Correlation of clinical and radiographic variables in cats with lower airway disease
Abstract
Background
Feline lower airway disease (FLAD) is frequently associated with radiographic abnormalities.
Objectives
To evaluate whether radiographic changes in cats with naturally occurring FLAD improve with treatment and if radiographic changes correlate with clinical signs.
Animals
Twenty-four client-owned cats newly diagnosed with FLAD, based on medical history, typical clinical signs, radiographic findings, and examination of bronchoalveolar lavage fluid, were included in the prospective study.
Methods
At 2 examination time points (days 0 and 60), an owner questionnaire, clinical examination, and thoracic radiography were carried out. Information from the questionnaire and clinical examination were evaluated on the basis of a 12-point clinical score. Radiographs were assessed using a 10-point radiographic score. Individual treatment was given to all cats over the study period, based on severity of the disease and compliance of the cat. Clinical and radiographic scores were compared statistically for both examination time points and evaluated for correlation.
Results
All cats showed radiographic abnormalities at initial presentation. In addition to significant improvement in clinical variables, the total radiographic score improved significantly (P = .01) during the study period, with significant improvement in the severity of bronchial (P = .01) and interstitial lung pattern (P = .04). Improvement of the clinical and radiographic score was not correlated.
Conclusion and Clinical Importance
In addition to clinical signs, repeated radiographic examination can be used as a diagnostic tool to evaluate treatment response in cats with FLAD.
Abbreviations
-
- BAL
-
- bronchoalveolar lavage
-
- BALF
-
- bronchoalveolar lavage fluid
-
- CB
-
- chronic bronchitis
-
- FA
-
- feline asthma
-
- FLAD
-
- feline lower airway disease
-
- IQR
-
- interquartile range
-
- LMU
-
- Ludwig Maximilian University
1 INTRODUCTION
Feline asthma (FA) and feline chronic bronchitis (CB) are collectively known as feline lower airway disease (FLAD) and cause clinical signs, ranging from chronic cough and expiratory wheeze to episodes of respiratory distress.1, 2 The consequences of chronic airway inflammation in FLAD are epithelial edema associated with infiltration of inflammatory cells, hypersecretion of the goblet cells, and hypertrophy of the mucosa and submucosal glands and, additionally in FA, bronchoconstriction caused by hyperreactivity of bronchial smooth muscle.3-5 In the long term, without control of inflammation, irreversible pathological remodeling processes occur in the airways, also known as airway remodeling.3, 5, 6 These changes result in airway obstruction, which leads to clinicopathological findings of FLAD.3, 7 The most common radiographic finding described in cats with FLAD is an enhanced bronchial pattern caused by thickening of the bronchial walls and increased mucus accumulation in small airways.3, 5-9 In addition, interstitial or alveolar patterns, hyperinflation of the lung field, and lobar atelectasis also have been described in affected cats.1, 7, 9-11 Radiographic signs suggestive of pulmonary hyperinflation include increased lung transparency and caudal flattening of the diaphragm.3, 5, 6, 8, 10 The right middle lung lobe is most commonly affected by atelectasis as a consequence of mucus accumulation because of its dorsoventral orientation within the bronchial tree, and exposure to the effects of gravity.1, 3
Standard treatment of cats with FLAD consists of glucocorticoids, sometimes with the addition of bronchodilators. The aim of treatment is to suppress airway inflammation and thereby eliminate the clinicopathological findings.3, 5, 6, 12-14 Previous studies that investigated radiographic findings in cats treated for FLAD showed controversial results.15, 16 One study found improvement of radiographic changes in research cats with mild CB after administration of inhaled fluticasone for 2 weeks.16 In contrast, a randomized study investigating radiographic findings in 9 cats with naturally occurring FLAD treated with systemic glucocorticoids for 7 days, followed by either inhaled fluticasone or systemic glucocorticoids, showed no improvement in radiographic variables after 8 weeks of treatment in either group.15
Our aim was to investigate whether radiographic variables correlate with clinical signs as assessed using a standardized clinical score in cats with naturally occurring FLAD, and whether radiographic abnormalities improve with individualized treatment. We hypothesized that radiographic variables would improve with appropriate treatment to control the underlying airway inflammation in cats with FLAD, and that radiographic improvement would correlate with clinical signs.
2 MATERIALS AND METHODS
The prospective observational study was approved by the Ethics Committee of the Centre for Clinical Veterinary Medicine of Ludwig Maximilian University (LMU) of Munich (No. 139-20-07-2018).
2.1 Inclusion criteria
Client-owned cats presented for diagnostic evaluation at the LMU Clinic for Small Animal Medicine between May 2018 and July 2021, showing typical clinical signs of FLAD, were considered for inclusion in the study. Enrollment was possible if the pet owner agreed to participate, the cat was stable enough for diagnostic evaluation, and the cat had not received antibiotics within 14 days before presentation. Cats with bacterial growth or positive Mycoplasma spp. PCR in bronchoalveolar fluid (BALF) was retrospectively excluded from the study. Patients were enrolled as a part of a previously published substudy.17
2.2 Study sample
Twenty-four cats, diagnosed with FLAD on the basis of medical history, clinical signs, and BALF examination findings, were included in the study.
2.3 Study design
All cats were presented at 2 examination time points (days 0 and 60). During the initial presentation on day 0, the cat's medical history was obtained using a standardized owner questionnaire modified from a prior study,18 and a thorough clinical examination was performed. Each cat was assigned a previously published 12-point clinical score,19 based on information derived from the owner questionnaire and clinical examination findings. In cats with access to the outdoors, a Baermann fecal examination was performed to exclude lungworm infection. Radiographs of the thorax were taken of each cat using 2 views and evaluated using a previously published 10-point radiographic score.20 Bronchoalveolar fluid was obtained according to a previously described protocol17 as a part of the initial assessment on day 0. Bronchoalveolar lavage (BAL) was performed blindly in 23/24 cats and under endoscopy in 1/24 cats, endoscopy being indicated because this cat showed focal alveolar infiltration radiographically. The same board-certified clinical pathologist (JP) performed the cytological examination of all samples. The type of inflammation was classified according to the predominant cell types present: eosinophilic inflammation (≥17% eosinophils, <7% neutrophils), neutrophilic inflammation (≥7% neutrophils, <17% eosinophils), and mixed inflammation (≥7% neutrophils and ≥17% eosinophils).21 Aliquots of BALF were sent to the LMU Institute for Infectious Diseases and Zoonoses for aerobic bacteriological culture and to external laboratories for Mycoplasma spp. PCR.
2.4 12-point clinical score
On both examination days, a total clinical score (0-12) was determined for each cat, using the previously published 12-point clinical score19 (Table S1). This score included information from the standardized owner questionnaire and the findings of the clinical examination at the corresponding examination time point.
2.5 10-point radiographic score
Thoracic radiographs were obtained from each cat on both examination days using 2 views (left lateral and ventrodorsal or dorsoventral) in the inspiratory phase of respiratory cycle, as far as possible and tolerated by the cat. Because radiographic examinations were performed exclusively on unsedated cats, optimal positioning of the patients could not always be achieved. A standard setting for thoracic radiographs of kilovoltage peak (KVp) 65 and milliamperage (mA) 160 was used for all images. All radiographs were taken using a Fujifilm FDR Smart X x-ray unit (FUJIFILM Europe GmbH, Ratingen, Germany), and stored and visualized using specialized software (VetPACS-Viewer 7.1., Softneta UAB, Kaunas, Lithuania). All radiographs were randomized and reviewed at a later time point in Digital Imaging and Communications in Medicine (DICOM) format using a DICOM viewer (RadiAnt DICOM Viewer v2021.1, Medixant, Poznan, Poland) by the same board-certified radiologist (SH), who was blinded to patient signalment, clinical signs, examination findings, examination time point, and treatment. The radiographs were evaluated using a previously published 10-point radiographic score20 (Table S2). Pulmonary hyperinflation was subjectively graded as present or absent based on flattening of the diaphragm, expanded lung fields, hyperlucency of the lungs, increased distance from the caudal margin of the cardiac silhouette to the diaphragm on the lateral projection, and excessive convexity of the thoracic wall, as well as increased distance between the cardiac silhouette and the diaphragm on the ventrodorsal or dorsoventral projection.
2.6 Treatment
Treatment was selected individually for each study patient according to the severity of the disease and the compliance of the cat. To facilitate recovery after anesthesia, 3/24 cats received a single injection of dexamethasone (0.4 mg/kg IV). Therapeutic agents administered throughout the study period are listed in Table 1. A list of all therapeutic agents for each cat is provided in Table S3. Initially, 20/24 cats were treated with systemic glucocorticoids (prednisolone, 0.5-1.6 mg/kg q24h PO) followed by, or in addition to, inhaled glucocorticoids. One cat was treated systemically with cyclosporine (Sporimune, 5 mg/kg q24h PO) in direct combination with inhaled glucocorticoids. Three cats received inhalative glucocorticoids only (fluticasone propionate, 250 μg 1 puff q12h or salmeterol and fluticasone propionate 25 μg/125 μg, 1 puff, q12h). A bronchodilator (terbutaline, 0.05-0.1 mg/kg q8h PO) was administered in 10/24 cats initially.
| Therapeutic agents | Number of cats |
|---|---|
| Initially prednisolone, followed by fluticasone propionate | 11/24 |
| Initially prednisolone + terbutaline, followed by fluticasone propionate | 8/24 |
| Initially prednisolone, followed by budesonide | 1/24 |
| Cyclosporine + fluticasone propionate | 1/24 |
| Initially terbutaline, followed by fluticasone propionate | 1/24 |
| Terbutaline + salmeterol and fluticasone propionate | 1/24 |
| Fluticasone propionate only | 1/24 |
The duration of the initial treatment was based on the clinical response and the duration of time it took for the cat to become accustomed to inhalation treatment. During the study period, inhalation treatment was started in all 24 cats. Inhalation was carried out using a spacing chamber and an oronasal mask (Aerokat, Trudell Medical International). The aerosols used were fluticasone propionate 125 μg 1 puff q12h in 1/24 cats, fluticasone propionate 250 μg 1 puff q12h in 21/24 cats, budesonide 200 μg 1 puff q12h in 1/24 cats, and salmeterol and fluticasone propionate 25 μg/125 μg 1 puff q12h in 1/24 cats. The dosage of the respective inhalant drug was maintained throughout the entire study period. At the same time, PO bronchodilators were discontinued and systemic glucocorticoids were gradually tapered and discontinued, because long-term treatment with inhaled glucocorticoids alone was the aim in all study cats, leaving only 1 cat on additional PO glucocorticoid treatment (prednisolone, 0.2 mg/kg q24-48 h PO) and 1 cat on additional PO cyclosporine (Sporimune, 5 mg/kg q24h PO) between days 45 and day 60.
2.7 Follow-up examination
On day 60, all cats were re-presented to obtain a 12-point clinical score and a 10-point radiographic score on the basis of the findings from the owner questionnaire, clinical examination, and thoracic radiographs under treatment.
2.8 Statistical analysis
The statistics software SPSS version 28.0.1.0 was used for data analysis. To test for parametric distribution, the Shapiro-Wilk test was applied. Data was presented as mean ± SD for normally distributed data or median and interquartile range (IQR) for non-normally distributed data. The Wilcoxon signed-rank test was used because data was non-normally distributed to compare the 12-point clinical score and the 10-point radiographic score at both time points. Effect size of the Wilcoxon signed-rank test as rank biserial (rrb) with 95% confidence interval (Cl95%) was calculated between days 0 and 60. Effect size was considered tiny (rrb < .05), very low (rrb = .05-.10), low (rrb = .10-.20), medium (rrb = .20-.30), large (rrb = .30-.40), and very large (rrb > .40). Comparison of the changes between the 2 examination time points of the radiographic score of the 3 inflammation subtypes was assessed by Kruskal-Wallis test because data was non-normally distributed. Subsequently, P-values were corrected using the Bonferroni method for multiple comparisons.
Correlations were analyzed using Kendall rank correlation coefficient r. Correlations were considered very weak (r = .00-.19), weak (r = .20-.39), moderately strong (r = .40-.59), strong (r = .60-.79) and very strong (r = .80-1.0). In addition, the coefficient of determination r2 considered very weak (r2 = .00-.20), weak (r2 = .20-.40), moderate (r2 = .40-.60), strong (r2 = .60-.80), and very strong (r2 > .80).
For all tests, the significance level was set at P < .05.
3 RESULTS
3.1 Study sample
Forty-five cats were considered suitable candidates for the study (Figure 1). Because of ≥1 of the following, 21 cats were not included in the study: upper respiratory tract disease (n = 4), positive Mycoplasma spp. PCR (n = 8) or positive bacteriological culture (n = 4) of the BALF, normal BALF cytology (n = 2), missed follow-up appointment (n = 5), and lack of radiographs on day 60 (n = 1).
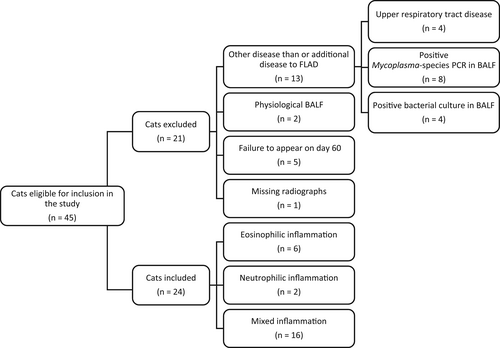
The study sample consisted of 24 cats with a mean age of 4 ± 3 years (range, 1-13 years) and a mean body weight of 4.8 ± 1.4 kg (range, 2.8-8.3 kg) on initial presentation. There were 14 females (13 spayed, 1 intact) and 10 males (8 neutered, 2 intact). Breeds included European Shorthair (n = 8), Abyssinian (n = 2), Ragdoll (n = 2), Siamese (n = 1), British Shorthair (n = 1), Bengal (n = 1), Siberian Forest (n = 1), Turkish Van (n = 1), Maine Coon (n = 1), and mixed-breed cats (n = 6).
3.2 Bronchoalveolar lavage fluid
The median total cell count in the BALF of all the cats was 2705 cells/μL (IQR, 1213-3275 cells/μL). The median cytological cell differentiation of the BALF was 46% eosinophils (IQR, 20%-58%), 13% neutrophils (IQR, 7%-43%), 33% macrophages (IQR, 17%-60%), and 0% lymphocytes (IQR, 0%-0%). Based on previously published classification,21 6 cats were diagnosed with eosinophilic inflammation, 2 cats with neutrophilic inflammation, and 16 cats with mixed inflammation.
3.3 12-point clinical score
The cats were part of a larger study and results of the 12-point clinical score in a larger number of cats have been published previously.17 A comparison of the 12-point clinical score on days 0 and 60 showed significant improvement in the total clinical score (5.5 [IQR, 4.4-7] vs 1.5 [IQR, 0-2.6]; rrb = 1.0; Cl95% [1.0, 1.0]; P < .001), coughing frequency (4 [IQR, 1.4-5] vs 0.5 [IQR, 0-2]; rrb = .97; Cl95% [.92, .99]; P < .001), frequency of respiratory distress (1 [IQR, 0-2] vs 0 [IQR, 0-0]; rrb = 1.0; Cl95% [1.0, 1.0]; P = .002), auscultation findings (1 [IQR, 0.9-1] vs 0 [IQR, 0-1]; rrb = .81; Cl95% [.55, .93]; P = .003), and general condition and appetite (0 [IQR, 0-1] vs 0 [IQR, 0-0]; rrb = 1.0; Cl95% [1.0, 1.0]; P = .02).
3.4 10-point radiographic score
All 24 cats showed a bronchial pattern on day 0, which was classified as mild (n = 14; 58.3%), moderate (n = 6; 25.0%) or severe (n = 4; 16.7%). Of these, 19/24 (79.2%) showed a bronchointerstitial pattern, with the degree of the interstitial component varying between mild (n = 9; 47.4%), moderate (n = 8; 42.1%), and severe (n = 2; 10.5%).
One cat (4.2%) had a focal alveolar infiltrate in the caudodorsal region of the lung on day 0 (Figure 2). For this reason, bronchoscopy was performed in this cat during initial evaluation, and in addition to BALF, 2 mucosal biopsy samples were taken in the area of the bifurcation for further investigation. Histopathological examination showed granulocytic inflammation and no evidence of infectious agents or neoplasia, BALF cytology also disclosed moderate eosinophilic and neutrophilic inflammation.
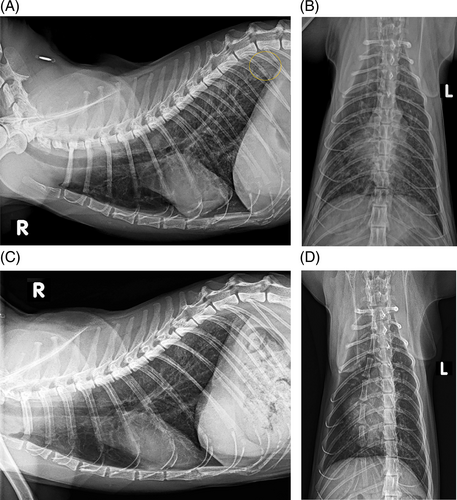
On day 0, 10/24 (41.7%) cats had radiographic evidence of pulmonary hyperinflation. One cat (4.2%) had atelectasis of the right middle lung lobe and an equivocal caudodorsal pulmonary nodule.
An investigation into whether the radiographic variables differed among cats with the 3 different subtypes of inflammation at both examination time points showed no significant differences among the 3 subgroups (Tables S4 and S5).
The results of the 10-point radiographic score are presented in Figure 3 and Table 2. The total radiographic score showed significant improvement when comparing the findings of day 0 and day 60 (rrb = .75; Cl95% [.48, .89]; P = .01). With treatment, 19/24 (79.2%) cats continued to show a bronchial pattern on day 60, being mild (n = 10; 52.6%), moderate (n = 8; 42.1%), or severe (n = 1; 5.3%). The severity of the bronchial pattern improved significantly (rrb = .82; Cl95% [.60, .92]; P = .01). Of these cats, 14/19 (73.7%) continued to have a mixed bronchointerstitial pattern. The interstitial component was classified as mild (n = 7; 50.0%), moderate (n = 6; 42.3%), or severe (n = 1; 7.1%), showing significant improvement over the study period (rrb = .71; Cl95% [.36, .88]; P = .04).
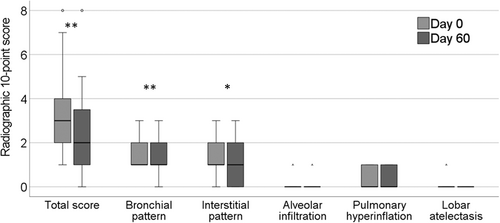
| Radiographic 10-point score | Day 0 | Day 60 | P value |
|---|---|---|---|
| Total radiographic score | 3 (2-4) | 2 (1-3.3) | .01 |
| Bronchial pattern | 1 (1-2) | 1 (1-2) | .01 |
| Interstitial pattern | 1 (1-2) | 1 (0-2) | .04 |
| Alveolar infiltration | 0 (0–0) | 0 (0-0) | 1.0 |
| Pulmonary hyperinflation | 0 (0–1) | 0 (0-1) | .18 |
| Lobar atelectasis | 0 (0–0) | 0 (0–0) | .32 |
- Note: Bold values indicate P-value <.05.
On day 60, 1 cat (4.2%) had a focal alveolar infiltrate in the ventral aspect of the left caudal lung lobe that had not been present on day 0. The alveolar infiltrate seen in the caudodorsal lung fields in 1 cat on day 0 was no longer visible on follow-up radiographs (Figure 2). Seven of the original 10 cats continued to show signs of pulmonary hyperinflation, whereas atelectasis was not noted in any of the radiographs. Atelectasis seen in 1 cat on day 0 had resolved on follow-up radiographs. A pulmonary nodule suspected in the same cat was no longer visible.
No significant difference was found in the extent of improvement of the radiographic variables over the study period among the 3 subgroups of inflammation (Table S6).
3.5 Correlation between BALF cytology and clinical and radiographic variables on day 0
Correlations between BALF findings and clinical and radiographic variables on day 0 are shown in Tables 3 and 4. A moderate correlation of the percentage of neutrophilic granulocytes in the BALF with total clinical score (r = .45; r2 = .20; P = .004) and abnormal auscultation findings (r = .47; r2 = .22; P = .004) was detected. No significant relationship could be found between neutrophilic granulocytes and total radiographic score (r = .25; r2 = .06; P = .12). A weak correlation was detected between the percentage of neutrophils and the severity of the bronchial score (r = .37; r2 = .14; P = .03). The number of eosinophilic granulocytes in BALF neither correlated with the total clinical score (r = −.21; r2 = .04; P = .17) nor with the total radiographic score (r = .04; r2 = .00; P = .8).
| BALF cytology | Clinical 12-point score | ||||
|---|---|---|---|---|---|
| Total clinical score | Coughing frequency | Respiratory distress | Auscultation score | General condition/appetite | |
| Total cell count (cells/μL) | r = −.02 r2 = .00 |
r = .012 r2 = .00 |
r = .02 r2 = .00 |
r = −.11 r2 = .01 |
r = −.21 r2 = .04 |
| Eosinophils (%) | r = −.21 r2 = .04 |
r = .08 r2 = .01 |
r = −.21 r2 = .04 |
r = −.32 r2 = .10 |
r = −.28 r2 = .08 |
| Neutrophils (%) | r = .45 r2 = .20 |
r = .22 r2 = .05 |
r = .09 r2 = .01 |
r = .47 r2 = .22 |
r = .17 r2 = .03 |
| Macrophages (%) | r = −.17 r2 = .03 |
r = −.38 r2 = .14 |
r = .18 r2 = .03 |
r = −.11 r2 = .01 |
r = .19 r2 = .04 |
| Lymphocytes (%) | r = .09 r2 = .01 |
r = −.09 r2 = .01 |
r = −.03 r2 = .00 |
r = .09 r2 = .01 |
r = .32 r2 = .10 |
- Note: Bold values indicate P-value <.05.
| BALF cytology | Radiographic 10-point score | |||||
|---|---|---|---|---|---|---|
| Total radiographic score | Bronchial pattern | Interstitial pattern | Alveolar infiltration | Pulmonary hyperinflation | Lobar atelectasis | |
| Total cell count (cells/μL) | r = .10 r2 = .01 |
r = .38 r2 = .14 |
r = .05 r2 = .00 |
r = .26 r2 = .07 |
r = .36 r2 = .13 |
r = .01 r2 = .00 |
| Eosinophils (%) | r = −.04 r2 = .00 |
r = .05 r2 = .00 |
r = −.13 r2 = .02 |
r = .04 r2 = .00 |
r = .12 r2 = .01 |
r = −.04 r2 = .00 |
| Neutrophils (%) | r = .25 r2 = .06 |
r = .37 r2 = .14 |
r = .20 r2 = .04 |
r = .17 r2 = .03 |
r = .06 r2 = .00 |
r = .14 r2 = .02 |
| Macrophages (%) | r = −.29 r2 = .08 |
r = −.38 r2 = .14 |
r = −.18 r2 = .03 |
r = −.24 r2 = .06 |
r = −.27 r2 = .07 |
r = −.14 r2 = .02 |
| Lymphocytes (%) | r = .09 r2 = .01 |
r = −.01 r2 = .01 |
r = .15 r2 = .02 |
r = −.08 r2 = .01 |
r = −.07 r2 = .01 |
r = −.08 r2 = .01 |
- Note: Bold values indicate P-value <.05.
The number of macrophages in BALF showed a weak negative correlation with coughing frequency (r = −.38; r2 = .14; P = .02) and the bronchial score (r = −.38; r2 = .14; P = .02). Total cell count in BALF showed a weak correlation with the detection of pulmonary hyperinflation on radiography (r = .36; r2 = .13; P = .04).
3.6 Correlation of clinical and radiographic variables
Correlations of the changes in clinical and radiographic variables between the 2 examination time points are presented in Table 5. No significant correlation was found between changes in the 12-point clinical score and the 10-point radiographic score.
| Changes in clinical 12-point score | Changes in radiographic 10-point score | |||||
|---|---|---|---|---|---|---|
| Total radiographic score | Bronchial pattern | Interstitial pattern | Alveolar infiltration | Pulmonary hyperinflation | Lobar atelectasis | |
| Total clinical score | r = .24 r2 = .06 |
r = .12 r2 = .04 |
r = .16 r2 = .03 |
r = .29 r2 = .09 |
r = −.10 r2 = .11 |
r = .28 r2 = .08 |
| Coughing frequency | r = .35 r2 = .12 |
r = .03 r2 = .00 |
r = .02 r2 = .00 |
r = .26 r2 = .07 |
r = −.24 r2 = .06 |
r = .15 r2 = .02 |
| Respiratory distress | r = .10 r2 = .01 |
r = .05 r2 = .00 |
r = .07 r2 = .01 |
r = .12 r2 = .01 |
r = .06 r2 = .00 |
r = .08 r2 = .01 |
| Auscultation score | r = .21 r2 = .04 |
r = .02 r2 = .00 |
r = .15 r2 = .02 |
r = .00 r2 = .00 |
r = .17 r2 = .03 |
r = .32 r2 = .10 |
| General condition/appetite | r = .27 r2 = .07 |
r = .30 r2 = .09 |
r = .25 r2 = .06 |
r = .00 r2 = .00 |
r = .04 r2 = .00 |
r = −.12 r2 = .01 |
- Note: All correlations were not statistically significant.
4 DISCUSSION
The purpose of our study was to evaluate whether radiographic variables in cats with naturally-occurring FLAD improve with individual treatment and correlate with clinical signs. The data provides evidence of significant improvement in radiographic abnormalities with anti-inflammatory treatment. However, no clinically relevant correlation with clinical improvement could be shown.
Before treatment on day 0, all cats had abnormalities on radiographic examination in addition to clinical signs. All cats had bronchial or bronchointerstitial lung patterns on radiographic examination on day 0, matching results of previous studies.9, 10, 22, 23 Accordingly, in the published literature, 9% to 94% of cats with FLAD showed radiographic changes.9, 22 In contrast, other authors have reported 17% to 23% of cats with FLAD as having unremarkable thoracic radiographs.10, 24
Alveolar infiltrates were only documented in 1 cat at each examination time point, which corresponds to a previous study in which alveolar infiltration was reported rarely.10 In contrast, another investigation showed alveolar patterns in 44% of cats with lower airway disease.25 Cats responding to antibiotics were included in that study, and concurrent bacterial respiratory tract disease resulting in alveolar patterns could not be excluded.
On day 0, lung hyperinflation was detected in 41.7% cats, with a more frequent occurrence compared with previous reports.10, 24, 25 The reason for more frequent hyperinflation, defined as an abnormal increase in lung volume at the end of tidal expiration,26 in our study is unclear. Lower airway obstruction can lead to hyperinflation of the lungs, because the affected cats are unable to fully exhale because of narrowed airways, resulting in air trapping and causing expiratory respiratory distress.6, 10 Thus, during episodes of respiratory distress, lung hyperinflation may be present, but between these episodes it may remain undetected radiographically.27 Discrepancies among different study results could be explained by differences in clinical stability when the radiographs were taken. Hyperinflation on radiographic imaging may have been more frequently detected, because only cats with obvious current clinical signs (including episodes of respiratory distress) were included in our study, and 70% of cats with radiographic findings of hyperinflation on day 0 showed obvious signs of respiratory distress before enrollment. Another study observed lung hyperinflation in cats with FLAD as the third most common radiographic abnormality,7 which is more consistent with our present data. This finding is somewhat subjective, and may be variably identified by different radiologists.
Some authors have reported that approximately 10% of cats with FLAD have atelectasis of the middle right lung lobe on radiographs, because of its dorsoventral orientation within the bronchial tree, which allows mucus to accumulate easily.3 Our study identified this radiographic abnormality in only 1 cat on day 0. However, it was no longer visible during treatment, consistent with results reported in other studies.10, 25
Comparing the prevalence of hyperinflation and atelectasis on radiographs among studies is challenging, because these pathological changes often were not reported in prior investigations. Furthermore, a standardized radiographic score would be necessary for direct comparison.
In addition to improvement in clinical variables, the total radiographic score improved significantly during treatment. This finding matches results of a previous investigation of research cats suffering from mild CB, treated with inhaled fluticasone (250 μg; q24h), after 2 weeks of treatment.16 Besides the total score, our current results show clinically relevant improvement in both bronchial and interstitial lung patterns. In comparison, a previous study15 did not report any improvement in radiographic variables in 9 cats with FLAD after PO (prednisolone, 5 mg q12h; and decrease after 14 days to prednisolone, 5 mg q24h in 4/9 cats) or combined PO and inhaled (prednisolone, 5 mg q24h PO for 7 days, followed by fluticasone 110 μg q12h in 5/9 cats) glucocorticoid treatment in a preliminary study. Because the follow-up examination during treatment also took place after 8 weeks, it is unclear why the results differed from those of our study. The small study sample and potential differences in disease severity could account for the discrepancy.15
Our study showed more improvement in the bronchial lung pattern as compared to the interstitial lung pattern. This result may indicate that bronchial changes improve more rapidly with adequate treatment than do interstitial changes. A possible explanation might be that cats with advanced FLAD are more likely to show interstitial lung patterns as a result of extension of the inflammation over the borders of the bronchial walls into the pulmonary interstitium,28 and therefore require more time to recover. For this hypothesis to be supported, it would be necessary to examine follow-up radiographs after a longer period of treatment.
Although not significant, the number of patients with hyperinflation and atelectasis on radiographic examination decreased over the study period. Because hyperinflation was still radiographically visible in 7/10 originally affected cats on day 60 despite treatment, in some cases treatment was modified on day 60 by adding a bronchodilator. A possible explanation for the fact that some cats continued to show radiographic hyperinflation even with treatment could be discontinuation of the bronchodilator after several weeks, because bronchodilators were administered only initially in most cases. However, because these cats showed further clinical improvement on day 60, with complete absence of respiratory distress in 6/7 cats and existing but marked improvement in respiratory distress episodes in 1/7 cats, it is also possible that radiographic findings lag behind clinical status, and may further improve with prolonged consistent treatment. In addition, ongoing subclinical inflammation could not be ruled out in our study, because a second BALF was not obtained on day 60 because of the risk involved in repeating the procedure under anesthesia in client-owned cats.
Cats with positive Mycoplasma spp. PCR on BALF examination were excluded from the study, because the pathogenicity and clinical relevance of these microorganisms in the context of FLAD are not yet clearly understood, and our aim was to investigate a population that was as homogeneous as possible. Whether the absence of Mycoplasma ssp. contributed to greater improvement in radiographic findings in our study and whether Mycoplasma spp. in the lower airways leads to more severe abnormalities on radiographs cannot be answered within the context of this study and should be further investigated in the future.
A correlation was found between the percentage of neutrophilic granulocytes in BALF and total clinical score, auscultation abnormalities and severity of bronchial lung pattern on day 0. This finding contradicts a previous study that did not show a correlation between clinical or radiological findings and BALF variables.20 In addition, we were able to show a negative correlation between percentage of macrophages in the BALF and coughing frequency and severity of bronchial pattern on radiography, which suggests that a higher coughing frequency and more severe bronchial pattern are associated with lower numbers of macrophages in the BALF. Because macrophages are the predominant cell type in the BALF in healthy cats,5 this result is not surprising. Thus, in cats suffering from FLAD with evidence of coughing and bronchial lung pattern on radiographs, lower numbers of macrophages and higher numbers of neutrophils or eosinophils are to be expected in BALF cytology. The total cell count in the BALF correlated only with the prevalence of lung hyperinflation. This observation contradicts the results of a previous study in which total cell count showed a significant correlation with almost all radiographic scores in experimentally-induced bronchial inflammation in cats.29 However, this study included both Ascaris suum-sensitized cats, as well as a healthy control group, which limits comparison with the findings of our study.
To investigate the relationship between clinical signs and radiological findings in cats with FLAD, the changes in the 12-point clinical score and the 10-point radiographic score over the study period were analyzed for correlation. No clinically relevant correlation was found between the clinical and radiographic variables, in accordance with results of previous studies.24, 25 In humans with asthma, a correlation between radiographic abnormalities and clinical signs also could not be proven.30 This result stresses the importance of reconciling radiographic abnormalities with medical history, physical examination and laboratory data at all times. Why the changes in clinical and radiographic variables failed to show a relevant correlation despite improvement in both scores over the study period remains unknown. A possible explanation could be that clinical signs likely respond faster to adequate treatment compared to radiological findings. Seven cats continued to show pulmonary hyperinflation radiographically at the follow-up examination despite substantial clinical improvement, which supports this assumption. Further evaluation of this finding would require more frequent follow-up examinations at shorter time intervals after initiation of treatment, which would be challenging in client-owned cats.
However, radiographic findings did improve as clinical signs improved, suggesting that radiographic examination is a sensitive tool for monitoring treatment response in cats with FLAD. Assessing therapeutic response by radiographic examination makes sense especially for cats where clinical evaluation may be limited (eg, in cats that spend most of the day unattended or outdoors). In addition, it is known that clinical signs can vary in cats with FLAD. Radiographic examination therefore may help assess treatment response and optimization in clinically healthy cats.
The lack of a standardized treatment protocol is a limitation of our study. We deliberately decided against a standardized approach to treatment, because individualized treatment is more responsive to the individual needs of the cats, as well as the owners. This approach mimics the situation in clinical practice, in which an individual approach to each patient is essential. In addition, all radiographs were interpreted by the same radiologist, which may involve a certain degree of subjectivity, and it was not possible to investigate interobserver variability. Furthermore, radiographic imaging during the inspiratory phase of the respiratory cycle is not always possible in cats that are awake, which may have influenced interpretation of the radiographs. Because radiographic examinations were performed exclusively on unsedated awake cats, optimal positioning could not always be guaranteed, which also may have had an impact on the quality of the radiographs. However, this situation reflects the reality in veterinary practice in many countries, in which cats are usually radiographed unsedated.
5 CONCLUSION
In addition to clinical signs, radiographic abnormalities could be detected in all cats with FLAD at initial presentation. With individualized treatment, both clinical and radiographic variables improved significantly. However, we failed to show a correlation between the improvement of clinical and radiographic findings. The results indicate that, in addition to clinical signs, repeated radiographic examination can be used to assess treatment response in cats with FLAD. However, differences exist in how clinical and radiographic variables improve in cats with FLAD undergoing treatment.
ACKNOWLEDGMENT
No funding was received for this study. Part of the study was presented orally at the European College of Veterinary Internal Medicine – Companion Animals (ECVIM-CA) 32rd Annual Congress, September 01-03, 2022, Gothenburg, Sweden. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Ethics Committee of the Centre for Clinical Veterinary Medicine of Ludwig Maximilian University, Munich (No. 139-20-07-2018) and informed owner consent was obtained for all cats before enrolment.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




