Accuracy of Crit-Line® monitors in predicting hematocrit and change in blood volume of dogs during hemodialysis
Abstract
Background
Ultrafiltration (UF) is an extracorporeal technique for treating fluid overload and is monitored with noninvasive blood volume monitors.
Hypothesis/Objectives
The primary objective was to determine the accuracy of Crit-Line® III and IV noninvasive blood volume monitors to estimate canine packed cell volume (PCV) and changes in blood volume during UF. A secondary aim was to determine accuracy of targeted ultrafiltration rates (UFR) compared to calculated delivered UFR.
Methods
An ex vivo study with a single Phoenix® X36 platform and canine packed red blood cells (pRBC) was performed. Through dilution and UF, clinically applicable PCV values were obtained and compared to Crit-Line® hematocrit (Hct). Blood volume was constantly measured and compared to targeted UFR. Systematic and proportional bias were calculated using a Bayes method.
Results
Crit-Line® III and IV reported Hct was significantly lower than PCV (n = 140, median 26%, range, 8%-50%) when PCV was >25% and >30%, respectively. Crit-Line® III and IV calculated change in blood volume (ΔBV%) was significantly different from measured blood volume changes at ΔBV% ±20% and >−20%, respectively. Comparing targeted and delivered UFR (mL/h), less than targeted UFR was removed at UFR100 through UFR400 and UFR0 adding volume.
Conclusions and Clinical Importance
Crit-Line® III and IV monitors provide accurate estimates of canine PCV and UF volume change within specific ranges and are useful in monitoring canine UF and hemodialysis. Veterinary hemodialysis services should evaluate individual machines for UFR inaccuracies, which can meaningfully affect small animals.
Abbreviations
-
- BLUP
-
- Bayes-derived best linear unbiased predictor
-
- Hct
-
- hematocrit
-
- kg/h
-
- kilogram per hour
-
- m2
-
- meters squared
-
- mEq/L
-
- milliequivalents per liter
-
- mEq/mL
-
- milliequivalents per milliliter
-
- mL
-
- milliliter
-
- mL/h
-
- milliliters per hour
-
- mL/h/mmHg
-
- milliliters per hour per millimeters of mercury
-
- mL/kg/h
-
- milliliter per kilogram per hour
-
- mL/min
-
- milliliters per minute
-
- mmHg
-
- millimeters of mercury
-
- mS/cm
-
- milliSiemens per centimeter
-
- P-B
-
- Passing-Bablok
-
- pRBC
-
- packed red blood cells
-
- RBC
-
- red blood cells
-
- rpm
-
- rotations per minute
-
- TMP
-
- transmembrane pressure
-
- UF
-
- ultrafiltration
-
- UFR
-
- ultrafiltration rate
-
- ΔBV
-
- change in blood volume
1 INTRODUCTION
Fluid overload is a life-threatening sequela of acute kidney injury and ureteral obstruction, and generally defined as fluid intake exceeding fluid output.1 As a result of the kidney's inability to excrete excess water, hypervolemia and circulatory overload develop, resulting in peripheral edema, hypertension, pulmonary edema, pleural effusion, ascites, and congestive heart failure.2 Critical illness in both humans and animals is significantly associated with risk for fluid overload, not only in renal disease but secondary to acute lung injury, sepsis, and major surgical procedures.1 Fluid overload increases morbidity and is negatively associated with survival in humans and animals.1, 3
Ultrafiltration (UF) is an extracorporeal technique applied during hemodialysis which removes plasma water through a semipermeable membrane along a pressure gradient. This technique allows for removal of excess fluid from an individual and is controlled through the hemodialysis machine. Slow continuous ultrafiltration is a technique which can be applied separately from hemodialysis, and has applications in treatment of fluid overload where concurrent blood purification is not needed.4 Overzealous UF can lead to hypovolemia and hypotension.5 This occurs when the rate of fluid removal from the intravascular compartment excessively exceeds the rate of refilling from the interstitial compartment. During UF, red blood cells and blood proteins are not removed and thus their relative concentration provides a noninvasive means to assess relative changes in blood volume.3 Serial monitoring of hematocrit (Hct) or hemoglobin and calculation of change in blood volume can predict adverse events, such as intradialytic hypotension, in humans and animals undergoing hemodialysis.6, 7 The Crit-Line® is a noninvasive, in-line monitoring device approved for people undergoing hemodialysis. It continuously measures Hct using a photo-optic technique. Because Hct and blood volume are inversely related, the Crit-Line® gives a hematocrit-based estimate of change in blood volume during an ultrafiltration session. In pediatric human patients, a comparison between the Crit-Line® device and laboratory measured hemoglobin and Hct found good correlation between the 2 measurement techniques.8 Two models of Crit-Line® are available: Crit-Line® III and Crit-Line® IV. Both models use the same equation to calculate change in blood volume (ΔBV% = {[Hctinitial/Hctcurrent] − 1} × 100) but differ only in the graphical display of data on the monitor. To date, there are no studies evaluating the accuracy of such noninvasive in-line blood volume monitors in estimating Hct and subsequently the relative change in blood volume in dogs.
During UF in low weight animals, such as small dogs or cats, total fluid removed and therefore ultrafiltration rate (UFR) are considerably smaller compared to a human patient. Generally, UFR of 5 to 10 mL/kg/h can be safely tolerated by dogs and cats.2 In both humans and small animals, UFR less than 10 mL/kg/h have lower risk of intradialytic morbid events.7, 9 Currently no studies compare targeted versus delivered UFR with commonly used hemodialysis platforms in small animals, who might be more sensitive to inaccuracies in delivered UFR. Because pet owner finances or ethics infrequently permit the more chronic hemodialysis common in human patients, it is especially critical that UF prescriptions are planned to avoid frequent intradialytic morbid events which might contribute to progression or chronic loss of renal function.7, 10
The primary objective of this study was to determine the accuracy of 2 Crit-Line® monitors to correctly estimate canine packed cell volume (PCV) over a range of clinically applicable PCV values and subsequently the accuracy in correctly reflecting changes in relative blood volume during UF in dogs. A secondary aim was to determine the accuracy of the targeted UFR controlled through the hemodialysis platform compared to the actual delivered rate of fluid removal under different linear UF settings and across various hemodialyzers. Using an ex vivo model with canine packed red blood cells (pRBC), we hypothesized that the Crit-Line® monitors will accurately predict canine PCV and changes in relative blood volume. Additionally, we hypothesized that a hemodialysis platform would deliver the targeted UFR ±2% as per the manufacturer's specifications.
2 MATERIALS AND METHODS
An ex vivo study was performed using donated canine pRBC from the hospital's own blood bank. Donated pRBC deemed unfit for animal use, but still less than 45 days from the draw date, were utilized in the study. A hemodialysis session was defined as 1 continuous 6-hour period of data collection. All sessions and data recording were performed by the first author. A starting volume of 100 to 420 mL of pRBC was placed in a 1000 mL graduated pitcher. The pitcher and contained blood product were considered the simulated dog. The pRBC were diluted with 0.9% saline with the aim of achieving a starting volume between 400 and 1000 mL which avoided aspiration of air by the hemodialysis catheter and did not exceed the volume measuring capacity of the pitcher. The diluted pRBC were continuously stirred at 900 rpm using a magnetic stirrer (King-Kong I Mini Magnetic Stirrer, Four E's Scientific, China) to keep the mixture in suspension. An 11.5Fr, 20 cm hemodialysis catheter (Hemo-Cath® ST, MedComp®, Harleysville, PA) was suspended in the pitcher and connected to a commercially available hemodialysis machine that is undergoing yearly preventative maintenance as required by the manufacturer (Gambro Phoenix® X36, Baxter®, Deerfield, IL). Two different hemodialyzers (Table S1) were used at 5 clinically applicable UFR (0, 100, 200, 300, 400 mL/h). Each combination of hemodialyzer and UFR was run in duplicate, resulting in a total of 20, 6-hour hemodialysis sessions. Only a single UFR was used during any given 6-hour session. UFR set by the hemodialysis machine was in kg/h units but was converted to mL/h for purposes of this study. All sessions were performed using the same hemodialysis prescription (Table S2) and with both the Crit-Line® III and IV blood chambers (Fresenius Medical Care North America®, Waltham, MA) inserted in series before the hemodialyzer, in the same order every session. The same 2 Crit-Line® monitors, Crit-Line® III and Crit-Line® IV, were used throughout the study. These models use the same equation to calculate change in blood volume (ΔBV% = {[Hctinitial/Hctcurrent] − 1} × 100) but differ only in the graphical display of data on the monitor (Figure S1).
Following subjective visual equilibration of the prime volume, approximately 1 to 2 minutes, both Crit-Line® monitors were turned on and began their graphical display. Time 0 was the time both Crit-line® monitors began to provide a graphical display and all first measurements were recorded. The following values were collected at time 0 and every 15 minutes thereafter for a total of 6 hours: volume of blood (mL) in the pitcher, change in blood volume (ΔBV%) recorded by Crit-Line® monitors III and IV, Hct (%) recorded by Crit-Line® monitors III and IV, and transmembrane pressures recorded by the hemodialysis platform. At time 0 and every 60 minutes thereafter for 6 hours, a 0.5 mL blood sample was collected from the pre blood pump port (equal to the pitcher blood draw) for PCV (%) measurement. Total sampled volume (3.5 mL) represented <1% of total volume at any given moment, and thus was considered inconsequential with respect to final volume calculations. PCV was measured in 4 microhematocrit tubes and the average of the 4 measurements was recorded. Any hemolysis in serum was noted and recorded subjectively as none, mild, moderate, or severe. A single operator (first author) performed all sessions and measurements in order to minimize operator dependent discrepancies in recording.
Aliquots of 0.9% saline were added hourly to the graduated pitcher when UFR 100 to 400 mL/h were utilized. Size of aliquot was selected in effort to maintain a pitcher volume between 400 and 1000 mL, but still allow for progressive volume change such that varying PCV values would be created. This allowed judicious use of pRBC for a full 6-hour period, such as would simulate a possible hemodialysis treatment length in a dog. Additionally, it allowed for testing a wide range of PCV values and ensured that air was not aspirated into the hemodialysis blood path. All added saline aliquots were factored into calculations of delivered UFR.
Following data collection, delivered UFR was calculated with the following equation: Delivered UFR = Volumecurrent − (Volume1 h prior + Volumesaline aliquot added). In order to compare relative blood volume change (ΔBV%) as calculated by the Crit-Line® monitor with the actual blood volumes measured in mL, volume measurements were converted to Volume Change (%) = {[(Volumecurrent/Volumeinitial) − 1] × 100}.
The Crit-Line® manufacturer specifications state that the hematocrit instrument range and accuracy when used in humans is 10 Hct to 60 Hct: ±1 SD. The Gambro Phoenix® X36 manufacturer specifications state an UFR range of 0 to 4.0 kg/h and accuracy of ±2%.
2.1 Statistical analysis
Scatter plots and Passing-Bablok (P-B) regression were used to assess corresponding Crit-Line® Hct and PCV data distribution and systematic/constant vs. proportional bias. Bland-Altman assessment of agreement was considered inappropriate due to repeated measures and violations of the assumptions of the Bland-Altman methodology.11, 12 Instead, agreement was assessed using an empirical Bayes method to compute the Best Linear Unbiased Prediction (BLUP) of the latent unit.13, 14 The BLUP uses for each subject repeated reference measurements of that subject to estimate the true value and additionally borrows information from the reference measurements of the other subjects in the sample. Constant and proportional bias were calculated by ordinary least squares after substituting BLUP for the true value xi. Results are graphed in a scatter/bias plot with 2 regression lines (for the reference and for the Crit-Line® Hct), which can be assessed visually for constant vs. proportional bias. The calculated results (constant bias + proportional bias) are also graphed as a total bias plot, with 95% confidence band, as a function of the BLUP of the PCV. Similar methodology was used to assess the accuracy in change volume. The association of the degree of hemolysis on the relationship between Crit-Line® and PCV, and for dialyzer on the relationship between delivered and targeted UFR, was assessed using linear mixed models with repeated measures within units. Statistical analysis was performed using Stata SE v18.0 (StataCorp, College Station, TX).
3 RESULTS
3.1 Accuracy of Crit-Line® Hct
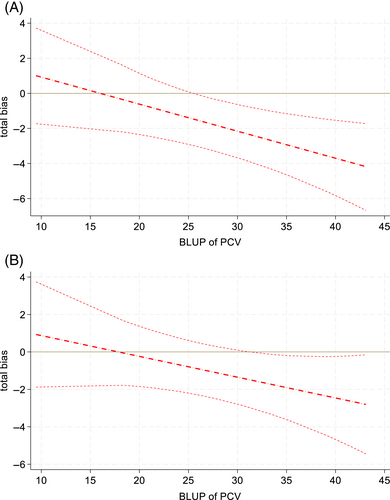
A total of 140 PCV samples were collected and compared to their respective Crit-Line® III and IV Hct readings. Median PCV was 26% with a range of 8% to 50%. The median starting PCV was 20.5% (range, 10%-46.5%). Two PCV samples of less than 8% occurred (7% and 6.25%, respectively); however, the Crit-Line® III was unable to perform a Hct reading at these values, thus they were excluded from overall descriptive analysis. The Crit-Line® IV was able to provide Hct readings at these PCV values (5.5% and 4.5%, respectively). P-B regression determined Hct measurements in both machines, compared to PCV, had systemic bias (intercepts) >0 [Crit-Line® III: 2.40 (95% CI: 1.74-3.10); Crit-Line® IV: 2.44 (95% CI: 1.61-3.34)]. In P-B regression, measurements in both machines also demonstrated proportional bias (slopes) ≠ 1 [Crit-Line® III: 0.850 (95% CI: 0.825-0.874); Crit-Line® IV: 0.875 (95% CI: 0.846-0.903)] although there were no significant deviations from linearity (P > .15). Using the empirical Bayes method, similar point estimates were calculated, but with wider confidence intervals. Systemic bias (intercepts) was >0 [Crit-Line® III: 2.47 (95% CI: 0.44-4.49); Crit-Line® IV: 1.98 (95% CI: −0.22 to 4.18)]; and proportional bias (slopes) <1 [Crit-Line® III: 0.846 (95% CI: 0.776-0.916); Crit-Line® IV: 0.889 (95% CI: 0.811-0.967)]. At the lowest PCVs, regression lines for Hct values were greater than the reference but, with increasing PCV value, were increasingly less than the reference (Figure 1). The absolute amount of bias ranged from approximately +1 (at lowest measured PCVs) to −3 (at highest measured PCVs). As there was less proportional bias in Crit-Line® IV than III, total bias less frequently differed from 0 in Crit-Line® IV than III (Figure 2). Assessing 95% confidence bands for total bias, measurements significantly differed from the reference PCV in Crit-Line® III when the PCV was >25 and in Crit-Line® IV when the PCV was >30.
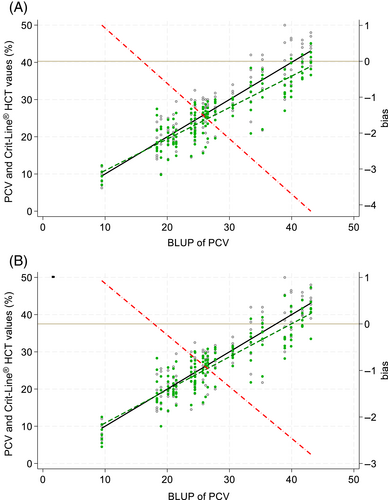
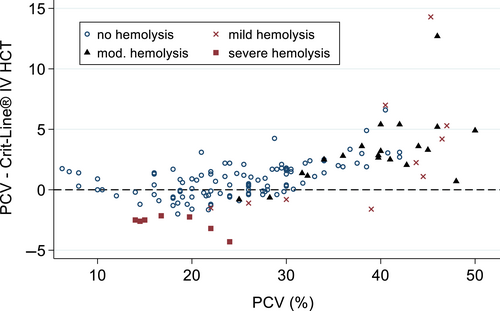
Hemolysis, assessed ordinally as none, mild, moderate, or severe, was not significantly associated with Hct measurements (P = .11; Figure 3).
3.2 Change in blood volume
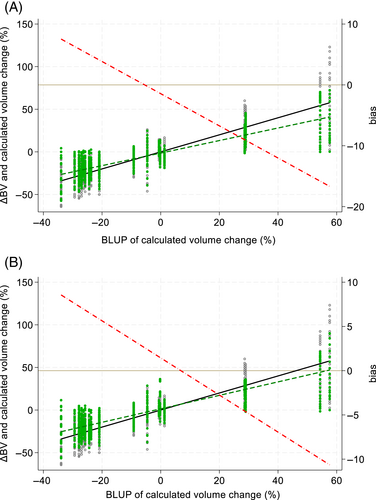
Agreement was assessed between the change in blood volume (ΔBV%) as depicted by the Crit-Line® monitor relative to serial volume measurements and calculated change in blood volume (%; Figure 4). Systemic/constant bias for Crit-Line® III was −1.44% (95% CI: −2.79 to −0.10) but was not significantly different from 0 for Crit-Line® IV [1.44% (95% CI: −0.09 to 2.97)] Proportional bias (slopes) deviated more from 1 in Crit-Line® III [0.736 (95% CI: 0.674-0.797)] than in Crit-Line® IV [0.790 (95% CI: 0.719-0.862)]. Total bias was significantly different from 0 in Crit-Line® III at larger ΔBV%, that is, approximately ±20%, but in Crit-Line® IV only in larger negative ΔBV%, that is, approximately greater than −20% (Figure 5).

3.3 Accuracy of ultrafiltration
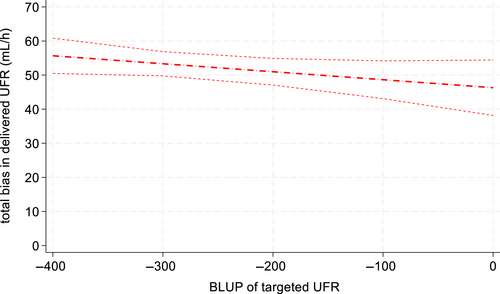
Delivered UF occurred at a constant rate, but with error, across targeted UFR (Figure 6, Table 1). Across all measurements, delivered UFR was significantly less than the targeted UFR (P < .05) with a constant bias of 46.3 mL/h (95% CI: 42.3-50.3) and proportional bias of 0.977 (95% CI: 0.963-0.991). However, at UFR 0 mL/h, volume was consistently added to the simulated dog, with a median volume added of 45 mL/h (range, +10 to +120 mL/h). The total mL/h difference between delivered UFR and targeted UFR progressively increased from targeted UFR100 through UFR400, with median volume removed of −340 mL/h (−310 to −390 mL/h) for UFR400 (Table 1). However, the average proportion of fluid removed progressively increased from UFR100 through UFR400, with delivered UFR achieving 60% (UFR100), 75% (UFR200), 83% (UFR300), and 85% (UFR400) of targeted UFR. When targeted and delivered UFR were also compared across hemodialyzer type, both the Polyflux and Revaclear dialyzers performed similarly (P = .29).
| Targeted UFR (mL/h) | TMP (mmHg) [median (range)] | Delivered UFR (mL/h) [median (range)] |
|---|---|---|
| 0 | 5 (−35 to 20) | 45 (10 to 120) |
| 100 | 0 (−35 to 20) | −60 (−90 to 10) |
| 200 | 5 (−20 to 15) | −150 (−260 to −120) |
| 300 | 10 (−20 to 25) | −250 (−340 to −200) |
| 400 | 10 (−15 to 15) | −340 (−390 to −310) |
- Note: Targeted UFR was controlled by the hemodialysis platform.
4 DISCUSSION
This study was designed to evaluate the accuracy of 2 different Crit-Line® monitors to correctly estimate canine packed cell volume (PCV) over a range of clinically applicable PCV values. It showed that Crit-Line® III and IV Hct accurately reflect canine PCV less than 25% and 30%, respectively. Additionally, we found Crit-Line® III ΔBV% accurately reflects actual volume gains or losses of less than 20%, while the Crit-Line® IV accurately reflects actual volume losses of less than 20% and actual volume gains up to 60%. These findings make the Crit-Line® blood volume monitor a useful tool to measure relative blood volume in dogs undergoing hemodialysis and ultrafiltration. The Crit-Line® IV consistently performed better than the Crit-Line® III, with accuracy at a wider range of both PCV and volume change. At the time of writing, Crit-Line® III production was discontinued by the manufacturer; however, the authors are aware that the Crit-Line® III is still in use in some veterinary hospitals performing hemodialysis, and therefore elected to include the data.
We elected to use PCV as the reference measurement in this study as it is a direct measurement theoretically subject to less error, whereas Hct is a calculated value based on RBC number and mean corpuscular volume and is considered subject to more error. In a study comparing canine PCV with Hct measurements, these values were found to have excellent correlation with no statistically significant difference between the 2.15 For our purposes, measurement of PCV was more cost effective than measurement of Hct on a complete blood count machine.
Hemolysis did not appear to influence Crit-Line® Hct readings when subdivided based on subjective degree of hemolysis, therefore samples with hemolysis were still included in this study. The Crit-Line® user manual reports that hemolysis can affect Hct readings by reporting higher Hct values. A limitation of this study is that it was not designed to determine the degree to which various levels of hemolysis affect the accuracy of the Crit-Line® device in dogs. While the use of stored and expired pRBC products likely contributed to cell fragility, the overall number of moderate or severely hemolyzed samples was low. Although in the current study hemolysis did not appear to affect Crit-Line® Hct, caution is still prudent before using Crit-Line® Hct readings to directly infer PCV in dogs with significant hemolysis.
Discarding or use of the hemodialysis circuit prime volume can affect the accuracy of relative blood volume monitors.16 In this study, the prime volume was not discarded; however, the Crit-Line® monitors were not started until after the prime volume was equilibrated with the blood in the graduated pitcher. Therefore, the prime volume had minimal effect on the initial hematocrit reading and therefore the relative blood volume calculations. Likewise, with addition of periodic saline aliquots, an equilibration period was allowed before obtaining new Crit-Line® readings.
Sodium content of the blood and dialysate can affect the Crit-Line® device. The Crit-Line® device was verified in humans using a serum sodium level of 137 mEq/L. The device user manual reports a 1% decrease in measured Hct for every 12 mEq/L increase in sodium from 137 mEq/L. In the current study, we used pRBC stored with sodium citrate, which has a sodium concentration of 165 mEq/L, and 0.9% NaCl, which has a sodium concentration of 154 mEq/L. During data collection, the hemodialysis prescription setting for sodium was 125 mEq/L and this lowest possible setting was selected to conserve resources. Because of the speed at which equilibration of sodium occurs when dialysis begins, we did not expect the higher starting sodium content of the solution used to meaningfully affect the Crit-Line® readings throughout the 6-hour sessions performed. However, the lower-than-normal sodium concentration might contribute to some bias in Crit-Line® Hct readings.
Large differences between blood and dialysate sodium concentrations could also affect delivered UF volumes, causing fluid shifts via osmotic drag as equilibration occurs.7 In this study, as saline was added in intervals to the simulated dog, an equilibration period was allowed, to minimize effect on measurements. Given the relatively high speed of dialysate and blood flow compared to the size of the simulated dog, we assume that equilibrium was reached rapidly. If the sodium differential between blood and dialysate did cause meaningful fluid shifts, we would have expected to see increased delivered UFR in the period immediately after adding the saline to the simulated dog; however, our data showed UFR occurring at a constant rate without these fluctuations. Additionally, the delivered UFR was consistently lower than targeted UFR and osmotic fluid drag between a high blood sodium and lower dialysate sodium should have contributed to an increased delivered UFR.
A secondary aim of this study was to determine the accuracy of UFR commonly utilized in dogs. The Gambro Phoenix® X36 platform allows an UFR of 100 mL/h as its lowest setting. This can be too aggressive for small animals, sometimes making it necessary to set the ultrafiltration to UFR0. An alternative practice is to apply a higher UFR while simultaneously administering fluids intravenously. Using a single Gambro Phoenix® X36 platform, it appears that proportionally, targeted and delivered UFR differ from each other by anywhere from 15% to 40%, with proportionally less fluid removed at lower UFR rates. For example, instead of removing 0.6 kg (600 mL) from a 10 kg dog during a 6 h session at UFR100, we might only remove 0.36 kg (360 mL) of fluid. Additionally in the current study, at minimum UF or UFR0, fluid was consistently added to the simulated dog. For example, in a 4 kg dog over a 6 h session at UFR0, we would add 0.24 kg in fluid weight, rather than an anticipated net zero fluid balance. These examples are specific to the machine tested and might not translate across other machines or models. This finding has important clinical implications for small animals receiving UF or even minimal UF as part of their extracorporeal prescription. In smaller animals the margin for error with UF becomes much narrower. Too aggressive UFR risks hemodynamic instability, intradialytic hypotension, end organ ischemic damage, and progression of renal injury with a likely outcome of euthanasia if ethically or financially an owner cannot commit to chronic hemodialysis.7, 10, 17 UFR which are too low risks lack of resolution of fluid overload, increased treatment time, and increase in treatment costs to the owner. This emphasizes that UF needs to be consistently monitored in an animal undergoing hemodialysis.
A single Phoenix® X36 machine was used for the entire study to limit the potential for variability between machines. A standard maintenance protocol and scheduled maintenance assessment schedule was followed during the entirety of the study period, making it unlikely that the discrepancy in targeted and delivered UFR was due to machine error. While the manufacturer literature states a UFR accuracy to ±2%, it is possible that individual machines within a model vary significantly between each other despite routine maintenance, in which case these data apply only to the machine tested. Because a single model intermittent hemodialysis machine was used, data in this study does not necessarily translate to other hemodialysis machine models. Veterinary facilities offering hemodialysis should consider testing accuracy of their individual machines before use. Additionally, a single operator recorded all study measurements, eliminating interobserver bias.
Due to difficulty obtaining blood products, we did not test all hemodialyzers commonly used in small animals, nor all hemodialysis platforms. Therefore, these results might not be applicable across other dialyzers and hemodialysis platforms.
Another limitation is the ex vivo nature of this study. An ex vivo model was chosen to safely and simultaneously evaluate UFR accuracy and Crit-Line® accuracy via precise measurement of volume and PCV, and across controlled comparison of UFRs and hemodialyzer with controlled prescription settings. It additionally allowed comparison to a wider range of PCV than might typically be seen in dogs undergoing UF. It is important to recognize that in vivo many other forces—such as oncotic pressure, vascular permeability, renal function, and the endothelial glycocalyx—will contribute to actual blood volume change in an animal receiving extracorporeal therapy.18 Thus, the ex vivo study design and use of relative blood volume monitoring here do not allow for direct comparison to actual blood volume changes in live dogs. Further assessment of UFR accuracy and Crit-Line® accuracy is indicated in small animals undergoing UF. It is interesting to note that human hemodialysis guidelines discourage use of UFR0, for similar reasons we see here—namely the risk of adding dialysate to the blood when no UF is applied.18 This is important as dialysate is not sterile, but at best ultrapure when an ultrapure filter is used, an uncommon practice in human medicine but standard in many veterinary hospitals.
Despite these limitations, we demonstrated that the Crit-Line® device provides an accurate estimate of canine PCV and volume changes within certain ranges, and therefore is a useful tool for monitoring hemodialysis and UF in dogs. Additionally, we noted clinically important differences between targeted and delivered UFR with an ex vivo model of hemodialysis, when using a single Phoenix® X36 platform. Minimum UF and lower UFR appear to be proportionally less accurate. Veterinary hospitals offering hemodialysis should consider evaluating individual machines for differences in targeted and delivered UFR.
ACKNOWLEDGMENT
No funding was received for this study. Partial data were presented as a research abstract poster at the 2021 American College of Veterinary Internal Medicine (ACVIM) Forum Virtual. The authors thank Julie Commons, LVT, VTS-SAIM for assistance in care, use, and troubleshooting of the Gambro Phoenix® X36 platform and for ordering and maintenance of supplies required for this study. The authors thank the Small Internal Medicine technician team for maintaining the donor blood bank and for saving blood samples no longer fit for animal use for this study.
CONFLICT OF INTEREST DECLARATION
George E. Moore serves as Consulting Editor for Experimental Design and Statistics for the Journal of Veterinary Internal Medicine. He was not involved in review of this manuscript. No other authors have a conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




