyl cellulose and was administered orally; or NANO, where 0.5 mg/kg of methadone was administered as lipid nanocarrier. The carboxymethyl cellulose was added to both formulations to avoid dripping during the administration. The total volume administered was the same (about 60 mL for each horse) for both groups.
The horses were fasted for 12 h prior to the experiment and were allowed to have water ad libitum. In the morning of the experiment, each animal was positioned into the paddock, in a quiet environment and was familiarized to the investigators. A 14-G catheter was placed in the left external jugular vein for blood sampling. The animal's buccal pH was measured before drug administration (pH indicator strips, Merck®). The oral administration was conducted directly from a 20-mL syringe into the buccal cavity of the animal.
Blood samples (10 mL) were obtained before drug administration and at 3, 5, 10, 15, 20, 30, 45, 60, 120, 240 and 360 min after the two formulations were given. The first 5 mL of blood was discarded, followed by a 10-mL sample collection. The catheter was flushed with 5 mL of 0.9% sodium chloride after each blood sampling. Blood samples were placed in tubes containing EDTA and centrifuged immediately. Plasma was harvested and frozen at −70 °C until analysis.
The pharmacokinetic data were analysed by noncompartmental and compartmental approaches using Excel® 2003 (Microsoft®) and Scientist® version 2.0 (MicroMath®).
The following parameters were determined by noncompartmental approach: Cmax1 and tmax2 were verified by visual inspection of the data from the concentration–time profile; the elimination rate constant (λz) was calculated by log-linear regression of methadone concentration–time data during the elimination phase and the t1/2z3 was calculated as 0.693/λz; the AUC0–t4 from time zero to the time of Clast5 was calculated by the linear trapezoidal rule; the AUC0–∞6 was obtained by the addition of AUC0–t; and the extrapolated area was determined by Clast/λz; CLtot/F7 was calculated from the quotient of the D8 and AUC0–∞; the AUMC0-∞9 was calculated by trapezoidal rule from a plot of Cxt vs. t up to Clast added to the extrapolated terminal area, calculated as Clast·t/λz + Clast/λz 2; the MRT10 was calculated by the ratio of the AUMC0–∞ and AUC0–∞; Vdss11 was determined as the product of CLtot and (MRT-MAT12 ). MAT was defined as the inverse of ka13 which was determined by Wagner-Nelson method (Shargel et al., 2012).
For the compartmental analysis, the individual pharmacokinetic profiles were modelled using one- and two-compartment models with and without weighting schemes. The best model to describe the data was selected using the MSC14, a modified AIC15; the correlation coefficient given by the software, the fitting of the data using the model and physiological meaning of the pharmacokinetic parameters estimated by the model.
Methadone bioanalytical method
Liquid chromatography (Shimadzu, Tokyo, Japan) in tandem with mass spectrometry (Quatro LC Micromass®, Waters Corporation, Milford, MA, USA) method was used for plasma sample analysis. The LC-MS/MS method described by Musshoff et al. (2006) and adapted by KuKanich and Borum (2008) was validated in the laboratory according to FDA16 guidelines (FDA Center for Drug Evaluation and Research, Center for Veterinary Medicine, 2001) with a linear curve over the methadone concentration range of 5–1000 ng/mL and the validation parameters such as intra- and interday precision and accuracy within the limits established. The lower limit of quantification (LOQ17 ) was 5 ng/mL. The intra- and interday variability were lower than 12.9%, and the accuracy was higher than 87% for all points of the calibration curve.
A Phenomenex C8 precolumn (4 × 3.0 mm) and a C8 (3 × 150 mm, 5 μm, XDB-C8 Agilent® Eclipse) column were used as stationary phase. The mobile phase consisted of 70:30 (v/v) MeOH: formic acid 0.1% aqueous solution at a 0.5 mL/min flow rate. The oven temperature was set at 30 °C. Source temperature was 130 °C, and desolvation temperature was 350 °C. Nebulization and desolvation gas flows were 75 and 750 L/h, respectively. Precursor ions were 310.1 and 337.1 m/z, and products were 104.7 and 188 m/z for methadone and fentanyl (internal standard), respectively.
Plasma sample extraction was conducted by liquid/liquid technique. For the calibration curves, plasma (100 μL) and internal standard (10 μL of fentanyl, 200 ng/mL) were added to 1.2 mL of ethyl acetate (Nuclear) and vortexed for 10 min. After centrifugation for 10 min at 6050 g and 4 °C, the supernatant was transferred to vacuum centrifuge tubes. The evaporated content of the tubes was re-suspended in 100 μL of MeOH:H20 (50:50), for a final injection volume of 20 μL. The animal's samples (100 μL) were processed in the same manner.
Pharmacodynamic investigation
The following pharmacodynamic parameters were evaluated: distance from the floor to animal's muzzle; heart rate, by auscultation; respiratory rate, by chest movement counts; and noninvasive arterial blood pressure, by a Doppler transducer (Model 812, Parks Medical Electronics, INC) placed in the coccygeal artery and corrected to the heart level (Taylor & Clarke, 2007). Two cuffs of 12 and 17 cm width were used, with a width-to-circumference ratio of 0.4 (Dura-cuff Critikon, GE Healthcare, Arlington Heights, IL, USA). Gastrointestinal sound scores were evaluated by abdominal auscultation (Teixeira Neto et al., 2004) and mechanical, thermal and electrical nociceptive thresholds as reported by Luna et al. (2015). Pharmacodynamic data were collected by an evaluator unaware of the treatment administered to each animal. These parameters were assessed at baseline (mean of three values) and at 10, 20, 45, 60, 80, 100, 120, 140, 160, 180, 210 and 240 min after dosing, simultaneously to blood sampling for the pharmacokinetic investigation.
Statistical analysis
Statistical analysis was performed using SIGMASTAT® v. 3.5, San Jose, CA, USA. For the pharmacokinetic data, the comparison between the pharmacokinetic parameters determined by noncompartmental and compartmental approaches was performed by Student's test assuming equal variances (α = 0.05). Comparison between ORAL and NANO groups was performed by paired t-test. Repeated-measures mixed models (Proc Mixed, SAS Institute, 2011) were performed to compare the means of the normally distributed variables between treatments and time points. An autoregressive covariance structure was used to model the correlation between repeated measurements within the same animals. Normality was tested, and Tukey's test was used to adjust the P-values resulted from multiple comparisons. For the nonparametric variables, the Wilcoxon signed-rank test and the Wilcoxon rank sum test (PROC NPAR1WAY, SAS Institute, 2011) were used for comparisons between time points, and between treatments within each time point, respectively. The Bonferroni method was used to adjust the P-values resulted from multiple comparisons.
Results
The buccal cavity pH values ranged from 8 and 9 in all animals and the formulation of nanoparticulated methadone had a pH of 7. Mean plasma concentration–time profiles after ORAL and NANO methadone administration at 0.5 mg/kg oral dose are shown in Fig. 1.
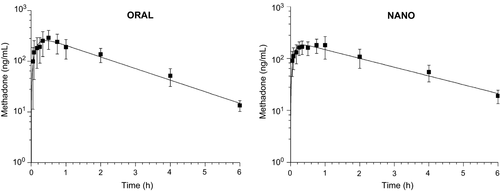
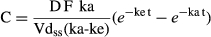
The mean plasma profile modelling resulted in MSC of 4.4 and 3.76 and a correlation coefficient of 0.996 and 0.993 for ORAL and NANO group, respectively.
The pharmacokinetic parameters determined by noncompartmental and compartmental approaches are shown in Table 1. The parameters determined by both model-independent and model-dependent approaches for each group were not statistically different (α = 0.05), confirming that the model selected was suitable to describe the experimental data.
| PK Parametersa | ORAL | NANO | ||
|---|---|---|---|---|
| Noncompartmental (Mean ± SD) | One compartment (Mean ± SD) | Noncompartmental (Mean ± SD) | One compartment (Mean ± SD) | |
| λz or ke (h−1) | 0.54 ± 0.10 | 0.49 ± 0.13 | 0.44 ± 0.10 | 0.45 ± 0.11 |
| t1/2z (h) | 1.3 ± 0.3 | 1.6 ± 0.7 | 1.6 ± 0.5 | 1.6 ± 0.4 |
| Cmax (ng/mL) | 334.6 ± 106.1 | 219.5 ± 111.6 | 212.1 ± 57.6b | 165.0 ± 53.9 |
| tmax (min) | 28.3 ± 10.3 | 42.6 ± 12.6 | 39.2 ± 18.6 | 40.0 ± 18.5 |
| AUC0-∞ (ng·h/mL) | 671.0 ± 206.3 | 617.9 ± 188.7 | 555.5 ± 174.6 | 502.1 ± 154.7 |
| ka (h−1) | 3.8 ± 2.5 | 6.8 ± 4.0 | 8.8 ± 9.7 | 10.5 ± 7.8 |
| MRT (h) | 1.9 ± 0.2 | – | 2.37 ± 0.3b | – |
| CLtot/F (L/h/kg) | 0.80 ± 0.21 | 0.88 ± 0.27 | 0.98 ± 0.30 | 1.09 ± 0.36 |
| Vdss/F (L/kg) | 1.18 ± 0.34 | 1.92 ± 0.77 | 2.09 ± 0.75b | 2.50 ± 0.81 |
- a λz, elimination rate constant (noncompartmental approach); ke, elimination rate constant (compartmental approach); t1/2z, half-life; Cmax, peak plasma concentration; tmax, time to peak concentration; AUC0-∞, area under the curve; ka, absorption rate constant; MRT, mean residence time; CLtot/F, apparent total clearance per fraction absorbed; Vdss/F, apparent volume of distribution per fraction absorbed.
- Values represent mean ± SD; n = 6/group.
- b P < 0.05 (statistical differences between groups for the noncompartmental analysis).
The extrapolated AUC (Clast/λz) was less than 20% for all animals in both groups, demonstrating that the sampling period was long enough to correctly characterize drug elimination after ORAL and NANO administration.
No statistical differences were observed in the pharmacokinetic parameters between groups except for Cmax, Vdss/F and MRT, determined only by noncompartmental analysis. Peak plasma concentration was significantly decreased after NANO (212.1 ± 57.6 ng/mL) dosing compared to ORAL (334.58 ± 106.05 ng/mL). Both peaks, however, were achieved around 1.3–1.6 h after administration. The larger Vdss/F for NANO (1.92 ± 0.77 L/kg) than ORAL (1.18 ± 0.34 L/kg) administration may be advocated incorrectly because F is unknown and the plasma exposure to methadone was similar after ORAL and NANO administration (AUC0-∞ = 671.0 ± 206.3 and 556.9 ± 174.6 ng·h/mL, respectively), indicating that the nanoencapsulation did not change the extent of drug absorption. The MRT, however, was significantly increased after NANO dosing (2.4 ± 0.3 h) in comparison with ORAL dosing (1.9 ± 0.2 h). The results of the pharmacokinetic analysis indicate that methadone nanoencapsulation may alter the drug's distribution in the body and the time of residence but does not cause impact on drug bioavailability, which was reduced in comparison with the ORAL dosing (Relative bioavailability – Frel = 0.82).
Table 2 shows the pharmacodynamic data for the antinociceptive stimuli (pressure, thermal and electrical threshold), gastrointestinal sound scores, noninvasive systolic arterial blood pressure, distance from the floor to animal's muzzle, heart rate and respiratory rate. No statistical differences in pharmacodynamics parameters were observed (α = 0.05).
| Pharmacodynamic parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sampling time | PT (Newtons) | TT (0 C) | ET (Volts) | GS (scale) | NIBP (mm/Hg) | F/M (cm) | HR (beats/min) | RR (breaths/min) |
| NANO | ||||||||
| Basal | 3.8 ± 2.1 | 14.7 (10.8–15.7) | 2 (1–7) | 14 (12–15) | 119 ± 9 | 80 ± 4.5 | 49 ± 7.8 | 14 ± 1.8 |
| 10 min | 3.5 ± 2.1 | 12.9 (7.6–17.8) | 3 (2–6) | 13 (11–15) | 129 ± 17.1 | 84.2 ± 11.1 | 48 ± 9.0 | 15 ± 2.4 |
| 20 min | 6.4 ± 5 | 14.6 (11.5–21) | 2 (2–5) | 14 (11–15) | 127 ± 13.4 | 84.2 ± 10.2 | 47 ± 9.9 | 14 ± 2.0 |
| 45 min | 7.2 ± 2.4 | 14.3 (10.1–21.2) | 3 (2–6) | 12.5 (10–14) | 119 ± 14 | 89.2 ± 2 | 47 ± 11.1 | 13 ± 2.4 |
| 60 min | 8.1 ± 2.7 | 15.5 (11.9–16.8) | 3 (2–5) | 13.5 (12–15) | 120 ± 9.2 | 85.0 ± 7.7 | 47 ± 10.5 | 13 ± 2.4 |
| 80 min | 6.8 ± 7.8 | 13.7 (11.3–17) | 4 (1–7) | 14.0 (11–15) | 125 ± 120 | 85.8 ± 3.8 | 46 ± 9.8 | 12 ± 2.0 |
| 100 min | 5.7 ± 6 | 15.6 (9.8–21.4) | 4 (2–11) | 13.5 (12–15) | 124 ± 11.6 | 84.2 ± 11.1 | 47 ± 8.6 | 13 ± 2.1 |
| 120 min | 5.4 ± 7.4 | 16.6 (10.7–19.8) | 3.5 (1–8) | 13.5 (11–15) | 118 ± 8.0 | 84.2 ± 5.8 | 48 ± 10.0 | 12 ± 1.3 |
| 140 min | 7.8 ± 6.3 | 16.1 (11.9–20.7) | 3 (1–11) | 12.5 (11–15) | 123 ± 9.3 | 81.7 ± 2.6 | 48 ± 10.2 | 12 ± 0.8 |
| 160 min | 6.6 ± 6 | 13.6 (5.6–22.2) | 3.5 (1–11) | 13.5 (13–15) | 120 ± 7.1 | 84.2 ± 3.8 | 47 ± 10.3 | 12 ± 2.0 |
| 180 min | 5.2 ± 5.1 | 13.7 (11.7–18.5) | 3.5 (2–11) | 13.0 (13–14) | 122 ± 8.4 | 82.5 ± 6.9 | 47 ± 11.1 | 13 ± 3.0 |
| 210 min | 5.4 ± 3.8 | 14.3 (12–16.8) | 4 (2–11) | 14.0 (12–15) | 116 ± 7.5 | 82.5 ± 6.1 | 48 ± 11.1 | 13 ± 2.1 |
| 240 min | 2.8 ± 2.3 | 13.3 (9.9–18.7) | 3 (2–11) | 14.0 (12–15) | 116 ± 5.1 | 85.8 ± 5.8 | 49 ± 12.0 | 12 ± 2.3 |
| ORAL | ||||||||
| Basal | 6.2 ± 1 | 22.7 (10.3–29.3) | 3 (2–6) | 14 (14–15) | 122 ± 15.3 | 87.5 ± 4.2 | 43 ± 3.0 | 16 ± 4.2 |
| 10 min | 5.4 ± 2.7 | 20.9 (10.3–29.4) | 30 (2–9) | 14.5 (11–16) | 128 ± 23.5 | 80.8 ± 13.2 | 43 ± 6.0 | 15 ± 4.8 |
| 20 min | 8.9 ± 6.4 | 22.7 (10.3–34.4) | 3 (3–10) | 15 (12–16) | 128 ± 17.8 | 82.5 ± 12.1 | 41 ± 2.7 | 16 ± 4.5 |
| 45 min | 9.6 ± 6.6 | 21.4 (11.2–31) | 3 (3–7) | 14.5 (12–15) | 131 ± 21.7 | 80.0 ± 16.4 | 43 ± 6.7 | 14 ± 3.7 |
| 60 min | 7 ± 7 | 19.6 (10.7–26.7) | 3.5 (2–6) | 15 (12–15) | 138 ± 27.5 | 84.2 ± 12.8 | 43 ± 7.1 | 14 ± 5.6 |
| 80 min | 7.4 ± 4.7 | 14.3 (9.1–24.9) | 4 (2–10) | 13.5 (12–15) | 131 ± 30.1 | 87.5 ± 5.2 | 42 ± 3.4 | 14 ± 5.7 |
| 100 min | 6 ± 5 | 16.2 (7.2–26.8) | 3.5 (2–6) | 14.5 (11–15) | 135 ± 21.8 | 82.5 ± 6.9 | 44 ± 7.6 | 13 ± 4.9 |
| 120 min | 6.4 ± 4.6 | 21.6 (7.1–29.3) | 3.5 (1–10) | 14 (11–16) | 130 ± 29.4 | 82.5 ± 5.2 | 43 ± 5.6 | 13 ± 3.9 |
| 140 min | 5.8 ± 3.3 | 21.8 (10.2–28.8) | 5 (2–7) | 15 (12–15) | 133 ± 28.6 | 82.5 ± 15.1 | 41 ± 4.7 | 14 ± 4.3 |
| 160 min | 5.9 ± 3.8 | 20.6 (9.3–26.7) | 4 (2–12) | 14 (13–15) | 132 ± 26.2 | 88.3 ± 7.5 | 43 ± 5.2 | 15 ± 5.0 |
| 180 min | 3.8 ± 1.9 | 19.6 (9.6–27.7) | 4 (3–5) | 14.5 (14–15) | 132 ± 28.6 | 90.8 ± 5.8 | 44 ± 6.1 | 15 ± 5.0 |
| 210 min | 3.3 ± 1.5 | 13.6 (8.6–22.4) | 2.5 (2–8) | 14.5 (14–15) | 127 ± 21.7 | 88.3 ± 6.1 | 41 ± 5.9 | 14 ± 2.2 |
| 240 min | 4.4 ± 2.5 | 11.1 (7.4–24.8) | 4 (2–6) | 15 (13–16) | 131 ± 19.9 | 90.0 ± 4.5 | 43 ± 8.5 | 14 ± 4.3 |
Discussion
Neither oral nor nanoencapsulated methadone produced antinociception; therefore, the hypothesis that oral administration of parenteral formulation of methadone produce antinociception in horses was rejected, in contrast to a previously published statement (Linardi et al., 2009). Although methadone nanoencapsulation did increase the drug's mean residence time, it did not improve absorption or potentiate or prolong the antinociceptive effect of oral methadone.
The same dose of methadone used in this study, administered intravenously or intramuscularly (i.m.) to horses, increased electrical, mechanical and thermal nociceptive threshold; however, adverse effects observed were relevant (Crosignani et al., 2013). The present study aimed to develop a new opioid formulation for analgesic treatment in horses, devoid of adverse effects, and to investigate whether the previously reported i.v. and i.m. methadone antinociceptive dose given orally would produce antinociception. Neither the parenteral formulation (ORAL) nor the nanoencapsulated methadone (NANO) produced antinociception in the animals, denying this study hypothesis and results previously published (Linardi et al., 2009).
Methadone analgesic plasma concentrations in humans range between 33 and 59 ng/mL (Gourlay et al., 1984), following intravenous injection, after a complete circulation cycle. Linardi et al. (2012) reported that oral methadone administration in horses produced higher plasma concentrations than those reported as analgesic in humans, and, therefore, suggested that oral methadone might be a possible route for opioid analgesia. The present study showed that although human analgesic-related plasma concentrations were achieved, no antinociception was observed. Methadone concentrations observed in the present study were similar to those reported by Linardi et al. (2012). In that study, methadone oral bioavailability was above 100% when blood was collected from the jugular vein.
Messenger et al. (2011) comparing blood buprenorphine concentrations in the jugular and lateral thoracic veins, after sublingual administration in horses, concluded that the jugular vein was inappropriate for pharmacokinetic studies, when the target drug is absorbed via oral transmucosal, because bioavailability was greater than 100% in jugular blood. Otherwise, blood buprenorphine concentrations at the lateral thoracic vein were below the lower limit of quantification. Similar results were reported by Sohlberg et al. (2013), when comparing plasma samples collected from the jugular or saphenous vein after oral administration of a particular drug. Plasma concentration at the jugular vein showed greater variation in maximum values and twofold greater bioavailability and fourfold greater concentrations than those determined in the saphenous vein.
Considering these previous studies showing that the bioavailability was greater than 100% when drugs were given orally and sampled in the jugular vein, this may be partially explained by a large oral methadone absorption locally, as the linguofacial vein drains the blood from the oral cavity into the jugular vein, and therefore, plasma concentration may be overestimated (Dyce et al., 2010; Messenger et al., 2011). For this reason, any correlation between methadone plasma concentrations determined from jugular blood samples after oral administration and antinociceptive effects would be without ground and was beyond the scope of this study.
Sampling from jugular vein might be a limitation of our study alike in previous ones (Linardi et al., 2009, 2012); however, we did not aim to correlate plasma concentration and antinociception, but to investigate and compare the local absorption and antinociceptive effects of the injection form of oral methadone vs. the nanoparticulated one.
Some studies investigated the analgesic and antinociceptive effects followed by oral administration of opioids in cats. Oral methadone increased force response thresholds; however, blood samples were collected from the jugular vein and antinociception was not related to plasma concentration (Ferreira et al., 2011). On the other hand, a clinical study showed that oral administration of buprenorphine was not so effective for postoperative analgesia when compared to i.v. or i.m. administration in cats undergoing ovariohysterectomy (Giordano et al., 2010).
Nanoparticles are strategic carriers for brain targeting. Several models demonstrated the ability of nanoparticles to cross the blood–brain barrier and deliver drugs specifically to the brain (Tosi et al., 2010).
Several opioids have been nanoparticulated and showed antinociceptive effect in mice, such as morphine (Betbeder et al., 2000) and buprenorphine (Wang et al., 2009) and even opioids that do not cross the BBB, like loperamide, reached the CNS and maintained the concentration for 24 h in mice (Alyautdin et al., 1997). Although oral administration of methadone did not have an antinociceptive effect in our study, it might be expected that nanoencapsulated methadone formulation would enhance the blood–brain barrier passage and therefore potentiate the analgesic effect of methadone. However, this formulation failed to produce or augment antinociception; therefore, it is not useful for clinical use at the dose and route tested in the present study.
Another possible reason why nanoparticulated methadone did not cause antinociception in this study would be the formulation itself. The method for producing nanoparticulated methadone has already been successfully used for other drug formulations (Müller et al., 2000) and is commercially available for diazepam and etomidate. The use of solid lipid nanoparticles is somewhat limited because of the physical instability. In this study, an aliquot of the formulation remained in the laboratory, to guarantee that at the same time of oral administration in horses, the size of the particles was measured, to ensure that the particles remained in nanosize scale.
For both formulations used in this study (ORAL and NANO), the final methadone concentration was 3 mg/mL for which 60 mL of drug needed to be administered to each animal in order to achieve the desired dose. Such a high volume was difficult to administer, and it may have been partially absorbed transmucosally besides swallowed. The alkaline pH of the equine buccal cavity and the alkaline pKa of methadone (8.9) facilitate buccal absorption of this opioid. Furthermore, several factors can reduce the bioavailability of methadone in the enteric environment, compared to the buccal cavity, like lower pH, enterocyte enzymes, membrane proteins, such as P-gP or hepatic first-pass metabolism (Linardi et al., 2009, 2012, 2013).
Conclusions
Neither methadone hydrochloride nor methadone lipid nanocarriers administered orally in horses produced electrical, thermal or mechanical antinociceptive effects. The nanoencapsulated formulation of methadone given orally to horses did not improve drug absorption.
Acknowledgments
Grant 2011/00892-3 and 2010/08967-0 from São Paulo Research Foundation (FAPESP/Brazil). Cristália® laboratory for the methadone donation and support. Ethical Institutional protocol number: 55/2011




