Presumptive phenobarbital-induced systemic lupus erythematosus in a domestic dog
Abstract
Case Description
We describe a case of presumptive acquired systemic lupus erythematosus secondary to phenobarbital administration in a dog, which resolved with withdrawal of the drug.
Clinical Findings
A 3.5 year-old poodle presented to a veterinary teaching hospital for Tier 1 idiopathic epilepsy and was treated with phenobarbital. The dog experienced fever, multiple cytopenias, and proteinuria in conjunction with a positive antinuclear antibody (ANA) titer.
Diagnostics
Serial CBCs, urine protein : creatinine ratios, and sternal bone marrow aspirates were performed to evaluate improvement.
Treatment and Outcome
Phenobarbital was withdrawn and levetiracetam initiated. All abnormalities resolved with supportive care, without initiation of immunosuppressive drugs. All cytopenias and proteinuria resolved and ANA test results became negative within 3 months. The patient recovered and did well clinically.
Clinical Relevance
Systemic lupus erythematosus is a disease of multiple autoimmune syndromes occurring concurrently or sequentially in conjunction with the presence of circulating ANA. It has been well described in dogs as an idiopathic condition, but in human medicine may occur secondary to drug reactions (drug-associated lupus) including as a reaction to phenobarbital. The findings in our case are consistent with the criteria for drug-induced lupus in humans and we suggest it as the first report of phenobarbital-induced lupus in a dog.
Abbreviations
-
- AKI
-
- acute kidney injury
-
- ANA
-
- antinuclear antibody
-
- CBC
-
- complete blood count
-
- MRI
-
- magnetic resonance imaging
-
- SLE
-
- systemic lupus erythematosus
1 INTRODUCTION
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by the presence of antinuclear antibodies and multisystemic immune-mediated inflammation.1-3 Drug-induced lupus refers to the development of autoimmune disease fulfilling lupus criteria as a result of administration of a medication; over 100 drugs have been implicated in human medicine.4 Specifically, lupus secondary to anticonvulsants has been described in association with phenobarbital, ethosuximide, trimethadione, and others.5-8
Lupus and lupoid syndromes have been well described in dogs,9 where the disease exists in both systemic and cutaneous forms, as well as several lupus-like (lupoid) conditions.2, 10 Drug-induced lupus has not been described previously in canine medicine, however antinuclear antibody formation has been noted in dogs secondary to hydralazine administration11 and in cats secondary to propylthiouracil.12
Adverse effects to phenobarbital have been extensively reported in veterinary medicine, and include on target effects such as sedation, ataxia, and lethargy, and off target or idiosyncratic drug reactions such as cytopenias and hepatopathy.13 Drug-induced lupus has not been described previously in canine medicine in association with phenobarbital administration.
This report describes an adult dog that developed proteinuria, acute kidney injury, multiple cytopenias, and a positive antinuclear antibody titer after administration of phenobarbital, all of which resolved without specific treatment.
2 CASE DESCRIPTION
A 3.5 year-old spayed female Standard Poodle presented to a veterinary referral hospital for evaluation of recurrent seizures. The dog was diagnosed with Tier 1 idiopathic epilepsy.14 A CBC and serum biochemistry panel performed a week before had been unremarkable, and PO treatment with phenobarbital (3.16 mg/kg q12h) was initiated.
The patient re-presented 3 weeks later for a reevaluation. No additional seizures had been noted at this time, and physical examination was unremarkable. Serum phenobarbital concentration was 102 μmol/L, and considered to be in the therapeutic range (70-170 μmol/L).
Two weeks later (5 weeks after commencement of treatment, day 0), the dog developed acute onset of lethargy and hyporexia. It initially was presented to the family veterinarian, where it was found to be febrile (39.5 C) and mildly dehydrated. A CBC disclosed low normal hematocrit (40%; reference interval [RI], 39-56%), moderate neutropenia (1.4 × 109/L; RI, 2.9-10.6 × 109/L), and marked thrombocytopenia (55 × 109/L; RI, 117-418 × 109/L), but a slide review was not performed at this time. Biochemistry disclosed azotemia, as indicated by increased serum creatinine (288 μmol/L; RI, 44-133 μmol/L) and BUN (42.4 mmol/L; RI, 7.3-11 mmol/L) concentrations. Serum symmetric dimethyl arginine concentration also was mildly increased (15 μg/dL; RI, <14 μg/dL). Urine specific gravity was 1.020, but the sample was collected after fluid therapy had begun. Urine protein : creatinine ratio was markedly increased at 11.4 (RI, <0.5) with an inactive urine sediment. The dog presented to the previous veterinary referral hospital, where these findings were confirmed (Table 1). The neutropenia was a persistent finding, no left shift or neutrophil toxicity was observed, and platelet clumps were not identified upon examination of a blood smear by a clinical pathologist (ie, marked thrombocytopenia was accurate). Direct Coombs testing was negative, as was an ELISA for vector-borne diseases (Dirofilaria immitis antigen, and Anaplasma spp., Ehrlichia spp., and Borrelia burgdorferi antibodies).
| Day 0 | Day 1 | Day 4 | Day 8 | Day 9 | Day 20 | Day 34 | Day 55 | Day 78 | |
|---|---|---|---|---|---|---|---|---|---|
| Urea [3.5-9.0] mmol/L | 32.5 | 13.8 | 12.2 | – | 5.4 | – | – | – | – |
| Creatinine [20-150] μmol/L | 203 | 150 | 140 | – | 87 | – | – | – | – |
| WBC [4.9-15.4] × 109/L | 3.8 | 2.8 | 1.6 | 2.4 | 3.8 | 2.6 | 7.9 | 6.3 | 6.4 |
| Hematocrit [39-56] % | 40 | 46 | 33 | 16 | 26 | 33 | 38 | 45 | 55 |
| Reticulocytes (<80) | 17.3 | 43.7 | |||||||
| Platelets [117-418] × 109/L | 36 | 17 | 7 | 30 | 46 | 268 | 300 | 205 | 167 |
| Seg neutrophils [2.9-10.6] × 109/L | 0.99 | 0.36 | 0.24 | 0.02 | 0.30 | 0.13 | 3.87 | 2.65 | 3.46 |
| Urine specific gravity | – | 1.020 | 1.017 | – | – | 1.056 | – | – | – |
| Urine protein : creatinine ratio [<0.5] | – | 11.4 | 4.8 | – | – | 0.3 | – | – | – |
| Urine sediment | – | IA | IA | – | – | IA | – | – | – |
| Urine protein | – | 3+ | 2+ | – | – | 2+ | – | – | – |
| Bone marrow aspirate | * | * | * | ||||||
| Antinuclear antibody | 1 : 640 | 1 : 10 |
- Note: All reported results are from the same referral laboratory. All platelet counts were confirmed by clinical pathologists. Asterisks indicate days bone marrow was sampled. Note, phenobarbital initiated on day −35 and discontinued on day 1.
- Abbreviation: IA, inactive.
Supportive care with IV fluids, broad spectrum antibiotics (ampicillin and enrofloxacin), and gastrointestinal (GI) support was initiated. Because of a suspicion of phenobarbital-induced bone marrow suppression, this drug was discontinued and levetiracetam initiated as a replacement. A positive response to treatment was noted; pyrexia resolved within 12 hours, and appetite returned the next day.
A sternal bone marrow aspirate was collected on day 2, as previously described15 (Figure 1, Supplemental Material 1). These findings were interpreted as indicating marked granulocytic hyperplasia and possibly megakaryocytic hyperplasia, with ineffective granulopoiesis and megakaryopoiesis. The underlying mechanism was speculated to be an adverse reaction to phenobarbital or underlying (primary or secondary) immune-mediated disease.
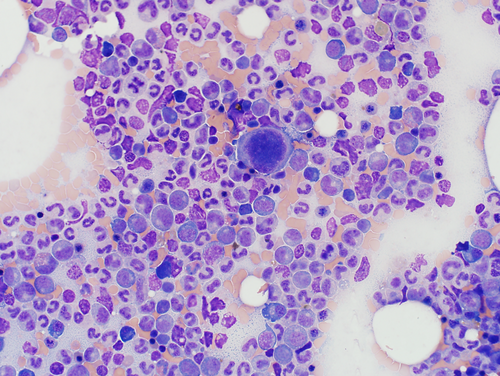
The dog remained stable in the hospital over several days. Azotemia resolved with IV fluid therapy, and proteinuria improved but persisted without specific treatment. Neutropenia and thrombocytopenia were unchanged and anemia developed (Table 1). Urine culture and urine Leptospira PCR were negative. Blood was collected on day 4 for an antinuclear antibody (ANA) titer, which was strongly positive at 1 : 640 (Cornell Diagnostic Laboratory, Ithaca, NY).
Because the dog became pancytopenic, sternal bone marrow aspiration was repeated on day 8 (Figure 2). The findings lent further support for a presumptive immune-mediated component to the cytopenias (Supplemental Material 1).
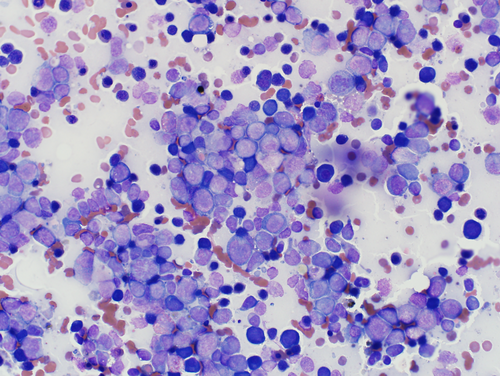
Although clinical presentation was strongly suggestive of an adverse reaction to phenobarbital, cytologic findings on the follow-up bone marrow raised concerns for a myelodysplastic or leukemic state. For this reason, flow cytometry was pursued to further investigate the population of cells in the marrow. This evaluation showed a predominance of monocytic precursors (Supplemental Material 2).
The patient received a blood transfusion on day 8 because of clinical signs associated with anemia. The transfusion improved hematocrit, and clinical signs improved by day 9. On day 10, the dog was discharged from the hospital, clinically stable and with good appetite. Treatment with amoxicillin-clavulanic acid and enrofloxacin was continued at home because of neutropenia.
Throughout management, immunosuppression was discussed because of a suspected immunologic reaction. Systemic lupus erythematosus (SLE) was not strongly considered, and the use of immunosuppression in phenobarbital induced dysmyelopoesis is not well established. In addition, the risks of immunosuppression in an already immunocompromised animal were of concern. Because of risk aversion of the part of the owner, a conservative monitoring plan was adopted, with intent to add immunosuppression if clinical signs deteriorated.
Several reevaluations were performed in the following weeks (Table 1). During this time, thrombocytopenia and proteinuria resolved, and anemia gradually improved. Neutropenia was the last cytopenia to resolve, eventually normalizing on day 34.
At the final reevaluation 11 weeks after initial detection of cytopenias, a CBC, sternal bone marrow aspirate, and ANA titer were repeated. Based on resolution of the cytopenias, bone marrow aspiration was not strongly indicated, but was performed because of previous concerns for acute leukemia and the owner's desire to confirm that all abnormalities had resolved before curtailing monitoring. All cytopenias had resolved and ANA had decreased markedly to 1 : 10. Bone marrow cytology was substantially improved. It was estimated to be 60% cellular, with adequate myeloid: erythroid ratio (2 : 1). Complete and synchronous granulocytic maturation was observed with relatively increased proportion of intermediate and late stage rubricytes, and numerous immature megakaryocytes. Based on these results, presumptive drug-induced lupus was determined to be in remission.
3 DISCUSSION
The American Rheumatologic Association defines criteria for the diagnosis of lupus in humans as a positive ANA and the presence of at least 10 points on a weighted scoring scheme.1 Although no universally recognized system for SLE diagnosis has been established in dogs, previous studies have routinely applied criteria used in humans.2, 3 In our patient, the presence of a positive ANA titer, fever (2 points), leukopenia (3 points), thrombocytopenia (4 points), and proteinuria (4 points) resulted in 13 points, consistent with a diagnosis of lupus by the classification scheme used in humans. Although seizures are a criterion for the diagnosis of lupus in humans, they predated other clinical signs in this dog and were considered unrelated. Although the criteria used for the diagnosis of SLE in humans have changed over time, the criteria utilized in our case were consistent with previous studies of SLE in dogs.2, 3, 9
The fever in our patient may have been multifactorial. Primary immune-mediated fever has been reported in humans with lupus and may have been the cause in our patient.1 Given the presence of neutropenia, fever secondary to low white blood cell numbers and inadequate host defense also is possible and supported by resolution after treatment with antimicrobials, but resolution correlated with withdrawal of phenobarbital.
Proteinuria likely was of renal origin based on the inactive sediment. Based also on its magnitude, glomerular origin was suspected. In human patients with SLE, proteinuria may occur as a result of antibody deposition within the glomeruli and may occur in a variety of forms (eg, membranous, proliferative, focal segmental glomerulonephritis). Confirmation of immune-mediated origin would have required renal biopsy, which was not performed in our patient. It is possible that systemic disease (eg, fever, low grade sepsis) also may have affected glomerular function and contributed to proteinuria.
The anemia in this case appeared initially to be mild and nonregenerative, but became regenerative with increased severity. No microscopic evidence of immune-mediated destruction was noted, and Coombs testing was negative. Consequently, a hemolytic cause was not thought likely. Possible causes may have included intestinal bleeding (not clinically noted, but potentially supported by the increased BUN concentration), lack of production because of bone marrow suppression or, less likely, lack of erythropoietin because of the renal insult.
Azotemia in our patient was suspected to be of prerenal origin given the response to IV fluids. Unfortunately, urinalysis was not performed before IV fluid administration and confirmation of pre-renal origin was not possible. Postrenal causes are unlikely given the absence of obstruction. Other causes may have included drug-induced acute kidney injury, immune-mediated renal disease, or pyelonephritis (but negative urine culture makes pyelonephritis less likely). Testing for Lyme disease and leptospirosis was negative.
The lack of hematologic changes before phenobarbital treatment makes it unlikely that clinical disease was present before anticonvulsants were started. Although a urine protein : creatinine ratio was not performed before phenobarbital treatment, the resolution of proteinuria with drug withdrawal makes it unlikely it was a preexisting condition.
Phenobarbital-induced multilineage cytopenias have been reported in the veterinary literature, both in response to acute overdoses16 or because of idiosyncratic drug reactions to therapeutic concentrations.17, 18 In particular, ineffective myeloid hyperplasia and neutropenia have been described in dogs treated with phenobarbital,18 as noted in our case. Because the dog's cytopenias persisted 8 days after discontinuing phenobarbital, a repeat bone marrow aspirate was performed and showed substantial expansion of immature myeloid precursors, but with few mature granulocytes. The lack of improvement of the cytopenias within this limited time frame was not unexpected, because a previous study described a median time of 14 days for the resolution of neutropenia after withdrawal of the drug.18 Furthermore, the half-life of phenobarbital in dogs is approximately 3 days,19 and it is expected to take 15-21 days (ie, 5-7 half-lives) to clear the drug in a healthy dog.
On flow cytometry, large mononuclear cells and lymphocytes made up approximately 40% and 25% of all cells, respectively. The large mononuclear cells expressed monocytic markers (MHCII and CD14) and were thought to represent monocytes and monocytic precursors (monoblasts and promonocytes). The shift toward monocytopoiesis was thought to reflect repopulation of the marrow, which tends to occur more rapidly than granulopoiesis in certain instances (eg, sepsis, viral infections). An acute monocytic leukemia was considered unlikely based on uniform positivity for MHCII.20 Given the paucity of granulocytic precursors and mature granulocytes, an immune-mediated process targeting this lineage also was considered.
In several cases of phenobarbital induced anemia, peripheral immunologic mechanisms have been considered less likely on the basis of negative Coombs testing.16-18 However, a negative Coombs test does not rule out peripheral or bone marrow level destruction of red cell precursors, as could be the case in many animals. It is possible that an immune-mediated etiology is present in some of these instances.
Phenobarbital may lead to adverse effects through a variety of mechanisms. These may be dose dependent effects of the drug, or idiosyncratic drug reactions. The cases of hematologic or immune-mediated disease caused by phenobarbital are generally idiosyncratic and likely represent a combination of drug effect, genetic predisposition, and other as yet unidentified factors.
The propensity for phenobarbital to stimulate drug reactions and autoimmune disease has been described in many epileptic patients.21 Phenobarbital, phenytoin, and carbamazepine are metabolized by hepatic P450 enzymes to form hydroxylated arene oxides because of their aromatic moiety bound to ternary or quaternary carbons, which readily bind biological macromolecules.22 These form haptens or prohaptens, which activate T-cells and stimulate an immune response.23 All of these drugs have been implicated to cause skin rashes, fever, hepatitis, and hematologic changes though immune-mediated mechanisms,24 and cross reactivity between among drugs has been reported at 70%.21
In nonimmunologic forms of anticonvulsant hypersensitivity, it may be the toxic effects of these arene oxides that cause bone marrow damage, skin necrosis, and other clinical signs. Carbamazepine, phenytoin and valproic acid have been implicated as possible nonimmunologic causes of hematologic disease.22 However, in immunologic reactions (such as drug-induced lupus) it is likely that an immunologic aspect plays a more important role.22, 25
In humans with phenobarbital sensitivity, lymphocyte subsets have been isolated that proliferate and produce cytokines rapidly in response to phenobarbital challenge.23 These cells can recognize both phenobarbital and its arene oxide metabolites, which in some cases is not MHC restricted or dependent on presentation by antigen presenting cells.23 As such, after sensitization, lymphocytes may proliferate rapidly when exposed to phenobarbital, contributing to immunologic disease. It is increasingly recognized that in anticonvulsant hypersensitivity syndrome of humans, it may be the anticonvulsant itself and not the arene oxide metabolites that cause sensitization.26
In the reported cases of phenobarbital-induced myelosuppression in veterinary medicine, urinary protein loss and ANA titers have not been described. In many case reports of drug-induced cytopenia in humans, no investigation for systemic autoimmune disease is reported. As such, it is possible that many of these cases represent drug-induced lupus that have not been previously diagnosed. Further investigation into ANA titers and concurrent autoimmune syndromes in animals with phenobarbital reactions may be warranted to determine if these represent drug-induced lupus.
The resolution of clinical and laboratory findings after withdrawal of a drug, and without the use of immunosuppressive medications, is consistent with a drug-induced disease. Ideally, rechallenge with the medication would be used to confirm this diagnosis, but this approach was not followed for ethical reasons. In human medicine, half of the cases of anticonvulsant-induced drug-associated lupus are reported to respond to withdrawal of medication, whereas the other 50% require immunosuppressive therapy. No specific factors have been identified to determine which individuals will require immunosuppression.7 In humans with drug-induced lupus, antibodies to double-stranded DNA, antibodies to histones, antiphospholipid antibodies, and positive Coombs test results also have been investigated. It does not appear that the presence of these antibodies is associated with the need for immunosuppressive therapy.27-29
We describe the first veterinary report of presumptive drug-induced SLE from phenobarbital treatment. This condition should be considered as a possible differential diagnosis when adverse reactions to phenobarbital are seen. In our case, immunosuppressive therapy was recommended but declined, and resolution of clinical abnormalities occurred within 3 months after drug withdrawal.
ACKNOWLEDGMENT
No funding was received for this study.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Standard of care treatments and diagnostics were performed on a client owned animal and no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




