Intestinal drug transport: ex vivo evaluation of the interactions between ABC transporters and anthelmintic molecules
Abstract
The family of ATP-binding cassette (ABC) transporters is composed of several transmembrane proteins that are involved in the efflux of a large number of drugs including ivermectin, a macrocyclic lactone (ML) endectocide, widely used in human and livestock antiparasitic therapy. The aim of the work reported here was to assess the interaction between three different anthelmintic drugs with substrates of the P-glycoprotein (P-gp) and the breast cancer resistance protein (BCRP). The ability of ivermectin (IVM), moxidectin (MOX) and closantel (CST) to modulate the intestinal transport of both rhodamine 123 (Rho 123), a P-gp substrate, and danofloxacin (DFX), a BCRP substrate, across rat ileum was studied by performing the Ussing chamber technique. Compared to the controls, Rho 123 efflux was significantly reduced by IVM (69%), CST (51%) and the positive control PSC833 (65%), whereas no significant differences were observed in the presence of MOX (30%). In addition, DFX efflux was reduced between 59% and 72% by all the assayed drug molecules, showing a higher potency than that observed in the presence of the specific BCRP inhibitor pantoprazole (PTZ) (52%). An ex vivo intestinal transport approach based on the diffusion chambers technique may offer a complementary tool to study potential drug interactions with efflux transporters such as P-gp and BCRP.
Introduction
The family of ATP-binding cassette (ABC) transporters includes several transmembrane proteins that extrude a broad range of hydrophobic compounds across the cellular plasma membrane by ATP-dependent process (Gottesman & Pastan, 1993). Located in a number of tissues related to the absorption, distribution and excretion, ABC drug transporters exert a physiological function by protecting the host against xenobiotic toxicity. Among them, P-glycoprotein (P-gp), breast cancer resistance protein (BCRP) and multidrug resistance-associated proteins (MRPs) are the best-characterized cellular transporter systems (Schinkel & Jonker, 2003). Due to its broad substrate specificity, these efflux proteins may substantially modify both the disposition and pharmacological effect of many therapeutically used drugs, playing a clinically relevant role in drug–drug interactions in humans (Ho & Kim, 2005).
Anthelmintic drugs are the most used practical tool in livestock production to control antiparasitic diseases. The interactions of different antiparasitics with some ABC transporters such as P-gp and BCRP were corroborated in vivo and in vitro. A number of in vitro approaches (Pouliot et al., 1997; Lespine et al., 2007) using recombinant cell lines and in situ (closed-loop models in rat intestine) (Laffont et al., 2002) have been performed to characterize the interaction between ivermectin (IVM) and P-gp. The contribution of P-gp to the in vivo IVM disposition kinetics was also demonstrated in different animal species including rat (Lifschitz et al., 2004), sheep (Ballent et al., 2007) and cattle (Lifschitz et al., 2010a,b).
The study of BCRP substrates and/or inhibitors has been steadily expanding since its discovery. The BCRP tissue distribution and function overlaps extensively with that of P-gp, suggesting a similar role in substrate transport in mammals (Schinkel & Jonker, 2003). It has been recently reported that IVM can block the BCRP-mediated albendazole sulphoxide efflux in Madin–Darbin canine kidney cells overexpressing mouse Bcrp (MDCKII-Bcrp) (Muenster et al., 2008). Besides, the interaction between moxidectin (MOX), another macrocyclic lactone and BCRP has been corroborated in pharmacokinetic studies using the knockout mice model (Perez et al., 2009). Therefore, P-gp and BCRP may play a relevant role in the modulation of disposition kinetics of different anthelmintic drugs.
The well-documented interactions of anthelmintics drugs with different ABC transporters derived from in vitro studies using overexpressing cell lines. The Ussing chamber has been proven to be a predictive ex vivo method to measure the active drug efflux in excised intestinal tissues. Thus, the objective of the current study was to characterize the effect of three anthelmintic drugs on the intestinal transport of the P-gp substrate Rh123 and the BCRP substrate DFX in an ex vivo rat model.
Materials and Methods
Diffusion studies
A series of ex vivo experiments were performed to evaluate the interaction of IVM, MOX and closantel (CST) with different ABC transporters. The potential interaction of these antiparasitic drugs on P-gp and BCRP intestinal activity was assayed in diffusion chambers using Rho 123 and DFX, respectively. Male Wistar rats (280–320 g), at least 4 (four) animals per set of experiments, were handled following to procedures and management protocols approved by the Ethics Committee according to the Animal Welfare Policy (act 087/02, Protocol N° 12/2013) of the Faculty of Veterinary Medicine, Universidad Nacional del Centro de la Provincia de Buenos Aires (UNCPBA), Tandil, Argentina. Under anaesthesia with ketamine hydrochloride (100 mg·kg−1, intraperitoneal) (Holliday-Scott, Buenos Aires, Argentina), the entire gastrointestinal tract was rapidly removed from the rats and the ileum–caecum valve identified. The first 30 cm of intestine from the ileocecal valve were defined as ileum. After this procedure, animals were killed by exsanguination. Then, the intestinal segment was cut open along the mesenteric border, and resulting flat sheets were mounted into the Ussing chambers providing an exposed area of 0.85 cm2. Both mucosal (M) and serosal (S) sides were filled with 11 mL of prewarmed and oxygenated Krebs buffer (pH 7.4), which was maintained at 37 °C. To ensure oxygenation and agitation, a mixture of 95% O2 and 5% CO2 was bubbled through each compartment. Measurement of transepithelial electrical resistance (Rt) values was conducted prior to the beginning and at the end of the experiments to access the integrity of the intestinal tissue. A maximum decrease of 30% of the initial Rt measurements was fixed as an acceptance criterion. After a 20-min equilibration period, the experiment was initiated by the addition of Rho 123 (5 μm), a P-gp substrate or DFX (50 μm), a BCRP substrate, to the M or S side of the chambers for measuring the absorption and secretion process, respectively. For inhibition studies, IVM, MOX and CST, at the equimolar concentration of 10 μm, were added both in the donor and acceptor sides 10 min prior to starting the transport study. To compare the effect obtained in presence of the different anthlemintic drugs, PSC833 and pantoprazole (PTZ) at 10 μm were used as specific P-gp and BCRP inhibitors, respectively. Drugs were dissolved in ethanol and diluted in the medium. In all cases, the final solvent concentration was <0.2%. Finally, aliquots from both compartments were collected at intervals of 15 min.
Rho 123 and DFX analyses
Samples (1 mL) were mixed with 2 mL of Krebs buffer solution to reach a final volume of 3 mL. Calibration curves were performed in a range between 0.12 and 125 pmol/mL for Rho 123 and between 0.39 and 200 nmol/mL for DFX (three replicates). The concentrations of Rho 123 and DFX were measured by a fluorescent spectrophotometer RF-5301PC (Shimadzu Corporation, Kyoto, Japan) set at an excitation and emission wavelengths of 485 and 520 nm (Rho 123) and 275 and 443 nm (DFX), respectively.
Intestinal efflux analysis
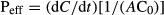
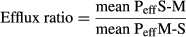
The Peff reflects the ability of one compound to permeate a cell layer. Permeability in the direction M-S represents the intestinal absorption. Permeability may also be determined from the S-M side and describes the intestinal secretion. A higher Peff S-M compared to the Peff M-S is indicative of carrier-mediated transport. Thus, substrates of efflux transporters expressed on the apical surface are transported more rapidly in the S-M direction, and their efflux ratios are >2 (Lennernäs, 1997).
Statistical analysis
All experiments were conducted at least in triplicate, and all the investigated parameters are expressed as mean ± standard deviation (SD). Statistical analysis was performed using one-way analysis of variance (anova) (Instat 3.0, Graph Pad software Inc., San Diego, CA, USA). A nonparametric Kruskal–Wallis test was used where significant differences among standard deviations were observed. Statistical differences were considered at P < 0.05.
Results
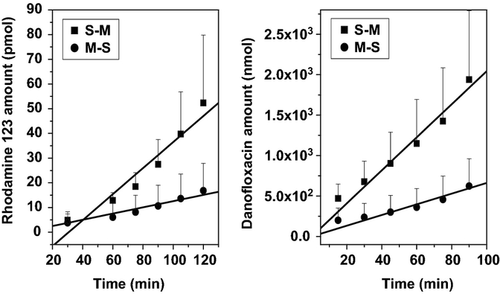
A complete validation of the analytical procedures for quantification of Rho 123 and DFX was performed before starting the analysis of experimental samples. The regression lines showed correlation coefficients ≥ to 0.99 and CV between 10 and 15% for both substrates. Diffusion studies using Rho 123 and DFX indicated an asymmetric transport of both markers across the rat ileum. In the absence of inhibitors, the secretion, expressed as apparent permeability (Peff S-M), was 3.21-fold (Rho 123) and 4.38-fold (DFX) higher than the absorption (Peff M-S) (Fig. 1).
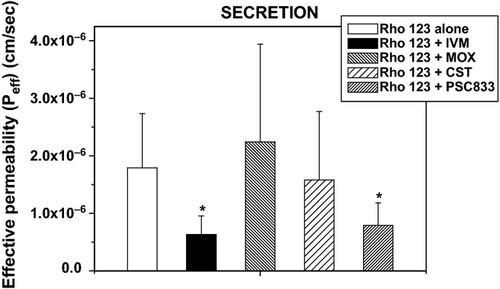
In the following set of experiments, the inhibitory effect of different antiparasitic drugs was evaluated. The absorption process of Rho 123 was not modified by PSC833, IVM or MOX. In the presence of these drugs, the Peff M-S was in a range between 0.62 × 10−6 ± 0.21 and 0.74 × 10−6 ± 0.29 cm/s, whereas the Peff M-S was 0.57 × 10−6 ± 0.29 cm/s without inhibitors. Interestingly, CST induced an increment in this parameter (0.78 × 10−6 ± 0.40 cm/s) compared with the incubation of Rho 123 alone.
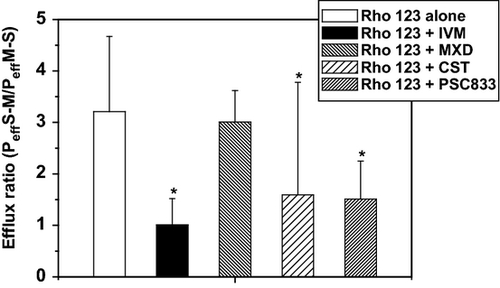
On the other hand, Rho 123 secretion was significantly reduced in the presence of PSC833 (0.79 × 10−6 ± 0.39 cm/s) and IVM (0.63 × 10−6 ± 0.32 cm/s) compared to the controls (1.79 × 10−6 ± 0.94 cm/s), whereas no differences were reached after its co-incubation with MOX (2.24 × 10−6 ± 1.76 cm/s) and CST (1.58 × 10−6 ± 1.19 cm/s). Figure 2 shows the comparative effect of IVM, MOX, CST and the PSC833 on Rho 123 secretion compared with its incubation alone. The calculated efflux ratio (Peff S-M/Peff M-S) decreased from 3.21 ± 1.46 (Rho 123 alone) to 1.01 ± 0.51 (Rho 123 + IVM), 1.59 ± 0.95 (Rho 123 + CST) and 1.51 ± 0.74 (Rho 123 + PSC833), whereas it was unaffected by co-incubation with MOX (3.01 ± 2.23) (Fig. 3).
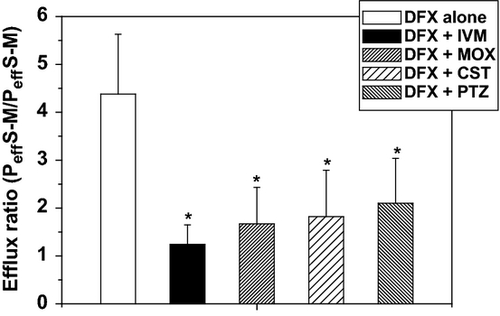
Additionally, results from the study of the intestinal activity BCRP mediated showed that DFX absorption was not modified by the drugs analysed. The Peff M-S of DFX was 1.63 × 10−6 ± 0.48 cm/s (control) and in a range between 1.75 × 10−6 ± 0.45 cm/s and 2.76 × 10−6 ± 1.38 cm/s after its co-incubation with the anthelmintic drugs. However, secretion process was significantly inhibited in the presence of all antiparasitics. A significant reduction in the Peff S-M was observed after co-incubation with IVM (1.24 × 10−6 ± 0.41 cm/s), MOX (1.67 × 10−6 ± 0.76 cm/s), CST (1.82 × 10−6 ± 0.97 cm/s) and PTZ (2.10 × 10−6 ± 0.94 cm/s) compared with the incubation of DFX alone (4.38 × 10−6 ± 1.25 cm/s). The efflux ratio values of DFX obtained after its incubation either alone or in the presence of the anthelmintics and the reference control PTZ is shown in Fig. 4.
Discussion
The interference between different drugs and ABC transporter proteins is a key mechanism underlying clinically important drug–drug interactions in humans (Marchetti et al., 2007). The inhibition of ABC transporters by several substances may also increase the drug bioavailability in animal species. The subcutaneous administration of loperamide increased both plasma and tissue disposition kinetic of IVM (subcutaneous treatment in rats) (Lifschitz et al., 2004) and MOX (intravenous and subcutaneous treatment) availability in cattle (Lifschitz et al., 2002). Likewise, the intraruminal administration of itraconazole enhanced IVM plasma and gastrointestinal disposition in sheep (Ballent et al., 2007). On the other hand, no differences in the oral bioavailability of different P-gp substrates were observed between P-gp null phenotype and ABCB1 wildtype dogs (Mealey et al., 2010). Altogether, these observations highlight the relevance to study potential drug interactions at intestinal level in the different animal species.
Besides the well-documented interactions between ML and P-gp, it was reported recently that ML interacts with other ABC transporters such as MRP in Caco-2 monolayers (Griffin et al., 2005) and A549 cell line (Lespine et al., 2006), and BCRP in both domestic species (Alvarez et al., 2006) and in vitro using Madin–Darbin canine kidney cells overexpressing mouse Bcrp (MDCKII-Bcrp) (Muenster et al., 2008). The current work is focus on the ex vivo drug interactions between anthelmintic drugs and two ABC transporter substrates.
The cellular cultures constitute an integrate system that offer a high expression levels of efflux proteins to perform in vitro transport studies. The methodology used here preserved the architecture of the intestinal mucosa and therefore may be useful to establish a potential ex vivo–in vivo correlation. The comparative capacity of IVM, MOX and CST to modulate Rho 123, a P-gp substrate, and DFX, a BCRP substrate, intestinal efflux was investigated.
As was expected, PSC833 significantly reduced the Rho 123 intestinal efflux. Similarly, IVM was able to reduce the Rho 123 efflux mediated by P-gp. The intestinal Rho 123 secretion was blocked 80% by IVM compared to the controls. Based on molecular modelling approaches, Garrigues et al. (2002) proposed the presence of two different, and partially overlapping, pharmacophores in which the ligands compete with each other. Authors also suggest that the molecular size of the ligands may affect their ability to compete for binding to P-gp. Because of the large molecular size of IVM, it is expected to IVM could be responsible for the overlapping in the binding of both P-gp recognition sites, leading to an increased P-gp affinity for this type of molecules (Lespine et al., 2007).
However, a different result was obtained with other ML such as MOX. The presence of MOX decreased 30% Rho 123 intestinal secretion. This observation was recently corroborated by different research groups. For instance, the concentrations of MOX necessary to reach the half-maximal inhibitory effect (IC50) on P-gp activity were ten times higher compared with IVM using a culture cell approaches (LLC-PK1-MDR1 cells) (Lespine et al., 2007). Similarly, results obtained by Griffin et al. (2005) showed that the potency of MOX to inhibit Rho 123 accumulation in Caco-2 cells was 100 times lower than that used for IVM. Furthermore, different affinities of ML by P-gp have been also corroborated in vivo using knockout mice (Kiki-Mvouaka et al., 2010). Whereas the P-gp deficiency led to a significant increase in the systemic availability of IVM (1.5-fold) and eprinomectin (EPM) (3.3-fold), MOX availability remained unchanged. Such molecular differences are relevant to explain the longer elimination half-life, large distribution volume and extensive elimination in milk during lactation observed for MOX in different animal species (Lanusse et al., 1997; Craven et al., 2002; Imperiale et al., 2004; Prichard et al., 2012).
Closantel is a salycilamide used against flukes and haematophagous nematodes in ruminants. The available information on the CST interaction with P-gp is scarce. Dupuy et al. (2010) reported a weak inhibitory effect of CST on Rho 123 accumulation using recombinant LLC-PK1-mdr1 cells, being its potency eightfold lower compared with IVM. Similarly, in our ex vivo approach, the potency of CST to inhibit the efflux of Rho 123 was 1.40-fold lower compared with IVM. Despite the fact that these in vitro and ex vivo studies indicated that CST may modulated the P-gp transport, a similar plasma disposition was observed after its co-administration with IVM compared with that obtained after the administration of each anthelmintic alone (Cromie et al., 2006).
Besides, the current work also focuses on the study of the BCRP-mediated intestinal transport. A marked inhibitory effect on DFX efflux was obtained after its co-incubation with all anthelmintic drugs. Transepithelial efflux mediated by ABC transporters has been proposed as an important elimination mechanism for fluoroquinolones (Griffiths et al., 1993; Lowes & Simmons, 2002). In the present study, the selected concentration of DFX was chosen based on the data previously reported. Žakelj et al. (2006) performed a transport assay with diffusion chambers using ciprofloxacin (CFX) at 50 μm. Ciprofloxacin has been described as a substrate for BCRP, but not for P-gp or MRP2 (Lowes & Simmons, 2002; Merino et al., 2006). Likewise, a common pathway in the intestinal transport of DFX and CFX has been proposed (Schrickx & Fink-Gremmels, 2007). In our experimental conditions, IVM, MOX and CST showed the same potency to inhibit DFX efflux BCRP mediated. As shown in Fig. 4, the calculated efflux ratio values were 3.53-fold (IVM), 2.62-fold (MOX), 2.40-fold (CST) and 2.08-fold (PTZ) lower compared to the controls. Table 1 summarizes the comparative inhibitory effect of the antiparasitic drugs and the reference inhibitors under study on both P-gp and BCRP intestinal activity.
| Rho 123 (% from control) | DFX (% from control) | ||
|---|---|---|---|
| IVM | 31 | IVM | 28 |
| MOX | 70 | MOX | 38 |
| CST | 49 | CST | 41 |
| PSC833 | 35 | PTZ | 48 |
- Rho 123, rhodamine 123; DFX, danofloxacin; IVM, ivermectin; MOX, moxidectin; CST, closantel; PTZ, pantoprazole.
The interaction of ML with BCRP has been investigated, though to a lesser extent than P-gp. Different in vitro approaches showed that IVM, used as BCRP inhibitor, was able to inhibit BCRP-mediated albendazole sulphoxide (Muenster et al., 2008) and DFX transport (Real et al., 2011) in overexpressing cells. These in vitro results correlated with recent pharmacokinetic studies that demonstrated the influence of IVM on the disposition kinetic of DFX in sheep (Real et al., 2011; Ballent et al., 2012). Both the plasma concentration profiles (Ballent et al., 2012) and the secretion of DFX into the milk (Real et al., 2011) were significantly modified by the co-administration with IVM. In addition, the interaction of MOX with BCRP has been studied using different experimental approaches and contradictory information was reported. The specific BCRP inhibitor fumitremorgin C did not modified MOX accumulation in cultures of rat hepatocytes (Dupuy et al., 2006). Similarly, Perez et al. (2009) showed a negligible transport of MOX mediated by BCRP in MDCKII transducer cells, resulting in efflux ratio values closed to 1.
Due to resistance appearance, several pharmaceutical formulations combining either two or three chemical entities have been developed and are available in the veterinary pharmaceutical market in different countries such as Australia, New Zealand and Uruguay. Some of these combinations addressed to be used in cattle and sheep include albendazole, ivermectin and levamisole; ivermectin and closantel; abamectin and ivermectin; albendazole, levamisole, closantel and abamectin. The potential pharmacokinetic interactions between drug components of these combinations highlight the need of deeper pharmacological-based research to identify the advantage/disadvantage of use of combined drug preparations for anthelmintic control in livestock. Thus, the understanding of drug interactions with these efflux transporters becomes crucial.
In conclusion, the work reported here describes the interaction of three different anthelmintic drugs on Rho 123 and DFX intestinal efflux using a predictive ex vivo method. Due to the limitation observed as a consequence of the high inter- and intravariability present in this type of methodologies compared to the in vivo situation, the correlation of the current results with intestinal transporter expression studies (Western blot and/or immunohistochemistry) should be assessed in the future. Further studies are also needed to corroborate both substrate specificity and absence/presence of cross-reactivity between the different ABC transporters that may be involved in the efflux of the current markers.
Acknowledgements
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 112-200801-01123) and Agencia Nacional de Promoción Científica y Técnica (ANPCyT) (PICT 1881, PICT 1432).




