Selective growth inhibition by suppression of F1Fo ATPase in canine malignant melanoma cell lines
Abstract
Canine malignant melanoma (CMM) is a highly aggressive and fatal neoplasm. To identify potential therapeutic compounds and/or targets, 320 compounds were screened for their growth inhibitory activity in a CMM line (CMM-1) using a chemical library known to target specific signaling pathways/cell growth-related molecules. Among the compounds screened, the F1Fo ATPase inhibitor oligomycin showed potent growth inhibitory effects in CMM-1 cells, while exhibiting less toxic effects in a non-neoplastic control cell line (MDCK cells). The growth inhibitory effect of oligomycin A was then examined using six CMM lines and MDCK cells. Three CMM lines were highly sensitive to oligomycin A, with around 3000–20 000 times lower IC50 compared with oligomycin A-resistant CMM lines and MDCK cells. Oligomycin A-sensitive CMM-1 cells exhibited much greater oligomycin A-induced decreases in cellular ATP compared to oligomycin A-resistant cell lines. Although the oligomycins are clinically unsuitable because of its in vivo toxicity, these findings implicate the potential of F1Fo ATPase as a therapeutic target in a subset of CMM.
Due to the aggressive nature of malignant melanoma in dogs, multidisciplinary therapy involving surgery, radiotherapy, and chemotherapy is often required. However, even with the use of these therapeutic modalities, malignant melanoma has a fair to poor prognosis, particularly in dogs that have metastasis at the time of diagnosis (Tuohy et al., 2014; Kawabe et al., 2015). Therefore, a new therapeutic approach is required for the treatment of canine malignant melanoma (CMM).
Discovering a compound that blocks specific targeted molecules on which CMM cells are crucially dependent for their survival and/or growth would represent a potential breakthrough therapeutic for CMM; however, the discovery of such a therapeutic compound/target has not been accomplished to date.
In the present study, a chemical library consisting of 320 compounds, which are used clinically in human medicine or known to target specific signaling pathways/cell growth-related molecules, was screened for compounds that produce potent growth inhibition of CMM cells.
The 320 compounds used herein are listed in Data S1. These compounds (the SCADS inhibitor kit) were kindly provided by the Screening Committee of Anticancer Drugs (SCADS, Japan). The compounds were dissolved in 50% methanol at 100 μm and stored at −30 °C until used. For screening of compounds that have growth inhibitory effect in CMM cells, two cell lines CMM-1 (derived from oral melanoma, kindly provided by Dr. Nakagawa, University of Tokyo) and non-neoplastic canine kidney cell line (MDCK, as a control) were used. Both cell lines were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal calf serum (Nippon Bio-Supply, Tokyo, Japan) (cDMEM).
The CMM-1 and MDCK cells were suspended in cDMEM, placed in 96-well plates (5 × 102 cells/well), and incubated for 24 h. The medium was then replaced with cDMEM containing 0.1 μm of each chemical compound or vehicle control (methanol; final concentration of 0.5%) and cultured for 72 h. After culturing, cell viability was evaluated using a WST-1 cell proliferation assay kit (Takara, Tokyo, Japan). To avoid selecting compounds that have nonspecific cytotoxic activity, compounds that produced >80% inhibition of CMM-1 cell growth but <40% inhibition of MDCK cell growth were selected.
Among the 320 compounds screened, only oligomycin, a potent mitochondrial F1Fo ATPase inhibitor, inhibited CMM-1 cell growth by more than 80% while producing less than 40% inhibition of MDCK cell growth (Fig. 1a).
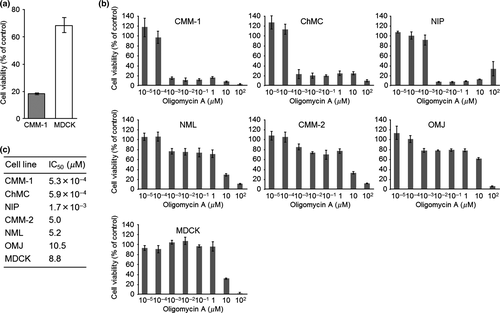
The screening experiments resulted in the selection of oligomycin, which was further evaluated for its ability to inhibit the growth of CMM cells. Because oligomycin in the SCADS inhibitor kit is a mixture of the A, B, and C isomers, we then examined the growth inhibitory effect of the most potent isomer (oligomycin A) using CMM-1, five other CMM cell lines (CMM-2 and ChMC, kindly provided by Dr. Nakagawa, University of Tokyo; NIP, NML, and OMJ, newly established in our laboratory), and MDCK. NML is derived from nail bed melanoma and the other CMM cell lines are derived from oral melanoma. All cell lines were maintained in cDMEM. Oligomycin A (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 100% dimethyl sulfoxide (DMSO) at 10 mm and stored at −30 °C until used.
CMM-1 CMM-2, ChMC, NIP, NML, OMJ, and MDCK were cultured in 96-well plates (5 × 102 cells/well) for 24 h in cDMEM and treated with different concentrations of oligomycin A (0–100 μm) for 72 h. Cell viability was then measured with a WST-1 cell proliferation assay kit (Takara) and the half-maximal inhibitory concentration (IC50) of oligomycin A for each cell line was calculated using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Among these cell lines, oligomycin A showed potent cell growth inhibition activity against three CMM lines (CMM-1, ChMC, and NIP) with IC50 values of 5.3 × 10−4–1.7 × 10−3 μm (Fig. 1b and c). In contrast, the three other CMM lines (NML, CMM-2, and OMJ) and MDCK were resistant to oligomycin A with IC50 values of 5.0–10.5 μm (Fig. 1b and c).
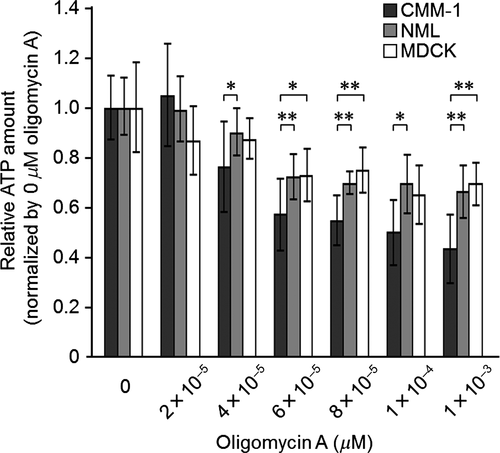
Because mitochondrial F1Fo ATPase is a major source of cellular ATP, the effect of oligomycin A on the cellular ATP levels in oligomycin A-sensitive and A-resistant CMM lines (CMM-1 and NML, respectively) and the oligomycin A-resistant non-neoplastic MDCK cell line was examined. For this experiment, CMM-1, NML, or MDCK cells were suspended in cDMEM and placed in 96-well plates at concentration of 5 × 102, 1 × 103, and 1 × 103 cells/well, respectively. The cells were then incubated for 24 h prior to being incubated for 48 h in the presence of various concentrations of oligomycin A (0–100 μm). The amount of cellular ATP was then measured using an ATP assay kit (Toyo B-Net, Tokyo, Japan). Statistical analysis was performed using an unpaired two-tailed Student's t-test. Significance was accepted at P < 0.05.
Cellular ATP levels were gradually decreased in an oligomycin A dose-dependent manner in all cell lines examined; however, CMM-1 cells were significantly more sensitive than NML (P < 0.05 or P < 0.01) and MDCK cells (P < 0.05 or P < 0.01) (Fig. 2).
By screening 320 compounds and a subsequent growth inhibition study, we identified three CMM cell lines (CMM-1, ChMC, and NIP) that are highly and selectively sensitive to oligomycin A, with 3000–20 000 times lower IC50 values than the oligomycin A-resistant CMM cell lines and MDCK cells. Consistent with these findings, cellular ATP was more severely depleted in CMM-1 cells compared with oligomycin A-resistant cell lines following treatment with oligomycin A.
F1Fo ATPase, the target of oligomycin A, is one of five multimeric enzyme complexes that form the mitochondrial respiratory chain and catalyze the production of ATP from ADP and orthophosphate by using the proton motive force (Jonckheere et al., 2012). This enzyme consists of two functional domains (Jonckheere et al., 2012); the F1 domain is the ATP synthesis region, is composed of five subunits (α, β, γ, δ, and ε), and is situated in the mitochondrial matrix; the Fo domain is responsible for proton flux and is composed of the c-ring, subunits a, b, d, and F6, and the oligomycin sensitivity-conferring protein (OSCP). A number of additional subunits (e, f, g, and A6L) are associated with Fo.
Our findings led to the hypothesis that there is a structural and/or quantitative difference in mitochondrial F1Fo ATPase that confers differential susceptibility toward oligomycin A among cell lines. We therefore examined the nucleotide sequences of cDNAs that encode OSCP and other functionally or structurally important subunits (α and β subunits, catalytic core; γ subunit, central stalk) in CMM-1, ChMC, NIP, NML, CMM-2, OMJ, and MDCK cells. However, nucleotide mutations that would alter amino acid sequences were not identified. Although we also compared the expression level of F1Fo ATPase among CMM-1, NML, and MDCK cells, no differences among these cell lines were found (Data S2).
F1Fo ATPase has been demonstrated to be located on the plasma membrane of certain tumor cells and may play an important role in maintaining normal intracellular pH in low extracellular pH environments (Chi & Pizzo, 2006). Moreover, inhibition of cell surface F1Fo ATPase prevented proliferation or caused death in certain tumor cells (Chi & Pizzo, 2006; Wang et al., 2008; Wen-Li et al., 2012). Therefore, we examined cell surface expression of F1Fo ATPase in CMM-1, NML, and MDCK cells. However, cell surface expression of F1Fo ATPase was not observed in these cell lines (Data S3).
Despite our efforts, we were unable to reveal the mechanism responsible for the selective susceptibility to oligomycin A in a subset of CMM cell lines. However, it is possible that other structural and/or functional differences exist that confer oligomycin A sensitivity among CMM cell lines, such as amino acid alteration in other F1Fo ATPase subunit(s) or differences in the posttranslational modification of the F1Fo ATPase subunit(s). Alternatively, differences in energy demand or in susceptibility to ATP depletion among the cell lines could underlie the differential sensitivity toward oligomycin A. Further studies are needed in order to elucidate the mechanism underlining the observed differences in susceptibility toward oligomycin A.
The oligomycins are a family of macrolide antibiotics that are clinically unsuitable in humans and animals because of its in vivo toxicity in rats (Kramar et al., 1984). Nonetheless, the remarkable selectivity of growth inhibition in certain CMM cell lines induced by oligomycin A implicates F1Fo ATPase as a potential therapeutic target for treating a subset of CMM.
Acknowledgments
The inhibitors were kindly provided by the Screening Committee of Anticancer Drugs supported by Grant-in-Aid for Scientific Research on Innovative Areas, Scientific Support Programs for Cancer Research, from The Ministry of Education, Culture, Sports, Science and Technology, Japan. This research was partially supported by a Grant-in-Aid for Scientific Research (No. 11050) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of this paper.




