Urinary cystatin B differentiates progressive versus stable IRIS Stage 1 chronic kidney disease in dogs
Abstract
Background
Early identification of dogs with progressive vs stable chronic kidney disease (CKD) might afford opportunity for interventions that would slow progression. However, currently no surrogate biomarker reliably predicts CKD progression.
Hypothesis/Objectives
Urinary cystatin B (uCysB), a novel kidney injury biomarker, predicts progressive disease in International Renal Interest Society (IRIS) CKD Stage 1.
Animals
Seventy-two dogs, including 20 dogs from 4 university centers with IRIS CKD Stage 1, with IDEXX symmetric dimethylarginine (SDMA) concentration up to 17 μg/dL and no systemic comorbidities, and 52 clinically healthy staff-owned dogs from a fifth university center.
Methods
A multicenter prospective longitudinal study was conducted between 2016 and 2021 to assess uCysB concentration in IRIS CKD Stage 1 and control dogs. Dogs were followed to a maximum of 3 years (control) or 25 months (CKD). Stage 1 IRIS CKD was classified as stable or progressive using the slope of 1/SDMA, calculated from 3 timepoints during the initial 90-day period. Dogs with slope above or below −0.0007 week × dL/μg were classified as stable or progressive, respectively. Mixed effects modeling was used to assess the association between uCysB and progression rate.
Results
Estimates of first visit uCysB results predictive of active ongoing kidney injury based on the mixed effects models were 17 ng/mL for control, 24 ng/mL for stable CKD, and 212 ng/mL for progressive CKD (P < .001).
Conclusions and Clinical Importance
Urinary cystatin B differentiated stable vs progressive IRIS CKD Stage 1. Identification of dogs with progressive CKD may provide an opportunity for clinicians to intervene early and slow progression rate.
Abbreviations
-
- AKI
-
- acute kidney injury
-
- CKD
-
- chronic kidney disease
-
- CysB
-
- cystatin B
-
- GFR
-
- glomerular filtration rate
-
- IRIS
-
- International Renal Interest Society
-
- LC-MS
-
- liquid chromatography-mass spectrometry
-
- SDMA
-
- symmetric dimethylarginine
-
- uCysB
-
- urinary Cystatin B
-
- UPC
-
- urine protein:creatinine ratio
1 INTRODUCTION
Chronic kidney disease (CKD) is defined as persistence of structural or functional abnormalities of the kidney for >3 months. Histological features include inflammation, fibrosis, and degeneration of the kidney parenchyma.1, 2 The parenchymal losses of CKD are irreversible and tend to be progressive in nature. Diagnosis of CKD typically is based on increased serum concentrations of functional markers, such as creatinine and symmetric dimethylarginine (SDMA), which also are used to monitor the disease progression. After diagnosis, guidelines from the International Renal Interest Society (IRIS) have been used to stratify CKD into 4 increasingly advanced stages.3 In IRIS CKD Stage 1, the earliest stage of CKD, functional markers often are insensitive for detecting disease progression because of their nonlinear relationship with glomerular filtration rate (GFR) and the relatively wide reference range for both creatinine and SDMA.4 Specific kidney injury markers to aid detection of progressive kidney disease are not routinely available in clinical practice but have been investigated extensively in the context of early diagnosis of acute kidney injury (AKI).5-8 Recent data suggest markers of active kidney injury also are increased in dogs with different stages of CKD.5, 6, 9-11 These studies indicate that active ongoing kidney injury also is present in dogs with CKD and has led to the suggestion that CKD and AKI are not completely separate processes and actually share common characteristics.4
Chronic kidney disease tends to be progressive in dogs, but the rate of progression varies substantially among dogs.4 Differences in progression can be observed in the rate of change of functional renal biomarkers (eg, creatinine, SDMA) vs time.12, 13 When assessing CKD progression, 1 approach is to convert functional renal biomarker results to their reciprocals (inverse biomarkers), from which linear slope changes (inverse slopes) can be calculated. Increased SDMA concentration is a well-documented risk factor for CKD progression in human medicine,14 and the 1/SDMA approach has been used for correlation analysis with kidney clearance estimates.15 In dogs, this approach distinguishes rates of progression and differentiates stable kidney function in healthy dogs vs dogs with progressive CKD.10, 16
These collective observations suggest the degree and duration of ongoing AKI in dogs with CKD assessed by kidney-specific active injury biomarkers might serve as surrogate markers for CKD progression rate. Preliminary data suggest the degree of active injury present in cats with CKD is associated with overall survival time.17
Cystatin B (CysB) is an 11 kD intracellular protein belonging to the family of cysteine protease inhibitors. It is found ubiquitously in many cell types, but because of its intracellular location, only trace concentrations are detected in serum in healthy subjects.18, 19 Presence of CysB in the urine (uCysB) predicts the presence of active renal tubular epithelial cell injury and death.20 Indeed, increased uCysB concentration was found in humans with diabetic nephropathy and preceded changes in other functional markers.21 In dogs, uCysB has been shown to be a marker of kidney injury after envenomation by the European adder22, 23 anesthesia,24 and after cardiopulmonary bypass surgery.25
We hypothesized that detection of increased uCysB concentration in dogs with IRIS CKD Stage 1 would predict ongoing active injury and could serve as a surrogate marker for progressive CKD. Our aim was to evaluate whether uCysB could differentiate dogs with stable vs progressive IRIS CKD Stage 1.
2 METHODS
2.1 Study population and clinical evaluation
Data for control dogs were collected from an archived longitudinal study of common vector-borne diseases in pets of students, faculty, and staff at the University Health Center at the University of Missouri. Data collection occurred between February 2012 and February 2015. All dogs were enrolled with owner consent. Institutional Animal Care and Use Committee approval for the study was obtained from University of Missouri. Dogs between 1 and 10 years of age, deemed healthy by their owners, clinical evaluation, and routine laboratory testing (including SDMA, serum biochemistry profile, CBC, and urinalysis with urine protein-to-creatinine ratio), were followed every 4-6 months for 3 years. Only dogs with data available for ≥2 visits and uCysB results were included in the study. The median number of visits during the study period was 9 (range, 5-10). However, on average, only 32% of each dog's visits had adequate residual urine volume available for uCysB testing. All dogs (n = 52) had uCysB determined at their first visit. For dogs with 2 visits (n = 29), most (n = 20) had their second measurement of uCysB at 6 months, whereas the remaining dogs (n = 9) had their second measurement 1-2 years later. For dogs with 3 visits (n = 11), the second visit with uCysB results occurred between 4 (n = 5) and 8 months (n = 8). Third visits occurred at approximately 8 months (n = 2), 20 months (n = 1) or 2 years (n = 8). For dogs with 4 visits (n = 12), uCysB was measured for all dogs at 4 (second visit), 8 (third visit), and 24 months (fourth visit). Dogs with IRIS CKD Stage 1 were enrolled prospectively at 4 additional academic institutions: University of Minnesota, North Carolina State University, Hebrew University, and University of California Veterinary Medical Center, San Diego. Data collection occurred between July 2016 and January 2021. Dogs >6 months of age presenting for evaluation of CKD were eligible for inclusion. Evaluation of enrolled dogs included physical examination, systolic blood pressure measurement, SDMA, serum biochemistry profile, CBC, urinalysis, urine culture, and urine protein-to-creatinine ratio at each visit. Diagnostic imaging was performed at the time of study inclusion for most (n = 15) dogs. Owner consent was required for enrollment, and Institutional Animal Care and Use Committee approval for the study was obtained from each academic institution. Only dogs with IRIS CKD Stage 1 were eligible for inclusion. Diagnosis of CKD was established by the Founders of the American College of Veterinary Nephrology and Urology at each site, and the dogs subsequently were staged in accordance with IRIS CKD staging guidelines.3 The diagnosis of CKD was based on a combination of diagnostic findings in a hydrated, stable dog: a persistent increase (>0.3 mg/dL) of serum creatinine concentration within the reference interval compared to an established baseline over a period of >2 weeks where no prerenal cause was apparent, a persistently increased SDMA concentration >14 μg/dL over a period of >2 weeks where no prerenal cause was apparent, abnormal kidney imaging, or persistent renal proteinuria with urine protein-to-creatinine ratio >0.5. Dogs with primary adrenal or exocrine pancreatic diseases, concurrent hepatic or cardiac diseases or other systemic diseases or inflammatory conditions expected to affect progression of CKD were excluded. Each dog was reassessed by board-certified veterinary internists, IRIS CKD was staged, and the dog was screened for risk factors for progression including proteinuria, hypertension, and anemia at follow-up visits.
2.2 Stage 1 stable vs progressive CKD classification
Classification of dogs with IRIS CKD Stage 1 into stable or progressive CKD categories was based on the slopes calculated using a least square regressions with inverse SDMA as the dependent variable and follow-up time as the independent variable for each patient.26 The regression was based on uCysB results on Day 0, 90 and a randomly chosen day between 0 and 90 days. Days were chosen randomly using the “sample_n” function in the “dplyr” package27 in R (version 4.1.0).28 An inverse SDMA slope cutoff of −0.0007 determined from the population of healthy control dogs with stable kidney function was used.16 Dogs with slopes ≥−0.0007 were classified as stable CKD, whereas dogs with slopes <−0.0007 were classified as progressive CKD. The same analysis was attempted using an inverse creatinine slope cutoff determined from the population of healthy control dogs with stable kidney function for comparison. When an inverse creatinine slope cutoff of ≥−0.0119 was used26 only 1 dog was categorized as progressive and thus further evaluation was not possible.
2.3 Analyte measurement
Serum aliquots for SDMA measurement were frozen at −80°C, shipped frozen in batches to IDEXX Laboratories, Inc., and stored at −80°C until testing in batch on the validated gold standard liquid chromatography-mass spectrometry (LC-MS) assay, as previously described.29 Measurement of uCysB was performed using a research sandwich format ELISA at IDEXX Laboratories, Inc. (Westbrook, Maine) as previously described.20 The research ELISA plate assay met all validation criteria including spike accuracy: ±20%, intra-assay precision: ≤15% coefficient of variation (CV), inter-assay precision: ≤15% CV, and sample freeze-thaw stability: ±20% of initial measurement. The dynamic range of the research ELISA plate assay was 15-2500 ng/mL. The reference range was ≤50 ng/mL based on a population of apparently healthy dogs (n = 78) as determined by normal serum SDMA and creatinine concentrations, CBC, serum biochemistry profile, and urinalysis (data on file at IDEXX Laboratories, Inc., Westbrook, Maine). Urine sample aliquots for uCysB measurement were frozen at −80°C, shipped frozen in batches to IDEXX Laboratories, Inc. (Westbrook, Maine), and stored at −80°C until testing in batch.
2.4 Statistical analysis
Descriptive analyses were performed to understand the dependence of serial samples taken from a single dog as well as how uCysB results differed within each CKD group. Kruskal-Wallis analysis of variance (ANOVA) was used to compare differences in the distribution of uCysB detection in dogs within a CKD group as well as between CKD study groups. Dunn's tests with Bonferroni correction for multiple comparisons were performed to identify which groups were different. Least square regressions between uCysB (dependent variable) and follow-up day (independent variable) were performed for each dog to identify the effect of CKD group on y-intercepts and slopes. The y-intercepts are an approximation of the first uCysB result of each dog. Post hoc descriptive analyses (without statistical analyses) were conducted to determine the potential utility of uCysB as an early biomarker of progressive CKD.
A linear mixed-effects model was used to assess the relationship between follow-up time and uCysB. All timepoints with uCysB results were used. A random slope was used to account for differences in CKD group. A random intercept was used to account for the non-independence of serial uCysB measurements within each individual. Regression lines and SE bars were plotted for each CKD group based on predicted uCysB results for each day of follow-up (Days 0-851). All statistical analyses were performed in R (version 4.1.0), using the lme4,30 tidyverse,31 and ggplot232 packages.
3 RESULTS
3.1 Demographics
There were 52 (72%) controls, 12 (17%) stable CKD dogs, and 8 (11%) progressive CKD dogs (Table 1). Over half (56%; 40/72) of the enrolled dogs were between 5 and 10 years of age. Most dogs were spayed females (47%; 34/72) or neutered males (46%, 33/72). Mixed breeds were most common.
| Demographic | Control (n = 52) (72%) | Stable CKD (n = 12) (17%) | Progressive CKD (n = 8) (11%) |
|---|---|---|---|
| Age (years) | |||
| <1 year old (n = 1) (1%) | 0 (0) | 1 (8) | 0 (0) |
| 1 to <5 years old (n = 22) (31%) | 15 (29) | 5 (42) | 2 (25) |
| 5 to <10 years old (n = 40) (56%) | 34 (65) | 5 (42) | 1 (12) |
| At least 10 years old (n = 9) (13%) | 3 (6) | 1 (8) | 5 (62) |
| Sex | |||
| Female (n = 2) (3%) | 1 (2) | 1 (8) | 0 (0) |
| Spayed female (n = 34) (47%) | 25 (48) | 5 (42) | 4 (50) |
| Male (n = 1) (1%) | 1 (2) | 0 (0) | 0 (0) |
| Neutered male (n = 33) (46%) | 25 (48) | 5 (42) | 3 (38) |
| Unknown (n = 2) (3%) | 0 (0) | 1 (8) | 1 (12) |
| Breed | |||
| Mixed (n = 20) (28%) | 16 (31) | 1 (8) | 3 (38) |
| Labrador retriever (n = 8) (11%) | 4 (8) | 2 (17) | 2 (25) |
| Golden retriever (n = 6) (8%) | 3 (6) | 3 (25) | 0 (0) |
| Dachshund (n = 4) (6%) | 4 (8) | 0 (0) | 0 (0) |
| Siberian Husky (n = 3) (4%) | 1 (2) | 1 (8) | 1 (12) |
| Other (n = 31) (43%) | 24 (46) | 5 (42) | 2 (25) |
3.2 CKD diagnosis inclusion criteria, uCysB measurement, and treatment summary
Stable CKD dogs were diagnosed with CKD based on persistent increases of SDMA (n = 2), persistent proteinuria (n = 2), and structural changes in the kidneys on ultrasound examination (n = 8). Structural changes in the kidneys of dogs in the stable CKD group included 4 dogs with small kidney size and diminished corticomedullary differentiation, and 1 dog each with evidence of prior infarct, pyelectasia, ectopic ureters, and unilateral kidney agenesis, respectively. Etiologies of CKD for dogs in the stable CKD group included juvenile onset CKD (n = 4), structural abnormalities (n = 3), persistent proteinuria (n = 2), hypertension (n = 1), renal infarct (n = 1), and unknown (n = 1).
Progressive CKD dogs were diagnosed with CKD based on persistently increased SDMA (n = 2), persistent proteinuria (n = 3), and structural changes in the kidneys on ultrasound examination (n = 3). Structural changes present in the kidneys in the progressive CKD group included 1 dog with mild bilateral degenerative nephropathy and 2 dogs with small kidney size with diminished corticomedullary differentiation. Etiologies of CKD reported for dogs in the progressive CKD group included juvenile onset CKD (n = 2), persistent proteinuria (n = 3), nephritis (n = 1), nephrolithiasis (n = 1), and unknown (n = 1).
Measurement of uCysB occurred at every visit, and visits occurred approximately monthly. Dogs with <3 study visits were excluded. Average duration of follow-up was 7 months and maximal duration of follow-up was 25 months. Treatments administered included correction of ectopic ureter (n = 1), angiotensin-converting enzyme inhibitors (enalapril, n = 2), angiotensin receptor blockers (telmisartan, n = 3; losartan, n = 1), antiplatelet agents (clopidogrel, n = 2), factor Xa inhibitors (rivaroxaban, n = 1), sympathomimetics (phenylpropanolamine, n = 4), parasympathomimetics (bethanechol, n = 1), calcium channel blockers (amlodipine, n = 2), omega-3 fatty acid supplementation (n = 1), cranberry supplementation (n = 2), and renal diet (n = 9). Laboratory testing for all dogs was performed by IDEXX Laboratories, Inc. (Westbrook, Maine). Numbers of dogs enrolled from each study center are listed in Table 2.
| Study center | n |
|---|---|
| University Health Center at the University of Missouri | 52 |
| University of Minnesota | 7 |
| North Carolina State University | 4 |
| Hebrew University | 2 |
| University of California Veterinary Medical Center, San Diego | 7 |
3.3 Descriptive analysis
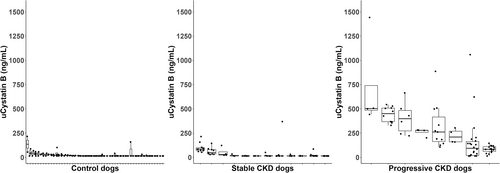
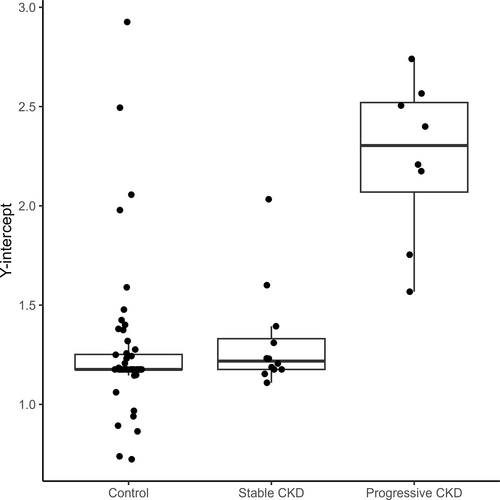
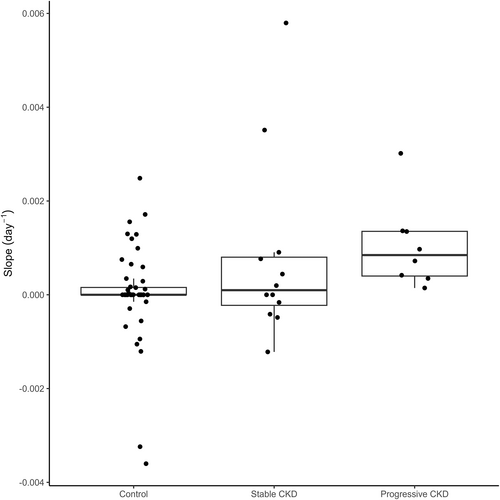
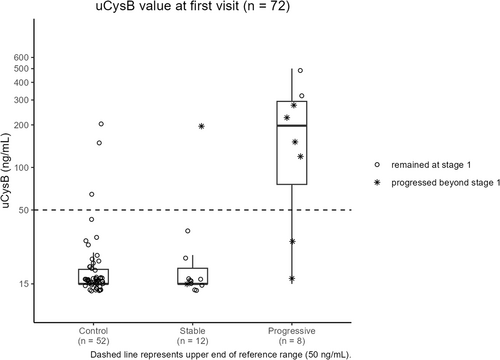
Urinary cystatin B results did not cluster around a common value for control (Kruskal-Wallis, P < .02), stable (Kruskal-Wallis, P < .001) or progressive (Kruskal-Wallis, P < .001) dogs (Figure 1). Urinary cystatin B results of dogs with progressive CKD were higher than those of control dogs and dogs with stable CKD (Dunn's test, P < .001). The y-intercepts calculated based on per-dog linear regressions also differed by study group (Kruskal-Wallis, P < .001; Figure 2). The y-intercepts of dogs with progressive CKD were higher than those of control dogs (Dunn's test, P < .001) and dogs with stable CKD (Dunn's test, P = .01). No significant difference between uCysB results of control dogs and dogs with stable CKD was detected (Dunn's test, P = .5). A difference in slopes among control, stable CKD and progressive CKD dogs was detected (Kruskal-Wallis, P < .001; Figure 3). Post hoc analysis (Figure 4) showed 75% (6/8) of dogs with progressive CKD had increased uCysB results already at their first visit compared to only 6% (3/52) and 8% (1/12) of control dogs and dogs with stable CKD, respectively. No differences were observed in first visit SDMA, systolic blood pressure, or urine specific gravity between stable and progressive dogs (Figures S1-S3). First visit urine protein-to-creatinine ratios were higher in dogs with progressive CKD (Wilcoxon rank sum test, P = .03; Figure S4).
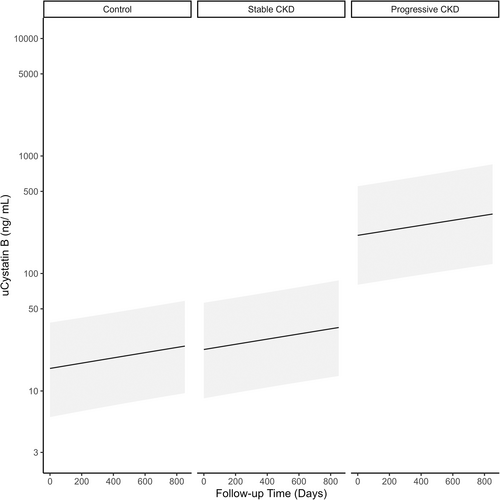
3.4 Linear mixed-effects model
A linear mixed effects model was used to determine the relationship between uCysB and follow-up time among different CKD groups (Figure 5; Table 3). Based on results of the descriptive analysis, random slopes were used to account for variability based on study group classification whereas random intercepts were used to account for variability based on differences among individual dogs. Being classified as progressive CKD was associated with a 2.55 (95% confidence interval [CI], 1.96-3.15) increase in log(uCysB) compared to control dogs. No significant difference was detected between dogs with stable CKD and control dogs (0.35; 95% CI, −0.02 to 0.33). The y-intercept analysis indicated that the approximate Day 0 values for dogs with progressive CKD were significantly higher than those of control dogs, whereas no differences were detected between stable and control dogs.
| Fixed effects | Coefficient | 95% CI | Random effect (SD) |
|---|---|---|---|
| Follow-up day | 0.00048 | 0.00018 to 0.00080 | - |
| CKD group | |||
| Control | - | - | - |
| Stable | 0.35 | −0.0082 to 0.71 | 0.54 |
| Progressive | 2.55 | 1.96 to 3.15 | 0.80 |
- Note: A random intercept was used to account for the non-independence of repeated measures within an individual. Urinary cystatin B was the dependent variable. Follow-up day was the independent variable. The effect of CKD group was allowed to vary randomly.
4 DISCUSSION
Our study suggests active ongoing kidney injury is continually present in some dogs with IRIS CKD Stage 1. This conclusion was evidenced by the high uCysB results in dogs identified with progressive CKD in the linear mixed effects model. Furthermore, the increased y-intercepts in the linear mixed effects model, as well as the increased Day 0 uCysB results in the post hoc analysis of dogs with progressive CKD, suggest individual assessments of uCysB may be predictive for progressive CKD. One suggestion for classifying dogs into stable or progressive CKD requires a slope calculation using multiple measurements of SDMA over a span of time.16 However, in the current cohorts, the first uCysB measurement during follow-up predicted the stable vs progressive CKD status of a dog for the entirety of the study (as shown by the increased y-intercepts of dogs with progressive CKD in the linear mixed model and the increased Day 0 uCysB results in the post hoc analysis). Evaluation of uCysB as an active kidney injury marker may be helpful to predict dogs likely to experience progressive CKD and should prompt a careful evaluation for underlying disease. Functional kidney biomarkers are likely to be within normal limits in IRIS CKD Stage 1, which may make prediction of progressive CKD difficult.3, 4 In our study, first visit urine protein-to-creatinine ratios were higher in the progressive dogs than in the stable dogs. However, our study was not designed to evaluate the predictive value of proteinuria in differentiating progressive from stable CKD.
Absolute uCysB concentrations are reported in our study rather than concentrations normalized to urine creatinine concentration because of the risk of overestimating or underestimating urinary biomarker concentration. The approach of normalizing a urinary biomarker to urinary creatinine concentration to control for differences in urine concentration is based on the assumption that creatinine is always produced at a steady rate, but such is not always the case.33-36 Normalization to urine creatinine concentration may be misleading for urinary biomarkers in clinical contexts where (a) different creatinine generation rates or extrarenal degradation rates exist; (b) when GFR is changing and a steady state has not yet been achieved; (c) when renal tubular creatinine secretion differs; or (d) when tubular backleak of creatinine differs across injured tubular epithelium.35 Several reviews of urinary biomarker studies with and without normalization to urine creatinine concentration showed little or no influence when normalization was completed.22, 37-39 Normalization to urine creatinine concentration is also of particular concern when uCysB results are undetectable or at the low end of the detection range in healthy dogs because differences then may be solely introduced by urine creatinine concentration. For these reasons, normalization to urine creatinine concentration was not performed in our study.
Comparative analysis based on the inverse creatinine slope cutoff categorized only 1 IRIS CKD Stage 1 dog as progressive, which was insufficient for further analysis. Whereas the inverse creatinine slope cutoff differentiated progressive dysfunction in a subset of dogs with CKD progression caused by progressive X-linked hereditary nephropathy,26 creatinine may be too insensitive in early CKD. Additional studies to explore potential hypotheses and considerations related to application of an inverse creatinine slope cutoff in IRIS CKD Stage 1 in comparison to later IRIS stages would be valuable.
Chronic kidney disease in dogs and cats is a multifactorial disorder with several implicated etiologies. An underlying cause cannot be identified or eliminated in the majority of dogs and cats with CKD. In the absence of an identifiable cause, treatment is mostly focused on slowing progression (eg, dietary intervention)40 and controlling risk factors for rapid progression (eg, hypertension, proteinuria, heart disease, anemia).4 Once the disease has progressed to more advanced stages (eg, IRIS CKD Stage 3), treatment becomes mostly symptomatic. Therefore, a major therapeutic goal is to identify CKD early in its course and slow its progression before substantial irreversible damage occurs.
Diagnosis of IRIS CKD Stage 1 often is challenging, and additional diagnostic methods may be needed. The diagnosis of CKD often cannot be established solely on the basis of functional markers, and early diagnosis may be based on persistently poorly concentrated urine, persistent renal proteinuria, and ultrasonographic changes in the kidneys (which are often subtle and inconclusive at this stage of the disease). Additional diagnostics include GFR measurement and kidney biopsy, but neither are routinely obtained in the clinical setting because of insufficient accuracy and invasiveness, respectively.
Presence of active kidney injury in dogs with apparently stable CKD has been proposed,4 and preliminary data also suggest an association between the degree of active injury and long-term outcome in cats with CKD.17 In the latter study, renal-related and nonrenal-related deaths were not classified, and therefore it is impossible to determine if cats died from CKD progression or from other causes.17 In our study, 75% of dogs with progressive CKD had high uCysB at their first visit compared to only 8% (1 of 12) of dogs with stable CKD. Of note, this stable dog with high uCysB at first visit was also 1 of the 2 stable dogs that later progressed beyond Stage 1 over the duration of the study. In our study, categorization of stable vs progressive IRIS CKD Stage 1 was made strictly on the basis of the inverse SDMA slope calculated from 3 timepoints during the initial 90-day period. This categorization did not preclude subsequent increases in SDMA that may have occurred after the initial 90-day period that would indicate later progression beyond Stage 1. Additional studies would be needed to better characterize and elucidate the frequency of this occurrence with larger numbers of Stage 1 dogs. In contrast, 67% of dogs with progressive CKD with increased uCysB progressed beyond IRIS CKD Stage 1 during the follow-up period. Thus, uCysB is a potential aid in the diagnosis of IRIS CKD Stage 1, but more importantly in differentiating stable vs progressive CKD. Our results suggest that the degree of active ongoing injury, regardless of underlying etiology, might be used as a surrogate marker for active kidney injury, eventually resulting in further loss of kidney function and CKD stage progression. It remains to be determined if current therapeutic interventions can slow this progression. However, the availability of a real time marker of kidney injury will facilitate assessment of various therapeutic interventions in animals with CKD.22, 23, 25 To date, therapeutic interventions are typically evaluated by serial measurements of functional markers, such as serum creatinine and SDMA concentrations, which change slowly. Thus, a long follow-up time is required to assess therapeutic interventions in animals with early progressive disease, during which time other factors might influence progression. Therapeutic interventions, such as dietary modifications, are most likely relevant in the early stages of disease before substantial irreversible changes are present.13, 40-42 Real time markers such as uCysB may allow better understanding of the intrarenal processes governing CKD as well as the utility of therapeutic interventions aimed to mitigate these processes.
In our study, the inverse slope of SDMA vs time was used to assess disease progression rate. Despite the high prevalence of CKD in dogs and cats, there are no definitions or established guidelines to differentiate animals with slow vs moderate or high progression rate. Percentage change or absolute change in serum creatinine or SDMA concentration might be used to define progression, but the time during which these changes occur is critical to determine progression rate. An increase in serum creatinine concentration of 0.5 mg/dL might be consistent with rapidly progressive CKD if occurring over a shorter time period (eg, 2 months) or slowly progressive if occurring over a longer period (eg, 3 years). In our study, the inverse slope was calculated based on 3 time points over at least 3 months.16
Our study had several limitations. First, uCysB is an intracellular protein found ubiquitously in other cells throughout the body, and thus it cannot be ascertained if its presence in the urine originated exclusively from damaged renal tubular epithelial cells. Damage of the extraordinarily sensitive renal tubular epithelial cells, however, is well documented in cases of kidney injury.43, 44 It also has been determined that uCysB is released from rupture of tubular epithelial cells in culture.20 Because CysB is an 11 kD molecule and could be freely filtered across the glomerulus, it cannot be excluded that uCysB originated in some dogs from other injured body organs. However, circulating concentrations of CysB are known to be very low in healthy individuals.18, 19 Dogs with CKD and concurrent inflammatory conditions or systemic diseases were excluded to further minimize the likelihood that uCysB originated from extrarenal cellular damage. A second limitation is that the number of dogs included with IRIS CKD Stage 1 was relatively low. This limitation was in part associated with the challenges in diagnosis of IRIS CKD Stage 1 as well as recruitment for participation in a prospective longitudinal study requiring a high level of owner commitment for several follow-up visits in dogs that may not appear clinically sick. In addition, not all CKD dogs were evaluated by ultrasound imaging and some pertinent renal findings may have been missed. Third, there is potential for bias in the control dog population because data were extracted from an archived study on seroprevalence of vector-borne diseases. For the small number of control dogs with increased uCysB at first or subsequent visits, it is not possible to determine with the current study design whether or not subclinical kidney injury may have been present at those particular visits. In addition, systolic blood pressure was not assessed in the healthy control cohort. On a related note, the control dogs in our study also were included in data that was used to generate inverse slope criteria in a companion manuscript.26 However, concentrations of uCysB in the control population are consistent with those of control populations in other published studies.22, 23 Fourth, progression rate was based on 3 timepoints during the initial 90-day period. Longer follow-up time to define progression would have been advantageous. Fifth, our study was not designed to assess the impact of age on uCysB. In our study, dogs categorized as having progressive CKD tended to be older than the dogs categorized as having stable CKD.
In conclusion, uCysB is a promising biomarker for detection of active intrarenal injury in dogs with CKD and a potential surrogate marker for progression rate. In IRIS CKD Stage 1, uCysB can differentiate stable vs progressive CKD, thus creating opportunities to slow progression and improve patient management.
ACKNOWLEDGMENT
Funding for the study was provided by IDEXX Laboratories, Inc.
CONFLICT OF INTEREST DECLARATION
G. Farace, D. Szlosek, Z. Ouyang, S. Peterson, M. Beall, and M. Yerramilli are employed by and have stock or stock options with IDEXX Laboratories, Inc. L. D. Cowgill, G. Segev, S. Vaden, S. Ross, C. Dufayet, L. A. Cohn, M. Nabity, and D. Polzin have received travel reimbursement and honoraria from IDEXX Laboratories, Inc. within the past 5 years. Shelly Vaden serves as Associate Editor for the Journal of Veterinary Internal Medicine. She was not involved in review of this manuscript.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Hebrew University IACUC approval, North Carolina State University IACUC approval, University of California Davis IACUC approval, University of Minnesota IACUC approval, and University of Missouri IACUC approval.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




