Osteopontin and fibronectin in lung tissue, serum, and bronchoalveolar lavage fluid of dogs with idiopathic pulmonary fibrosis and control dogs
[Correction added after first online publication on 17 November 2023. Corrected the spelling of Mutien-Marie Garigliany.]
Abstract
Background
Idiopathic pulmonary fibrosis (IPF) affects West Highland white terriers (WHWTs). Osteopontin (SPP1) and fibronectin (FN1) are associated with human IPF and are overexpressed by bronchoalveolar lavage fluid (BALF) macrophages in dogs with IPF.
Objective
To investigate the value of these proteins as biomarkers of IPF.
Animals
West Highland white terriers (WHWTs) with IPF, control WHWTs, and terriers.
Methods
Cross-sectional observational study. Immunohistochemistry was used to localize SPP1 and FN1 in lung tissue. Serum and BALF SPP1 and FN1 concentrations were measured using canine ELISA kits and compared between groups.
Results
Osteopontin stained ciliated epithelial cells, smooth muscular cells, and macrophages of all included dogs, and type-II pneumocytes and extracellular matrix of all 12 diseased WHWTs, 4/6 control WHWTs, and none of the 3 terriers. Osteopontin serum concentration was higher in diseased WHWTs (n = 22; 2.15 ng/mL [0.74-5.30]) compared with control WHWTs (n = 13; 0.63 ng/mL [0.41-1.63]; P = .005) and terriers (n = 15; 0.31 ng/mL [0.19-0.51]; P < .0001), and in control WHWTs compared with terriers (P = .005). Osteopontin BALF concentrations were higher in diseased (0.27 ng/mL [0.14-0.43]) and control WHWTs (0.25 ng/mL [0.14-0.40]), compared with terriers (0.02 ng/mL [0.01-0.08]; P < .0001 and P = .003, respectively). Fibronectin (FN1) serum concentrations were lower in diseased dogs (1.03 ng/mL [0.35-1.48]) and control WHWTs (0.61 ng/mL [0.24-0.65]) compared with terriers (2.72 ng/mL [0.15-5.21]; P < .0001 and P = .0001, respectively). There was no difference in FN1 immunostaining and FN1 BALF concentrations between groups.
Conclusions
Results suggest that SPP1 is involved in pathogenesis of IPF and could predispose that breed to the disease. Osteopontin serum concentration could serve as a diagnostic biomarker of IPF.
Abbreviations
-
- 6MWD
-
- 6-minute walked distance
-
- 6MWT
-
- 6-minute walking test
-
- AUC
-
- area under the curve
-
- BALF
-
- bronchoalveolar lavage fluid
-
- 95% CI
-
- 95% confidence interval
-
- ECM
-
- extracellular matrix
-
- F
-
- female
-
- FN1
-
- fibronectin
-
- HRCT
-
- high-resolution computed tomography
-
- IPF
-
- idiopathic pulmonary fibrosis
-
- IQR
-
- interquartile range
-
- M
-
- male
-
- PaO2
-
- arterial partial pressure of oxygen
-
- PBS
-
- phosphate-buffered saline
-
- PCIIs
-
- type-II pneumocytes
-
- ROC
-
- receiver operating characteristics
-
- SPP1
-
- osteopontin
-
- TCC
-
- total cellular count
-
- WHWTs
-
- West Highland white terriers
1 INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) affects old West Highland white terriers (WHWTs) and is characterized by lung interstitial collagen deposition which progressively deteriorates lung function and ultimately leads to respiratory failure.1-5 It shares similar features with human idiopathic pulmonary fibrosis (IPF).4-6 IPF diagnosis relies on the presence of compatible clinical signs, thoracic high-resolution computed tomography (HRCT) findings, and the exclusion of other lung and cardiac diseases.4-6 Diagnosis is confirmed by histopathological examination of lung tissue which is usually obtained after the animal death.4-6 No validated biomarkers are available to facilitate the diagnosis and the follow-up of the disease as well as to assess dog's prognosis.4, 5 Thus, it appears useful to identify reliable markers and therapeutic targets against IPF for which only symptomatic treatments are currently available.4, 5
Recently, profibrotic monocytes and monocyte-derived macrophages clusters have been identified in bronchoalveolar lavage fluid (BALF) of dogs affected with IPF and are suspected to play a role in pathogenesis of IPF as they are enriched in transcripts playing a role in fibrosis development.7 Profibrotic transcripts of interest preferably expressed by those monocytes/macrophages include among others osteopontin (SPP1) and fibronectin (FN1).7
SPP1 is an extracellular matrix (ECM) glycoprotein that promotes fibroblasts proliferation and ECM development and activates myofibroblasts, especially by inducing and activating TGF-β1 which is 1 of the major drivers of fibrosis.8-11 In humans, SPP1is a biomarker of pulmonary fibrosis and a target for future promising therapies.8, 10-13 FN1 is also a glycoprotein and is 1 of the most dominant components of the ECM.14, 15 It mediates cell-matrix adhesion by binding ECM proteins, stimulates epithelial-mesenchymal transition of alveolar epithelial cells and promotes fibroblasts recruitment and myofibroblast differentiation causing lung architectural distortion.8, 14, 16, 17 FN1 is also found in abundance in the lung tissue of IPF human patients.14
The aim of this study was to investigate the potential of these glycoproteins as potential biomarkers of IPF disease by measuring their concentrations in serum and BALF, determining cut-off values for diagnosis of IPF, and localizing them by immunohistochemistry on lung tissue. In addition, SPP1 and FN1 concentrations in serum and BALF of WHWTs affected with IPF were correlated to markers of disease severity namely the 6-minute walked (6MWD) and the arterial partial pressure of oxygen (PaO2). To reach this goal, 3 groups were compared: WHWTs affected with IPF, control WHWTs, and control terriers other than WHWTs. We hypothesized that SPP1 and FN1 concentrations will be higher in WHWTs affected with IPF compared to control dogs and will correlate to markers of disease severity in WHWTs with IPF. Osteopontin and FN1 could also potentially be higher in control WHWTs compared to control terriers.
2 MATERIALS AND METHODS
2.1 Study samples
2.1.1 Lung tissue
FN1 and SPP1 immunohistochemical assessment was performed on lung tissue samples obtained from 12 WHWTs affected with IPF (diagnosis confirmed on histological evaluation), 6 control WHWTs, and 3 control terriers (Table 1). Control dogs were euthanatized for diseases other than respiratory disease and had normal lung architecture on histopathological examination performed by 1 of 2 European board-certified veterinary pathologists (MJD and MMG). Causes of death in the group of control dogs were various (Table 1). Dogs were enrolled in this cross-sectional observational study between April 2008 and April 2020 under the umbrella of the project about IPF in WHWTs conducted in the Small Animal Veterinary Clinic of the University of Liège and approved by the ethical committee of the University of Liège (approval numbers #1435 and #2245). All dogs were privately owned and were recruited with the written owner's consent.
| WHWTs affected with IPF (n = 12) | Control WHWTs (n = 6) | Control terriers (n = 3) | |
|---|---|---|---|
| Sex, M/F | 7/5 | 3/3 | 3/0 |
| Age, years | 12 (11-14) | 12 (11-13) | 15 (10-15) |
| Body weight, kg | 10.0 (9.4-10.8) | 7.8 (7.5-9.1) | 7.0 (5.9-8.2) |
| Breeds | WHWTs | WHWTs | Jack Russel terriers (n = 2), Yorkshire terrier (n = 1) |
| Cause of death | IPF | AKI (n = 2), mammary tumor, IMTP, chronic intestinal disorders, and neurological symptoms | Aggressiveness, severe lameness, liver mass |
- Note: Data are expressed as median and interquartile range.
- Abbreviations: AKI, acute kidney injury; F, female; IMTP, nonassociative immune-mediated thrombocytopenia; IPF, idiopathic pulmonary fibrosis; M, male; WHWT, West Highland white terrier.
2.1.2 Serum and BALF
Serum and BALF samples were obtained from dogs of the WHWT breed either affected with IPF (n = 22) or control (n = 13) and control dogs from other terrier breeds (n = 15) also enrolled in the study under the umbrella of the project about IPF in WHWTs (approval ethical numbers #1435 and #2245) between April 2013 and May 2020. Most dogs (15/22) were different from dogs used for immunohistochemical assessment of lung tissue. Principal characteristics of the groups are reported in Table 2.
| WHWTs affected with IPF (n = 22) | Control WHWTs (n = 13) | Control terriers (n = 15) | P-value | ||
|---|---|---|---|---|---|
| Sex, M/F | 10/12 | 9/4 | 10/5 | .164 | |
| Age, years | 11 (9-12)*,** | 9 (7-11)* | 8 (7-10)** | .002* .008** |
|
| Body weight, kg | 9.6 (8.8-10.8)* | 9.2 (8.4-9.8) | 6.5 (5.3-7.4)* | .0001* | |
| Breeds | WHWTs | WHWTs | Jack Russel terriers (n = 10), Yorkshire terriers (n = 5) | ||
| BALF | TCC, cells/μL | 2050 (1528-2755)*,** | 760 (447-930)* | 410 (235-573)** | .002* <.0001** |
| Macrophages, % | 78 (64-84) | 72.5 (55.8-78.8) | 79 (72-83) | .431 | |
| Neutrophils, % | 12 (7-17)* | 5 (3-8)* | 9 (7–10) | .001* | |
| Lymphocytes, % | 6 (3-16)* | 15.5 (12.8-34)* | 11 (9-13) | .011* | |
| Eosinophils, % | 1 (0-1) | 0 (0-2.3) | 1 (0–2) | .950 | |
| 6MWD, m | 374.1 (356.1-432) | 482.4 (452.4-516.4) | / | <.0001 | |
| PaO2, mmHg | 64.1 (59.2-68.2) | 86.7 (83.3-95.4) | / | .014 | |
- Note: Data are expressed as median and interquartile range. In West Highland white terriers (WHWTs) affected with idiopathic pulmonary fibrosis (IPF) and control WHWTs, the 6-minute walked distance (6MWD) was evaluated in 21 and 10 dogs, the arterial partial pressure in oxygen (PaO2) in 20 and 6 dogs. Stars indicate paired statistical differences between groups when more than 2 groups were compared. A P-value < .05 is considered statistically significant.
- Abbreviations: BALF, bronchoalveolar lavage fluid; M, male; F, female; TCC, total cell count; /, not assessed.
The diagnosis of IPF was established based on compatible clinical signs and physical examination and compatible thoracic HRCT images either alone (n = 13) or in combination with histopathological examination of lung tissue obtained after the animal's death (n = 9). Mean clinical signs duration at the time of the diagnosis was 9 months (range: 1-43 months). Clinical signs included exercise intolerance in 5/22 dogs (23%), cough in 4/22 dogs (18%), and a combination of both in 13/22 dogs (29%). Moreover, 11/22 dogs (50%) had dyspnea and 3/22 (14%) exhibited cyanosis. At study inclusion, 4 dogs were under treatment and received respectively theophylline (Xanthium, SMB S.A., Bruxelles, Belgium), theophylline (Xanthium, SMB S.A., Bruxelles, Belgium), and codeine (Paracodine, Teofarma S.r.l., Pavia, Italy), sildenafil and propentofylline (Vitofyllin, WDT, Garbsen, Germany), and prednisolone. At physical examination, crackles were heard on thoracic auscultation in all dogs affected with IPF, and a positive laryngotracheal reflex was reported in 7/22 dogs (32%). A 6-minute walking test (6MWT) was performed in 21/22 WHWTs affected with IPF (Table 2). Echocardiography was performed in all dogs and excluded primary cardiac disorder. An arterial pulmonary hypertension was reported in 54% (12/22) of dogs with IPF, based on the estimation of the systolic pulmonary artery pressure derived from the peak tricuspid regurgitation velocity, the pulmonary vein-to-right pulmonary artery ratio and on the appearance of anatomic echocardiographic sites, such as ventricles, pulmonary artery, right atrium, and caudal vena cava.18, 19 Results about PaO2 (n = 20) and BALF analysis (n = 22) are reported in Table 2.
The control status of WHWTs and dogs from other breeds than WHWT was determined based on complete history, physical examination, blood analysis (hematology and biochemistry), bronchoscopy, and BALF analysis. No dogs were receiving treatment at their inclusion. Results of the BALF cell analysis are reported in Table 2. In addition, in control WHWTs, a thoracic HRCT was performed at the time of sampling in 11/13 dogs and did not reveal any abnormalities except the presence of a grade 1 tracheal collapse in 3/11 dogs. The remaining 2 other control WHWTs without thoracic HRCT underwent normal histopathological examination of lung tissue after death for 1 dog and normal thoracic radiography at the time of sampling for the other. A 6MWT was also performed in 10/13 control WHWTs, PaO2 was available in 6/13 control WHWTs, and echocardiography was performed in 11/13 control WHWTs without any abnormalities (Table 2).
2.2 Samples collection
Lung tissue biopsies were collected within 1 hour of euthanasia, and directly fixed in 3.5% neutral buffered formalin for 24 to 72 hours, embedded in paraffin wax, and stored until use.
Serum and BALF samples from WHWTs affected with IPF collected at the time of the diagnosis were used. Blood samples were collected from the jugular vein in plain tubes. After clotting, tubes were centrifuged at 4°C for 15 minutes at 1300×g. Serum was collected, transferred into 1.5 mL plastic cryotubes, and stored at −80°C until analysis. Bronchoalveolar lavage was performed through flexible Pediatric Video-74 Bronchoscope EB-530S (FUJINON©) as already described and BALF total and differential cell count analyses were done as previously described directly after sampling.20 BALF supernatants were kept at −80°C until use.
2.3 Immunohistochemistry
For immunohistochemical study, the lung sections were sectioned at 5 μm, dewaxed with xylene, and rehydrated through graded concentrations of ethanol to distilled water. Antigen retrieval was undertaken by microwaving sections in 10 mM citrate buffer, pH 6.0, for 15 minutes. Tissues were incubated with 0.30% hydrogen peroxide for 5 minutes at room temperature to inhibit endogenous peroxidase activity and subsequently washed with distilled water and then phosphate-buffered saline (PBS) for 5 minutes. To reduce nonspecific staining, sections were blocked with 0.25% casein (Protein block, #X0909, Dako, Carpinteria, CA) in PBS for 10 minutes. Tissue sections were then incubated in a humid chamber overnight at 4°C with the primary antibodies directed to SPP1 (dilution 1:1000, #Ab8448, Abcam, Waltham, MA) and mouse Fc block (dilution 1:1000, BD Pharmingen, Erembodegem, Belgium) or directed to FN1 (dilution 1:1000, #Ab2413, Abcam, Waltham, MA) and bovine serum albumin at 0.50%. As negative controls, primary antibodies were substituted with rabbit preimmune serum at similar antibody concentration. As positive control a dog's cartilage sample was used.21-23 Sections were washed with PBS 3 times for 5 minutes and incubated with ImmPress (Peroxidase) Polymer Anti-Rabbit IgG Reagent (#MP-7451, Vector Laboratories, Newark, CA) for 30 minutes. After washing with PBS 2 times for 5 minutes, the ImmPACT AEC (3-amino-9-ethylcarbazole) HRP Substrate (#SK-4205, Vector Laboratories, Newark, CA) was applied as the chromogen for 10 minutes. Sections were subsequently washed in distilled water for 5 minutes and counterstained with Mayer's hematoxylin for 2 minutes. The slides were washed in water, mounted with commercial mounting medium (Glycergel mounting medium, Dako, Carpinteria, CA), and evaluated by light microscopy.
2.4 Protein concentration analysis in serum and BALF
Serum and BALF supernatant measurement of SPP1 and FN1 concentrations were performed using commercially available canine quantitative sandwich ELISA kits (ELISA kit for osteopontin, #SEA899Ca, Cloud-clone Corporation, Wuhan, China; FN1 ELISA kit, #OKEH03951, Aviva Systems Biology Corporation, San Diego, CA), according to the manufacturer's instructions and tested in duplicated wells. Serum samples were diluted twice for SPP1, but not diluted for FN1 measurement. Supernatant BALF samples were neither diluted for SPP1, nor for FN1 measurement.
2.5 Statistical analysis
Statistical analyses were performed using XLstat software (Addinsoft SARL, Paris, France). Osteopontin or FN1 concentrations below the detection limit of the ELISA kit were excluded from analyses. Normal distribution of the data was tested using the Shapiro-Wilk test. A Kruskal-Wallis test with Conover-Iman test and Bonferroni-corrected P values for multiple pairwise comparisons was used to test group differences if more than 2 groups were compared. A Mann-Whitney test was used to test group differences if only 2 groups were compared. Data were expressed as median and interquartile range (IQR). Proportions were compared using Chi-squared test or Fisher exact test if <5 data per group. Correlations between glycoprotein concentrations and 6MWD and PaO2 were assessed in WHWTs with IPF using Spearman test.
In case of differences in SPP1 or FN1 concentrations between groups, receiver operating characteristics (ROC) curves were created to assess the ability of SPP1 or FN1 concentrations to diagnose IPF in all dogs and in control WHWTs and WHWTs affected with IPF. The area under the curve (AUC) with the 95% confidence interval (95% CI) and the SPP1 or FN1 concentrations that resulted in the greatest combination of sensitivity and 1-specificity were identified using easyROC software (v.1.3.1).
For all analyses, P-value ≤ .05 was considered statistically significant.
3 RESULTS
3.1 SPP1 and FN1 immunoreactivity in lung tissues
Diffuse mature fibrosis was present in the lung interstitium of WHWTs affected with IPF (Figure 1A,E,G,H,I) compared to control dogs (Figure 1B-D), accompanied with some degree of type-II pneumocytes (PCIIs) hyperplasia and atypia (Figure 1H,I) in WHWTs affected with IPF.
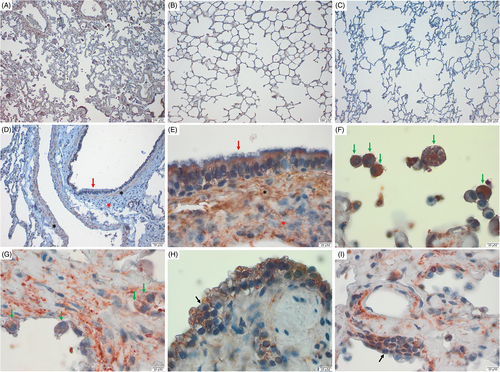
On histological sections of lung tissue from all dogs either IPF-affected or control, immunostaining for SPP1 was observed in ciliated epithelial cells (Figure 1D,E), smooth muscular cells surrounding large vessels, and bronchi/bronchioles (Figure 1D,E) and some alveolar macrophages (Figure 1F,G). The PCIIs (Figure 1H,I) and the ECM (Figure 1A,E,G,I) were labeled in all WHWTs affected with IPF, 4/6 (67%) control WHWTs (Figure 1B), and none of the control terriers (Figure 1C,D).
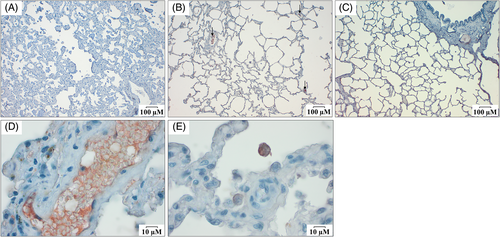
For FN1 immunostaining, the lung interstitium appeared slightly labeled (Figure 2A-C) as well as some alveolar macrophages (Figure 2E) in all groups. A strong FN1 labeling was identified in blood vessels which probably corresponds to plasmatic FN1 associated with fibrin (Figure 2D). Differences in FN1 immunoreactivity in lung tissue sections between groups were not noticed.
3.2 Protein concentrations
Results about SPP1 and FN1 concentrations in serum and BALF are presented in Figure 3.

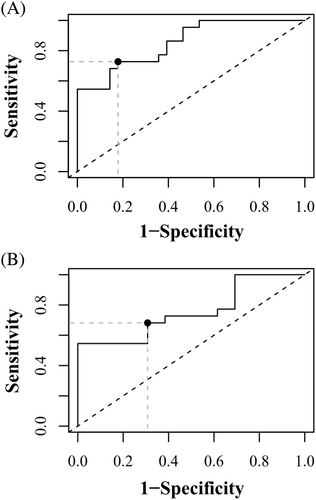
SPP1 serum concentration was higher in WHWTs affected with IPF (median 2.15 ng/mL and IQR [0.74-5.30]) in comparison with control WHWTs (0.63 ng/mL [0.41-1.63]; P = .005) and control terriers (0.31 ng/mL [0.19-0.51]; P < .0001). Higher serum concentrations were also found in control WHWTs compared with control terriers (P = .005). A cut-off value of 1.17 ng/mL can be used to differentiate WHWTs affected with IPF from all control dogs with a specificity of 82.1% and a sensitivity of 73% (AUC of 85% [CI 95%: 75-96]; P < .0001; Figure 4A). When only the WHWTs are considered, a cut-off value of 1.27 ng/mL can be used to differentiate WHWTs affected with IPF from control WHWTs with a specificity of 69.2% and a sensitivity of 68% (AUC of 76% [CI 95% 59-92%], P = .0002; Figure 4B).
In BALF, 37% of the samples tested for SPP1 were below the detection limit of the ELISA kit. Results above the detection limit were recorded in 14 WHWTs with IPF (64%), 5 control WHWTs (38%), and 11 control terriers (73%). The frequency of positive results did not differ between the groups (P = .154). Higher BALF SPP1 concentration was found in WHWTs affected with IPF (0.27 ng/mL [0.14-0.43]) and control WHWTs (0.25 ng/mL [0.14-0.40]) compared with control terriers (0.02 ng/mL [0.01-0.08]; P < .0001 and .003, respectively). No significant difference was found between WHWTs with IPF and control WHWTs (P = .953; Figure 3).
Serum FN1 concentration was undetectable in 4 of the 22 WHWTs affected with IPF (18%) and in 3 of the 13 control WHWTs (23%). Fibronectin concentration was higher in control terriers (2.72 ng/mL [2.15-5.21]) compared with control WHWTs (0.61 ng/mL [0.24-0.65]; P = .0001) and WHWTs affected with IPF (1.03 ng/mL [0.35-1.48]; P < .0001; Figure 3). No difference in FN1 concentration was found between control and IPF-affected WHWTs (P = .625; Figure 3).
No differences were found in BALF FN1 concentrations between control terriers (1.36 ng/mL [0.65-1.56]), control WHWTs (0.92 ng/mL [0.86-1.30]), and WHWTs affected with IPF (1.72 ng/mL [0.94-1.95]; P = .134; Figure 3).
3.3 Correlations with the 6MWD and the PaO2
Correlations between the 6MWD and the PaO2 and SPP1 and FN1 concentrations in serum and BALF from WHWTs affected with IPF are reported in Table 3.
| Serum SPP1 | BALF SPP1 | Serum FN1 | BALF FN1 | |||||
|---|---|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | r | P-value | |
| 6MWD (n = 21/22) | .129 | .575 | −.027 | .935 | .201 | .438 | −.094 | .686 |
| PaO2 (n = 20/22) | −.463 | .041a | .104 | .737 | .026 | .925 | .191 | .418 |
- Abbreviations: 6MWD, 6-minutes walked distance; BALF, bronchoalveolar lavage fluid; FN1, fibronectin; IPF, idiopathic pulmonary fibrosis; PaO2, arterial partial pressure in oxygen; r, Spearman coefficient of correlation; SPP1, osteopontin; WHWTs, West Highland white terriers.
- a Statistically significant result.
4 DISCUSSION
The present immunohistochemical study revealed that SPP1 was mostly expressed in WHWT dogs in PCIIs and in the lung ECM. Osteopontin concentration was greater in the serum of WHWTs affected with IPF, when compared with control WHWTs and terriers. A higher concentration was also found in control dogs from the WHWT breed compared with control dogs from other terrier breeds in BALF and serum. Finally, serum SPP1 concentration moderately negatively correlated with PaO2, a marker of decreased cardiopulmonary function. None of the hypothesized modifications in FN1 were confirmed.
In all dogs with IPF, SPP1 immunoreactivity was present in PCIIs. This was also observed in most of the control WHWTs, but in none of the control terriers. In addition, PCIIs showed a marked hyperplasia in diseased dogs. In dogs with IPF, the presence of a high proportion of hypertrophic and hyperplastic PCIIs is described.6 Those atypical cells are often localized in areas of more severe interstitial fibrotic changes suggesting a role of PCIIs in development of IPF.6 In human with IPF, PCIIs are involved in fibrotic process.24, 25 Hyperplastic PCIIs, which are also localized near honeycombed regions of the lung of patients with IPF, have impaired renewal capacity and produce pro-fibrotic and pro-inflammatory factors that contribute to fibrogenesis.24, 25 In human IPF and murine bleomycin-induced lung fibrosis models, there is SPP1 overexpression in PCIIs.10, 12 In PCIIs cell culture exposed to bleomycin, doxorubicin, or tunicamycin, SPP1 expression is increased, promotes proliferation of other PCIIs and migration of lung fibroblasts, and induces further SPP1 production that might be associated with the abnormal repair contributing to fibrogenesis.26 In dogs with IPF, the exact role of PCIIs in fibrosis development and progression remains to be studied but based on our results, SPP1 expression by PCIIs could represent 1 of the mechanisms at the origin of IPF development, perpetuation, or both.
In the WHWT breed, 1 hypothesis would be that all dogs with still unknown specific genetic particularities and submitted to specific environmental triggers will ultimately develop the disease, unless they die from another disease.4 The immunoreactivity found in PCIIs of WHWTs considered as control in this study might be an indicator of early disease or disease predisposition.
SPP1 immunoreactivity was also found in smooth muscular cells, ciliated epithelial cells, and macrophages in all lung samples. In human and lung fibrosis mouse models, macrophages overexpressing SPP1 are suggested to activate myofibroblasts and to promote ECM production and hence the development of fibrosis in the lung.8-11, 27 Although no difference in macrophages labeling was observed between groups, slight differences between the number of macrophages and the amount of SPP1 production by macrophages might be present and contribute to IPF development since it is suggested in a study assessing the expression of SPP1 in macrophages between healthy and diseased WHWTs.7
Pulmonary hypertension, which was identified in 54% of WHWTs with IPF, might also be 1 of the factors that explained the higher SPP1 concentration in WHWTs with IPF compared with healthy WHWTs. Indeed, in humans, the presence of pulmonary hypertension is associated with higher circulating SPP1 levels compared to healthy patients.28 However, development of pulmonary hypertension might also be secondary to the higher SPP1 level found in dogs with IPF as SPP1 might induce vascular remodeling.28
SPP1 was higher in the serum of WHWTs affected with IPF compared with control WHWTs and other control terriers and could be used as a diagnostic biomarker with a moderate diagnostic accuracy. Moreover, the use of SPP1 in conjunction with other previously published promising diagnostic biomarkers29-35 might be useful to increase the sensitivity and the specificity for diagnosis of IPF.
The higher concentrations of SPP1 found in serum and BALF of control WHWTs compared with control terriers might also be among the factors that contribute to the development of the disease in that breed. Indeed, SPP1 promotes fibrogenetic capacities including angiogenesis, cell proliferation, cell adhesion, cell activation, cell survival, cell cytokine expression as well as cytoskeletal-related functions including cell motility and fusion.36, 37 Because of all these profibrogenic effects, SPP1 could be an interesting target for therapy which was already proposed in humans affected by IPF.11
Finally, SPP1 moderately correlated with PaO2, but not with the 6MWD, in the sub-group of WHWTs affected with IPF. PaO2 and 6MWD were selected as they have been proposed to monitor cardiopulmonary function and IPF progression.1 The correlation with PaO2 suggests a more pronounced gas diffusion impairment in WHWTs affected with IPF in association with high SPP1 blood value. As SPP1 expression and production have been showed to be induced by hypoxic conditions, that association seems logical.38
FN1 labeling in lung tissue was not found except in the lung interstitium, in some rare macrophages, and inside blood vessels. The labeling of FN1 in vessels is probably because of blood clots forming after death. Fibronectin cross-links to fibrin in the forming clot and participates in the stabilization of platelet-platelet interactions in thrombus.39 It indicates that the staining was successful, at least to label FN1 present in the plasma and produced by platelets during the clot formation.15 The reason why we were not able to demonstrate specific FN1 labeling in IPF WHWTs is unclear. Possible explanations for the slight labeling of lung interstitium in this study include that FN1 epitopes might have become unavailable when inserted into the lung ECM, or that some epitopes might have been destroyed during sample storage, or that high antibody dilution might have prevented FN1 detection. However, lower dilution of FN1 antibodies could not be used because they resulted in a too strong and nonspecific labeling.
The absence of differences found in FN1 concentration in BALF and serum might be related to high amounts of FN1 in the lung that stimulate its complexion into matrix preventing the detection of soluble FN1.14, 15 However, this hypothesis is in contradiction with immunohistochemistry results. Even if FN1 transcript is overexpressed in monocytes and macrophages in BALF collected from WHWTs affected with IPF,7 it could not be associated with an increase in protein secretion in serum and BALF in the present study.
Limitations of the present study were the lack of matched samples for SPP1 and FN1 detection in serum, BALF, and lung tissue from the same animals, which could have been interesting to correlate the different results from various biological material together. It is difficult to obtain lung tissue after animal death because owners do not always accept the sampling of the lungs even when euthanasia is scheduled. Moreover, death can occur abruptly preventing to collect the blood, BALF, and lung samples in an adequate time.
Another limitation of our study was that no quantification/scoring system was performed neither for SPP1 or FN1 immunoreactivity nor for establishment of the degree of fibrosis. Quantitative pathology software exists that establishes scoring analysis that would be interesting to compare with our immunohistochemical qualitative visual description.
Despite the complete history, clinical signs, and complementary examinations performed, it remains possible that subclinical diseases were missed, both in healthy and in dogs with IPF, that might have induced modifications in SPP1 and FN1 concentrations.40, 41 A full necropsy was not performed and only lungs were submitted to histopathological examination.
Finally, treatments like sildenafil or prednisolone received by some IPF-affected WHWTs could have altered at least SPP1 concentrations.42-45 However, doses administered were quite small (0.5 mg/kg twice a day and 0.3 mg/kg every 3 days, respectively) and exclusion of dogs receiving treatments from the analyses did not change the results (data not included).
In conclusion, this study showed that SPP1 but not FN1 has a potential in terms of diagnostic biomarker of the disease, although additional studies are required to validate the use of SPP1 in serum and BALF for such purposes. Besides the demonstration of higher serum and BALF SPP1 concentrations in the control WHWTs compared with terrier breeds can be related to a predisposition of this breed for development of IPF.
ACKNOWLEDGMENT
Funding provided by Personal Funds and Special funds for research – Uliège. The authors gratefully thank Joelle Piret from the Department of Morphology and Pathology of the University of Liège for her technical assistance for immunohistochemistry and ELISA analyses. The authors thank Phan Kim-Thu, Albert Belinda, Di Biago Eugenia, and Romijn Sylvain from the Department of Clinical Sciences of the University of Liège for their help in samples collection and storage.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the ethical committee of the University of Liège (approval numbers #1435 and #2245) with written owner's consent.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




