Pharmacokinetic parameters for single- and multi-dose regimens for subcutaneous administration of a high-dose ceftiofur crystalline-free acid to neonatal foals
Abstract
The objective of this study was to determine the pharmacokinetics of single- and multi-dose ceftiofur crystalline-free acid (CCFA) administered subcutaneously at a dose of 13.2 mg/kg to 12 neonatal foals 1–3 days of age. Six foals received a single subcutaneous dose, while 6 additional foals received 4 doses of CCFA at 48-h intervals. Blood samples were collected at pre-determined times following drug administration, and plasma concentrations of ceftiofur free acid equivalents (CFAE) were measured using high-performance liquid chromatography. Following single-dose administration of CCFA, the mean ± standard deviation maximum observed plasma concentration was 3.1 ± 0.6 μg/mL and observed time to maximal plasma concentration was 14.0 ± 4.9 h. Following multi-dose administration of CCFA, the mean ±standard deviation times above CFAE concentrations of ≥0.5 μg/mL and ≥2.0 μg/mL were 192.95 ± 15.86 h and 78.80 ± 15.31 h, respectively. The mean ± standard deviation area under the concentration vs time curve (AUC0→∝) was 246.2 ± 30.7 h × μg/mL and 172.7 ± 27.14 h × μg/mL following single- and multi-dose CCFA administrations, respectively. Subcutaneous administration of CCFA at 13.2 mg/kg in neonatal foals was clinically well- tolerated and resulted in plasma concentrations sufficient for the treatment of most bacterial pathogens associated with neonatal foal septicemia. Multi-dose administration of four doses at dosing interval of 48 h between treatments maintains appropriate therapeutic concentrations in neonatal foals.
Bacterial infections continue to be a major cause of morbidity and death in neonatal foals (Koterba et al., 1984; Cohen, 1994; Paradis, 1994). The relative frequency of isolation of individual bacterial species from septicemic foals varies between different reports, but gram-negative microorganisms, especially Escherichia coli, are the predominant organisms isolated from neonatal foals with septicemia. Other micro-organisms, such as Actinobacillus spp., Klebsiella spp., Enterobacter spp., Proteus spp., Salmonella spp., Pasteurella spp., Pseudomonas spp., Enterococcus spp., Streptococcus spp., Staphylococcus spp., Clostridium spp., Acinetobacter spp. and Citrobacter spp., have also been isolated from the blood of septicemic foals (Koterba et al., 1984; Wilson & Madigan, 1989; Paradis, 1994; Marsh & Palmer, 2001; Hollis et al., 2008; Sanchez et al., 2008; Theelen et al., 2014). The definitive diagnosis of septicemia requires positive results of bacteriologic culture of blood, which requires a minimum of 48–72 h, yielding a positive result in only 12–80% of cases, and this diagnostic service may not always be available (Paradis, 1994). Therefore, neonatal foals are often subjected to extensive diagnostic evaluation and empirical systemic antimicrobial treatment, because the prognosis for sepsis largely depends on early identification and appropriate treatment.
Ceftiofur crystalline-free acid (CCFA) is an extended-release formulation of ceftiofur recently approved by the US Food and Drug Administration (FDA) for use in adult horses for the treatment of Streptococcus equi subsp. zooepidemicus pneumonia (Supplement NADA 141-209, 2009). A recent study determined that a single subcutaneous administration of CCFA at the FDA approved label dose of 6.6 mg/kg in neonatal foals did not achieve plasma concentrations sufficient to treat most gram-negative bacteria associated with neonatal infections for more than 24 h (Hall et al., 2010).
The objective of this study was to determine the pharmacokinetics of single and multi-dose CCFA administered subcutaneously at a dose of 13.2 mg/kg to neonatal foals.
Twelve neonatal foals (six fillies and six colts) with the age range from 1 to 3 days were determined to be healthy on the basis of physical examination and plasma IgG levels. Sepsis scores were assigned to each foal based upon a previously described sepsis scoring system (Weber et al., 2015). Foals resided at a single premise and were housed with their mares in individual stalls during the study period. Foals were permitted to nurse ad libitum throughout the study period. The study was approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
Foals were assigned to one of two groups based upon birth order. The first 6 foals received a single dose of CCFA (Excede, Zoetis, Florham Park, NJ, USA) at 13.2 mg/kg administered subcutaneously in the right axillary region just caudal to the triceps muscles. Heparinized blood samples were obtained through an indwelling catheter placed in the right jugular vein at times 0 (prior to administration), 0.5, 1, 2, 4, 8, 12, 24, 36, 48, 60, 72, 96, 120, 144, 168, 192, 240, and 288 h after administration of CCFA. All blood samples were placed immediately on ice and then centrifuged at 2800 g for 10 min after which plasma was separated and stored at −80 °C until analysis.
The second group of 6 foals received CCFA administered subcutaneously at a dose of 13.2 mg/kg every 48 h for a total of 4 doses alternating between the left axillary region and right axillary region just caudal to the triceps muscles. Heparinized blood samples were obtained via venipuncture of the jugular vein at times 0 (prior to administration), 12, 24, 36, 48, 60, 72, 84, 96, 108, 120, 132, 144, 144.5, 145, 146, 148, 152, 156, 168, 180, 192, 204, 216, 240, 246, 288, 312, 360, 408 h after administration of the first of four CCFA doses. Samples were handled and processed as previously described.
Foals from both groups were monitored twice daily by means of a physical examination during each of the study periods. Mentation, nursing activity, vital parameters, fecal consistency and reaction at the injection sites were closely monitored.
Plasma samples were analyzed as previously described using a high performance liquid chromatography assay method for quantifying ceftiofur free acid equivalents (CFAE; Jaglan et al., 1990). Briefly, ceftiofur and desfuroylceftiofur-like metabolites were extracted from plasma samples as desfuroylceftiofur following the addition of dithioerythritol solution. Desfuroylceftiofur was captured by use of a C-18 solid-phase extraction column, and desfuroylceftiofur converted to desfuroylceftiofur acetamide by derivatization with iodoacetamide as previously described (Benson et al., 2003). The limit of quantitation was 0.1 of CFAE/mL of plasma. Further, the intra-run variability and inter-run variability of the method were 5.5% and 9.4%, respectively. Concentrations of ceftiofur and metabolites were expressed as μg/mL of plasma. Data were analyzed using compartmental and non-compartmental approaches using a commercial software program (WinNonLin version 5.2; Pharsight Corporation, Mountain View, CA, USA).
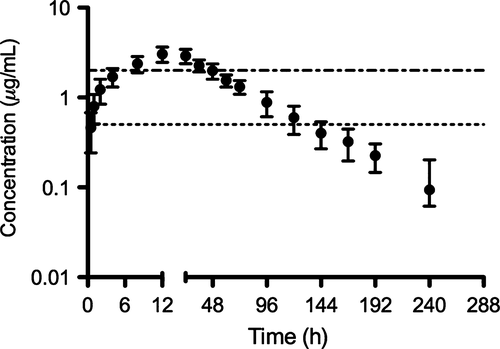
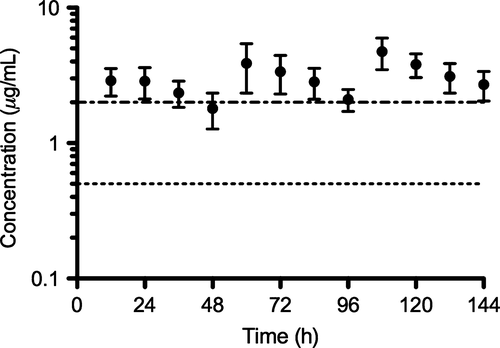
No systemic adverse clinical signs were observed in any of the study foals. Only mild non-painful swelling was observed in two foals following the subcutaneous administration of a single dose of CCFA at 13.2 mg/kg. Non-compartmental pharmacokinetic parameters calculated for individual foals receiving a single subcutaneous dose of CCFA at 13.2 mg/kg are reported in Table 1. The mean ± SD plasma concentration vs. time profile for a single subcutaneous administration for CCFA at 13.2 mg/kg is shown in Fig. 1. Compartmental pharmacokinetic parameters calculated for foals in the multiple dosage regimen study (13.2 mg/kg subcutaneously q 48 h) are reported in Table 2. For the multiple dose data, a one compartment model best-fit the data. Mean ± SD plasma concentration vs. time profile for CCFA administered subcutaneously at a dose of 13.2 mg/kg every 48 h for a total of 4 doses is shown in Fig. 2. The average ± SD time above a ceftiofur MIC ≥0.5 μg/mL and ≥2.0 μg/mL was 192.95 ± 15.86 h and 78.80 ± 15.31 h, respectively.
| PK parameter (units) | Animal no. | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Mean | SD | |
| λz (1/h) | 0.014 | 0.016 | 0.008 | 0.011 | 0.019 | 0.020 | 0.015 | 0.005 |
| t½λz (h) | 49.92 | 42.83 | 87.99 | 62.97 | 37.03 | 33.90 | 47.20 | 15.14 |
| AUC0−∞ (μg × h/mL) | 247.0 | 289.2 | 260.5 | 221.2 | 202.5 | 257.0 | 246.2 | 30.69 |
| AUC0−∞ % Extrapolated | 5.54 | 2.99 | 8.28 | 5.34 | 3.96 | 2.66 | 4.80 | 2.07 |
| Observed Cmax (μg/mL) | 3.22 | 2.98 | 2.81 | 2.25 | 3.15 | 4.02 | 3.07 | 0.58 |
| Observed Tmax (h) | 12 | 24 | 12 | 12 | 12 | 12 | 14 | 4.90 |
- λz, elimination rate constant; t½ λz, elimination half-life; AUC0−∞, area under the plasma concentration vs time curve extrapolated to infinity; AUC0−∞ % Extrapolated, % of AUC extrapolated; Observed Cmax, observed maximum plasma concentration; Observed Tmax, time to observed maximum plasma concentration.
| PK parameter (units) | Mean | SD |
|---|---|---|
| Non-compartmental | ||
| AUC144–192 (μg × h/mL) | 172.7 | 27.14 |
| Time ≥0.5 μg/mL | 192.95 | 15.86 |
| Time ≥2.0 μg/mL | 78.80 | 15.31 |
| Compartmental | ||
| K01 (1/h) | 0.236 | 0.045 |
| t½K01 (h) | 2.939 | 0.532 |
| K10 (1/h) | 0.013 | 0.001 |
| t½K10 (h) | 54.02 | 5.521 |
- AUC144–192, area under the plasma concentration vs time curve from 144 to 192 h following last injection; K01, absorption rate constant; t½K01, absorption half-life; K10, elimination constant; t½K10, elimination half-life.
The results from this study showed that the single- and multi-dose subcutaneous administrations of CCFA at 13.2 mg/kg subcutaneously in 12 neonatal foals was not associated with any significant clinical adverse effects such as injection site reactions or changes in fecal character. The results are in contrast with a previous study determining the pharmacokinetics of CCFA at 6.6 mg/kg in foals where four of six foals developed transient, self-limiting diarrhea 48–240 h post-antimicrobial administration (Hall et al., 2010). However, the diarrhea in the four foals was suspected to be related to foal heat, based on the absence of fever, and not CCFA administration.
Subcutaneous administration of antimicrobials is ideal as foals have a decreased muscle mass compared with adult horses. The site selected for subcutaneous administration of CCFA to foals in this study was chosen based upon the ease with which a single person could perform the injection and the same site was used in a previous study evaluating the single-dose administration of CCFA at 6.6 mg/kg (Hall et al., 2010). In the present study, there were only two foals which demonstrated very mild flocculent, non-painful swelling following a single injection of subcutaneous CCFA, which is in contrast to experimental subcutaneous injections in cattle and goats as well as intramuscular injections in adult horses where mild-to-moderate reactions have been reported (Supplement NADA 141-209, 2006; Supplement NADA 141-209, 2009; Doré et al., 2011).
In comparison with the intramuscular administration of CCFA to adult horses at a dose of 6.6 mg/kg, a single subcutaneous administration dose of 13.2 mg/kg in foals had a higher AUC0−∞ (adult: 157 ± 19.1 h × μg/mL; foal: 246.2 ± 30.7 h × μg/mL), longer observed Tmax (adult: 21.6 ± 5.8 h; foal: 47.2 ± 15.1 h), and a higher observed Cmax (adult: 0.78 ± 0.19 μg/mL; foal: 3.07 ± 0.58 μg/mL) (Supplement NADA 141-209, 2009). In comparison with the 2010 study by our group evaluating CCFA administered subcutaneously at 6.6 mg/kg to neonatal foals, administration at 13.2 mg/kg resulted in a higher AUC0→∝ (6.6 mg/kg subcutaneously: 139.53 ± 22.63 h × μg/mL; 13.2 mg/kg subcutaneously: 246.2 ± 30.69 h × μg/mL), a longer terminal elimination half-life (6.6 mg/kg subcutaneously: 39.7 ± 14.7 h; 13.2 mg/kg subcutaneously: 47.2 ± 15.14), higher Cmax(obs) (6.6 mg/kg subcutaneously: 2.52 ± 0.35 μg/mL; 13.2 mg/kg subcutaneously: 3.07 ± 0.58 μg/mL), and longer Tmax(obs) (6.6 mg/kg subcutaneously: 11.33 ± 1.63 h; 13.2 mg/kg subcutaneously: 14.0 ± 4.9 h)(Hall et al., 2010).
Gram-negative bacteria, particularly Enterobacteriaceae, are considered the most common isolates from neonatal foals with sepsis. However, in recent years the prevalence of gram-positive bacteria has increased (Theelen et al., 2014). This observation highlights the importance to choose an antimicrobial for the treatment or prevention of neonatal septicemia with activity against both gram-negative and gram-positive bacteria. This is even more important considering that bacteriological culture requires a minimum of 48–72 h, yielding a positive result in only 12–80% of cases, and may not always be an available service (Theelen et al., 2014). A ceftiofur MIC ≥2.0 μg/mL is a conservative value shown to include the greatest percentage of organisms (over 90%) isolated from neonatal foals, while a ceftiofur MIC ≥0.5 μg/mL has recently been shown to be sufficient to successfully target 79% of gram-positive and gram-negative organisms isolated from foals with bacterial septicemia (Meyer et al., 2009). In the present study, subcutaneous administration of CCFA at 13.2 mg/kg achieved plasma concentrations greater than 2 μg/mL for >90% of the first 48 h after administration of a single dose. Given that the time above MIC should be greater than 50% of the dosing interval for bactericidal time-dependent antimicrobials (Cruz et al., 2006), it seems that a dosing interval of 48–72 h would be appropriate for the treatment of susceptible pathogens in most septic foals, especially when blood culture results are negative. Dosing interval may be extended based on culture and antibiogram results; however, future studies are needed to determine the inter-dose intervals for bacteria with an MIC <0.5 μg/mL.
In conclusion, the subcutaneous administration of CCFA in neonatal foals at a dose of 13.2 mg/kg achieved plasma concentrations above the MIC for bacterial organisms commonly isolated in septicemic foals. A dosing interval of 48–72 h allowed for the maintenance of plasma concentrations above the targeted MIC plasma concentration up to 72 h after the last dose. The broad antimicrobial spectrum of ceftiofur makes this a good therapeutic choice for the treatment and prevention of neonatal septicemia because it increases the ease of on-farm administration, eliminates injection site irritation observed with intramuscular administration, and has a reputation for reduced nephrotoxicity. However, the time to reach maximal drug concentration after the first dose may be too long to solely rely on CCFA in a septic foal. In this situation, the administration of a single dose of ceftiofur sodium at 5 mg/kg either IV or SC concurrently with CCFA will shorten the time to reach maximal concentration to less than 30 min (Hall et al., 2010).
Acknowledgments
We would like to thank Zoetis for providing the ceftiofur crystalline-free acid (Excede) used in the study and financial support for study sample analyses. We would also like to thank the staff in the UC Davis branch of FARAD for running the analyses and the staff at the Center for Equine Health, UC Davis for their support during this study.




