Localization and characterization of atrial depolarization waves on the surface electrocardiogram in dogs with rapid supraventricular tachycardia
Abstract
Background
Supraventricular tachycardias (SVTs), despite having various anatomical substrates and pathophysiological mechanisms, frequently show similar electrocardiographic presentations.
Objectives
To locate and characterize atrial deflections (ADs) on 12-lead electrocardiograms in dogs with sustained rapid SVT and assess the utility of different electrocardiographic variables in differentiating types of tachycardia.
Animals
Ninety-two dogs with orthodromic atrioventricular reciprocating tachycardia, 17 with atrial flutter, 33 with focal atrial tachycardia recorded and confirmed by electrophysiological study, and 40 dogs with sinus tachycardia.
Methods
Atrial deflection position on the 12-lead surface electrocardiogram was assessed according to the sequence of intracardiac activation. Its features were evaluated together with the relationship between AD and QRS complex interval (AD-R) and QRS complex and AD interval (R-AD).
Results
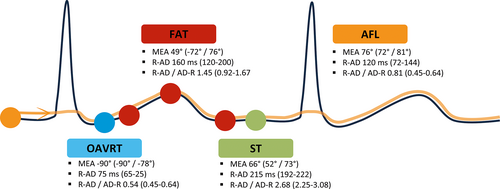
Orthodromic atrioventricular reciprocating tachycardia was characterized by an AD-AD interval of 213 ± 30 ms, mean electrical axis (MEA) of AD of −90 (−90/−78)°, R-AD interval of 75 (65-80) ms, and R-AD/AD-R of 0.54 (0.45-0.64). Atrial flutter was characterized by an AD-AD interval of 199 ± 57 ms, MEA of 76° (72/81), R-AD of 120 (72-144) ms, and R-AD/AD-R of 0.81 (0.63-1.13). Focal atrial tachycardia was characterized by an AD-AD interval of 270 ± 38 ms, MEA of 49 (−72/76)°, R-AD of 160 (120-200) ms, and R-AD/AD-R of 1.45 (0.92-1.67). Sinus tachycardia was characterized by an AD-AD interval of 292 ± 31 ms, MEA of 66° (52/73), R-AD of 215 (192-222) ms, and R-AD/AD-R of 2.68 (2.25-3.08).
Conclusions and Clinical Importance
Analyzing AD on 12-lead electrocardiogram is helpful in differentiating the most common SVTs in dogs.
Abbreviations
-
- AD
-
- atrial deflection
-
- AFL
-
- atrial flutter
-
- AUC
-
- area under the curve
-
- AV
-
- atrioventricular
-
- AVNRT
-
- atrioventricular nodal reciprocating tachycardia
-
- BPM
-
- beats per minute
-
- CS
-
- coronary sinus
-
- EPS
-
- electrophysiological study
-
- FAT
-
- focal atrial tachycardia
-
- MEA
-
- mean electrical axis
-
- NPV
-
- negative predictive value
-
- OAVRT
-
- orthodromic atrioventricular reciprocating tachycardia
-
- PJRT
-
- permanent junctional reciprocating tachycardia
-
- PPV
-
- positive predictive value
-
- ST
-
- sinus tachycardia
-
- SVT
-
- supraventricular tachycardia
1 INTRODUCTION
Supraventricular tachycardias (SVTs) have been defined in human medicine as tachycardias in which the mechanism involves tissue from the His bundle or above.1 Traditionally, the term SVT has been used to describe all types of tachycardia apart from ventricular tachycardia and atrial fibrillation. It has therefore included tachycardias such as orthodromic atrioventricular reciprocating tachycardia (OAVRT), which is not, in essence, a supraventricular rhythm.1 Specifically, sinus tachycardia (ST) is related to an enhanced normal automaticity of sinus pacemaker cells2; OAVRT results from the presence of an accessory pathway, which contributes to the formation of an anatomical macroreentrant atrioventricular (AV) circuit3, 4; atrial flutter (AFL) is an SVT that is perpetuated by large anatomical macroreentrant circuits in the right atrium or in the cranial vena cava5-7; focal atrial tachycardia (FAT) originates from ectopic foci in the atria or in the veins that are directly connected to the atria and are typically automatic8 and focal junctional tachycardia results from enhanced automaticity or triggered activity in the junctional area.9 Lastly, atrioventricular nodal reciprocating tachycardia (AVNRT) develops along an anatomical reentry circuit that includes the AV node and the surrounding atrial tissue,1 but has not been documented to occur spontaneously in the dog. Despite being characterized by different anatomical substrates and different pathophysiological mechanisms, SVTs often have similar electrocardiographic presentations; therefore, the electrocardiographic diagnosis can sometimes be challenging. The proper recognition of these tachycardias is of clinical importance because therapeutic options for SVTs vary according to the tachycardia type, and definitive treatment with radiofrequency catheter ablation is currently a viable option in dogs.3, 4
Endocavitary mapping has been performed during electrophysiological studies (EPS) in people to locate atrial deflections (AD) on superficial electrocardiograms and to define the relationships between AV and ventriculoatrial intervals.10-12 These data made it possible to build an efficient diagnostic algorithm, although surface ECG has sometimes failed to lead to a specific diagnosis.13 According to the European Society of Cardiology guidelines, the regularity of the RR interval first should be assessed.1 SVTs then should be classified as having short or long RP intervals; a very short ventriculoatrial interval in humans can be seen in AVNRT (in which the A is often nearly simultaneous with the V) or FAT (less common). Atrioventricular nodal reciprocating tachycardia typically has a VA interval that is shorter than the corresponding AV interval, but the A falls within the ST segment or T wave of the preceding QRS. Focal junctional tachycardia is a rare arrhythmia that also may have a short VA interval. A cut-off interval of 90 ms has been shown to be useful12 and is currently used in the diagnostic algorithm of the European Society of Cardiology.1 In veterinary medicine, a previous study analyzed the ECG appearance of narrow QRS complex tachycardias in a small sample of dogs affected by FAT and OAVRT. In this study, atrial depolarization wave position, axis on the frontal plane, heart rate, and presence of QRS alternans was useful in differentiating these 2 SVTs.14
We aimed to localize and characterize AD on a 12-lead ECG in a larger sample of dogs affected by different types of sustained SVTs and to assess their utility in differentiating the tachycardia type. We hypothesized that the mean electrical axis (MEA) and position of AD, defined using the R-AD interval and R-AD/AD-R ratio, would be useful in differentiating among the most common types of SVT.
2 MATERIALS AND METHODS
2.1 Study sample
We examined our hospital database retrospectively (from October 1, 2003 to October 1, 2021), and included the data of dogs affected by sustained SVT with a ventricular rate > 180 beats per minute (bpm) in which the ECG diagnosis was confirmed by EPS.
Breed, sex, age, body weight, previous and current antiarrhythmic treatments, and definitive diagnosis determined by EPS were obtained from medical records.
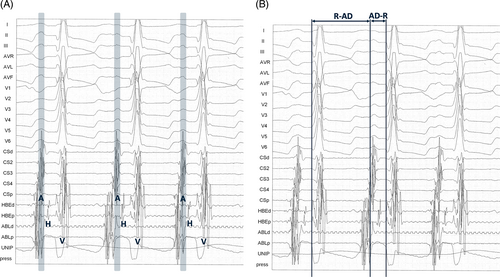
Twelve-lead surface ECG and intracardiac recordings were retrieved on an electrophysiologic system (EMS, 16 channels, Mennen Medical, Manta, Genova, Italy) and printed at a speed of 150 mm/s and amplitude of 1 mV/cm; all traces had to be simultaneously recorded on 1 sheet to allow for accurate identification of AD on a 12-lead surface ECG by referring to the sequence of intracardiac activation (Figure 1A). Surface and intracardiac electrograms were recorded with filter settings ranging from 50 to 500 Hz.
Regarding the EPS, antiarrhythmic drugs had to be discontinued for at least 5 elimination half-lives before the procedure; dogs with OAVRT that received constant rate infusions of IV lidocaine hydrochloride (Accord Healthcare, Milano, Italy) to control arrhythmias also were included (dose range 25-60 μg/kg/min) as long as lidocaine was stopped 60 minutes before the procedure. The anesthetic protocol for EPS was standardized for all patients: preanesthetic medication was administered as midazolam 0.2 mg/kg IV (Accord Healthcare, Milano, Italy), general anesthesia was induced using a 4 mg/kg IV bolus of propofol (Propovet, Zoetis, Roma, Italy), and all patients were maintained on a mixture of isoflurane (1%-2%) and oxygen (100%).
The precordial leads during the EPS had to be positioned according to Wilson's precordial lead system modified by Santilli et al, in which V1 is positioned at the first right intercostal space at the level of the costochondral junction, and the sixth intercostal space is used for all left-side leads (V2-V6).15, 16 All patients had to be positioned in right lateral recumbency during the EPS.
The following signals had to be available for analysis when analyzing intracardiac electrograms: 5 coronary sinus (CS) signals from a decapolar catheter placed into the great cardiac vein to provide mapping from CS proximal (CSp) to CS distal (CSd; Polaris X, 7-F, 2/5/2, Boston Scientific Corp, Genova, Italy); 2 His signals from a quadripolar catheter placed at the His bundle (Explorer 360, 5-F, 5/5/5, Boston Scientific Corp, Genova, Italy); 2 ablation site signals; 1 unipolar recording from the distal pole of the ablation catheter (Polaris C, 4 mm, 7-F, Boston Scientific Corp, Genova, Italy), and finally, 5 optional signals from a decapolar catheter positioned along the right atrial lateral wall to record potentials from the anterolateral aspect of the crista terminalis or high right atrium with the proximal pair of electrodes (HRA1-2), the mid-lateral right atrial (MRA) potentials with the middle pair, and the low posterior-lateral right atrial (LRA1-2) potentials with the distal pair (Polaris X, 7-F, 2/5/2, Boston Scientific Corp, Genova, Italy).
All ECG and EP recordings were evaluated for quality and lead-positioning adequacy by board-certified cardiologists (RAS and MP).
SVTs conducted with aberrancy (QRS complex duration > 80 ms) were excluded, as well as SVTs with heart rate < 180 bpm (focal junctional tachycardia and nonparoxysmal junctional tachycardia) and tracings with artifacts that prevented accurate measurements.
Group 1 was represented by dogs with OAVRT that were diagnosed based on previously established electrophysiological criteria.3, 4 Group 2 represented dogs affected by AFL.5-7 Dogs with sustained FAT were included in the third group.8 The fourth group included dogs with permanent junctional reciprocating tachycardia (PJRT).17 Finally, a control group of awake dogs with ST and heart rate > 180 bpm also was included. All patients had to be positioned in right lateral recumbency during the ECG. The presumptive diagnosis of ST was based on the P-wave axis on the 12-lead surface ECG, and on the behavior of the heart rate and RR interval during the 5 minutes.
2.2 Electrocardiographic measurements
Measurements were manually performed by a cardiology resident (SB) on printed tracings by using a caliper and ruler with 0.5 mm graduations on 3 randomly selected consecutive beats. Each measurement was included in the statistical analysis as a repeated measure.
The AD amplitude (mV) was measured in all 12 leads, and the AD axis on the frontal plane was calculated using the following equation: arctan (Iamp, aVFamp) × 180/π, where Iamp is the amplitude of AD in lead I, and aVFamp is the amplitude of AD in lead aVF.18
The R-AD interval was measured from the beginning of the QRS complex to the beginning of the AD, and the AD-R interval was measured from the beginning of the AD to the beginning of the QRS complex. The ratio between these 2 values (R-AD/AD-R) then was calculated (Figure 1B).
The AD position was classified into 4 groups: AD on R if AD was superimposed on the QRS complex, short R-AD if the R-AD interval was <50% of the RR interval, AD on T wave if AD was superimposed on the T wave, and long R-AD if the R-AD interval was >50% of the RR interval. The AD duration was measured in lead II. The AD-AD interval duration (ms) was measured from the beginning of AD to the beginning of the following AD. The AD-AD interval regularity then was assessed and classified as regular, cycle length irregularity (defined as variation of tachycardia cycle length, not on a beat-to-beat basis of ≥20 ms),14 or cycle length alternans (defined as beat-to-beat oscillation of tachycardia cycle length of ≥20 ms).14
The RR interval (ms) between 2 different R waves was measured and classified in the same way as regular, cycle length irregularity, and cycle length alternans.
The AV conduction ratio was assessed and classified as 1:1, 2:1, and variable.
For each ECG tracing, the appearance and duration of the QRS complex were analyzed to exclude SVTs conducted with aberrancy from the beginning. The presence of QRS alternans, which was defined as a beat-to-beat variation in QRS amplitude of ≥0.1 mV in at least 1 lead, was recorded.14
2.3 Statistical analysis
Statistical analysis was performed using a freeware statistical software package (JMP Pro, v15, SAS Institute, Milano, Italy).
Continuous data first were assessed for normality using the Shapiro-Wilk and Anderson-Darling tests.19 We considered data approximately normal in distribution if there was a failure to reject the null hypothesis for 1 of these tests. The data then were assessed for homoscedasticity using Levene's test.20
A repeated-measures mixed-model procedure for continuous data was applied.21 The model with fixed effects was the AD groups (OAVRT, AFL, FAT, ST). In all models, each animal was considered an experimental unit and used as a random variable.
The correlation between repeated measurements recorded in the same animal over time was assumed to be accounted for in the compound symmetry covariance structure.22
Thus, the residuals were checked for an approximately normal distribution. Variables that did not comply with the above assumptions were normalized using the BoxCox transformation.23
When significance was detected (P < .05), the results were separated using Tukey's multiple comparison test.
Normally distributed data are expressed as mean ± SD. Data with a nonnormal distribution are expressed as the median (25th/75th percentiles). To quantify the extent of the variability in the 3 measurements, the relative variability for R-AD interval was calculated by the ratio between the interquartile range (IQR) and median, and for AD-R interval using the ratio between the SD and mean.
Univariate analysis of nominal data was performed with a nominal logistic model by using the same model effect as in the previous analysis, and a Chi-square test was applied to assess differences among AD groups.24 Nominal data were expressed as occurrence probability percentages.
Variables that were statistically significant (P < .05) were considered relevant for multivariate analysis by using a stepwise logistic regression technique, with tachycardia type as the dependent variable and ECG criteria as the independent variables. The most parsimonious final model was selected using backward elimination with a Wald P-value of .05 as the removal threshold, given an acceptable log-likelihood ratio test value. Model fit was evaluated using Pearson's goodness-of-fit test and the Hosmer-Lemeshow test.
To assess discrimination and offer specificity and sensitivity prediction values, together with positive (PPV), and negative predictive values (NPV), areas under the receiver operating characteristic curve were employed for the variables that were significant in the multivariate analysis.
3 RESULTS
3.1 Study sample
The overall study sample consisted of 182 dogs, of which 92, 17, 33, and 40 were affected by OAVRT, AFL, FAT, and ST, respectively. Four dogs with PJRT were excluded from the analysis because of the small number of dogs in the group.
Among the dogs with OAVRT, 53 had a right posteroseptal accessory pathway, 26 had a right posterior accessory pathway, 11 had a right anterior accessory pathway, and 2 had a left posterior accessory pathway. Among the dogs with AFL, 12 had atypical AFL, 3 had typical AFL, and 2 had reverse typical AFL. Among the dogs with FAT, 23 had activation from the right atrial roof, 5 had activation from the right atrial floor, 3 had activation from the left atrial floor, and 2 had activation from the left atrial roof.
Table 1 shows the demographic data of the study sample. Among dogs affected by OAVRT, Labrador Retrievers were the most represented breed (46%), median age was 1 (0.8/2) year, mean body weight was 28 ± 7.6 kg, 71% were male and 29% were female. Among dogs affected by AFL, Bernese mountain dogs were the most commonly represented breed (35%), median age was 8 (5.3/9) years, mean body weight was 38 ± 15.8 kg, 65% were female, and 35% were male. Among dogs affected by FAT, Boxers were the most commonly represented breed (18%), median age was 4 (1.8/7.5) years, mean body weight was 37 ± 7.6 kg, 64% were male, and 36% were female.
| Orthodromic atrioventricular reciprocating tachycardia (92 dogs) | Atrial flutter (17 dogs) | Focal atrial tachycardia (33 dogs) | Sinus tachycardia (40 dogs) | |
|---|---|---|---|---|
| Breed | 42 Labrador Retrievers (46%), 9 Golden Retrievers (10%), 5 crossbreed (5%), 36 other breeds (39%) | 6 Bernese mountain dogs (35%), 2 English bulldogs (12%), 2 English setters (12%), 7 other breeds (41%) | 6 Boxers (18%), 5 Labrador Retrievers (15%), 3 Bernese Mountain dogs (9%), 19 other breeds (58%) | 12 Chihuahua (30%), 5 crossbreed (17%), 3 Dachshund (7%), 20 other breeds (46%) |
| Age | Median: 1 year IQR: 1.2 25th-75th percentiles: 0.8-2 |
Median: 8 years IQR: 3.7 25th-75th percentiles: 5.3-9 |
Median: 4 years IQR: 5.7 25th-75th percentiles: 1.8-7.5 |
Median: 10 years IQR: 5.5 25th-75th percentiles: 7.2-12.7 |
| Body weight | Mean: 28 kg SD: 7.6 |
Mean: 38 kg SD: 15.8 |
Mean: 37 kg SD: 7.6 |
Mean: 9 kg SD: 8.4 |
| Sex | 22 females (25%), 4 neutered females (4%) 62 males (67%) 4 neutered males (4%) | 3 females (18%), 8 neutered females (47%), 6 males (35%) | 9 females (27%), 3 neutered females (9%), 21 males (64%) | 6 females (15%), 19 neutered females (48%), 14 males (34%), 1 neutered male (3%) |
Among the control group with ST, Chihuahuas were the most commonly represented breed (30%), median age was 10 (7.2/12.7) years, mean body weight was 9 ± 8.4 kg, 63% were female, and 37% were male.
3.2 Atrial deflection features
The MEA of the AD had median values of −90° (−90°/−78°) in OAVRT, 76° (72°/81°) in AFL, 49° (−72°/76°) in FAT, and 66° (52°/73°) in ST.
Regarding AD position, all dogs with OAVRT were characterized by short R-AD intervals. Dogs with AFL showed variable positions of AD in 59% of cases; 18%, 18%, and 5% of cases had a long R-AD, a short R-AD, and an AD on T wave, respectively. Dogs with FAT had a long R-AD in 52% of cases, an AD on the T wave with the classical camel signs in 33% of cases, a short R-AD in 12% of the cases, and a variable R-AD in 3% of the cases. All ST dogs had a long R-AD.
The duration of AD was 46 (40/54) ms in OAVRT, 50 (40/60) ms in AFL, 50 (40/52) ms in FAT, and 41 (35/45) ms in ST. Dogs with AFL had a significantly longer duration of F waves in comparison to P waves of ST (P = .0004; Figure 2A).
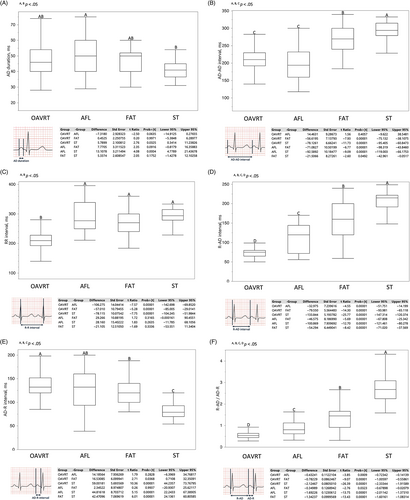
Table 2 presents the AD amplitudes in the 12 leads.
| Orthodromic atrioventricular reciprocating tachycardia | Atrial flutter | Focal atrial tachycardia | Sinus tachycardia | |
|---|---|---|---|---|
| I | 0 (0/0.05)B | 0.05 (0.05/0.1)B | 0.05 (0/0.1)B | 0.14 (0.12/0.19)A |
| II | −0.29 ± 0.18C | 0.32 ± 0.21A,B | 0.10 ± 0.24B | 0.38 ± 0.12A |
| III | −0.30 (−0.40/−0.20)A | 0.25 (0.15/0.30) C | 0.08 (−0.10/0.24)B | 0.27 (0.21/0.38)C |
| aVR | 0.20 (0.10/0.30) A | −0.15 (−0.20/−0.10)C | −0.10 (−0.20/0.09)B | −0.25 (−0.31/−0.19)C |
| aVL | 0.22 ± 0.14A | −0.12 ± 0.08C | −0.02 ± 0.13B | −0.12 ± 0.11C |
| aVF | −0.28 ± 0.20C | 0.27 ± 0.14A | 0.08 ± 0.23B | 0.34 ± 0.11A |
| V1 | −0.10 (−0.15/0.00)B | 0.15 (0.10/0.20)A | 0.15 (0.05/0.20)A | −0.13 (−0.19/−0.08)B |
| V2 | −0.09 ± 0.22B | 0.19 ± 0.14A | 0.14 ± 0.15A | 0.16 ± 0.07A |
| V3 | −0.10 ± 0.17B | 0.19 ± 0.12A | 0.15 ± 0.16A | 0.21 ± 0.10A |
| V4 | −0.10 ± 0.16C | 0.20 ± 0.12A,B | 0.14 ± 0.17B | 0.24 ± 0.10A |
| V5 | −0.10 ± 0.16C | 0.18 ± 0.09A,B | 0.14 ± 0.17B | 0.26 ± 0.10A |
| V6 | −0.10 (−0.16/0.00)C | 0.15 (0.10/0.20)A,B | 0.20 (0.06/0.25)B | 0.27 (0.2/0.33)A |
- Note: Normally distributed data are expressed as mean ± SD. Data with non-normal distribution were expressed as median (25th/75th percentiles). Statistically significant differences between groups (P < .05) are represented with capital letters.
3.3 Atrial deflection intervals
The AD-AD interval was 213 ± 30 ms in OAVRT, 199 ± 57 ms in AFL, 270 ± 38 ms in FAT, and 292 ± 31 ms in ST. Therefore, AFL was characterized by the shortest atrial cycle length, and it was regular in 100% of cases; however, it was not statistically different from the atrial cycle length in OAVRT, which was regular in 96% of cases and alternant in 4% of cases.
Dogs with FAT had a longer atrial cycle length than the first 2 groups (OAVRT vs FAT, P = .00001; AFL vs FAT, P = .00001), and it was regular in 55% of cases and regular with cycle length irregularity in 45% of cases. The atrial cycle length in ST also was significantly longer than that in the other groups (ST vs OAVRT, P = .00001; ST vs AFL, P = .00001; ST vs FAT, P = .05; Figure 2B).
Similar results were found for the R-R interval: 210 (192/228) ms in OAVRT, 277 (250/339) ms in AFL, 270 (240/300) ms in FAT, and 294 (279/315) ms in ST, with the only difference being OAVRT (OAVRT vs all other groups, P = .00001). Regarding AFL, 65% of cases had a regular RR interval and 35% of cases showed cycle length irregularity related to the AV conduction ratio (Figure 2C).
The AV conduction ratio was 1:1 in all dogs with OAVRT and ST. Dogs with AFL showed a 1:1 conduction ratio in 41% of cases, a 2:1 ratio in another 41% of cases, and a variable ratio in 18% of cases. In FAT, 97% of the cases showed a 1:1 conduction ratio, and only 3% of the cases showed a conduction ratio of 2:1.
The R-AD interval values were 75 (65/80) ms in OAVRT, 120 (72/144) ms in AFL, 160 (120/200) ms in FAT, and 215 (192/222) ms in ST, and there were significant differences among the 4 groups (P = .00001; Figure 2D). The extent of the variability in the 3 measurements, based on the relative variability of R-AD interval, was lower in ST (14%), followed by OAVRT (20%), FAT (50%), and AFL (60%).
The AD-R interval was 140 ± 32 ms for OAVRT, 126 ± 43 ms for AFL, 124 ± 29 ms for FAT, and 81 ± 16 ms for ST, and there were significant differences among OAVRT, FAT, and ST (OAVRT vs FAT, P = .04 and other groups differences, P = .00001; Figure 2E). The relative variability resulted lower in ST (20%), followed by OAVRT (23%), FAT (24%), and AFL (34%).
The R-AD/AD-R ratio was 0.54 (0.45/0.64) in OAVRT, 0.81 (0.63/1.13) ms in AFL, 1.45 (0.92/1.67) ms in FAT, and 2.68 (2.25/3.08) ms in ST, and there were significant differences among groups (OAVRT vs AFL, P = .001; AFL vs FAT, P = .03; other group differences, P = .00001; Figure 2F).
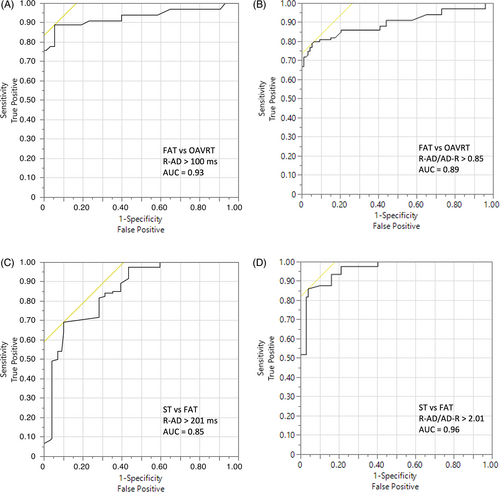
In the multivariate analysis, an R-AD interval > 100 ms differentiated FAT from OAVRT with a sensitivity of 89%, specificity of 95%, area under the curve (AUC): 0.93, PPV of 85% and NPV of 96% (Figure 3A), and an R-AD/AD-R ratio > 0.85 differentiated FAT from OAVRT with a sensitivity of 95%, specificity of 79%, AUC: 0.89, PPV of 93% and NPV of 84% (Figure 3B). For differentiation between ST and FAT, the R-AD interval had to be >201 ms (sensitivity 70%, specificity 90%, AUC 0.85, PPV 89%, NPV 71%; Figure 3C), and the R-AD/AD-R ratio had to be >2.01 (sensitivity 86%, specificity 96%, AUC 0.96, PPV 96%, NPV 85%; Figure 3D).
3.4 Additional data
The median QRS duration was 60 (50/63) ms for OAVRT, 56 (50/60) ms for AFL, 60 (52/60) ms for FAT, and 52 (48/60) ms for ST; there were no significant differences among the 4 groups.
QRS alternans was mainly present in dogs with OAVRT (46% of cases) and mainly was visible in the left precordial leads. Twelve percent of dogs with FAT also showed QRS alternans, which was mainly visible in the inferior leads and V3 (Table 3).
| Orthodromic atrioventricular reciprocating tachycardia (46% of cases) | Atrial flutter | Focal atrial tachycardia (12% of cases) | Sinus tachycardia | |
|---|---|---|---|---|
| I | 6% | — | 0% | — |
| II | 79% | — | 100% | — |
| III | 76% | — | 100% | — |
| aVR | 36% | — | 25% | — |
| aVL | 6% | — | 0% | — |
| aVF | 76% | — | 100% | — |
| V1 | 15% | — | 0% | — |
| V2 | 91% | — | 75% | — |
| V3 | 94% | — | 100% | — |
| V4 | 91% | — | 50% | — |
| V5 | 79% | — | 50% | — |
| V6 | 30% | — | 50% | — |
- Note: Numbers reported refer to the probability to find QRS alternans in that particular lead, in the animals that were characterized by QRS alternans, defined as a beat-to-beat variation in QRS amplitude of ≥0.1 mV in at least one lead.
The median QT interval was 180 (160/194) ms in OAVRT, 200 (170/220) ms in AFL, 198 (180/200) ms in FAT, and 167 (159/178) ms in ST. There was a significant difference between the ST and AFL groups (difference: −31.44; SE: 7.23; t ratio: −4.35; P = .00001; lower 95%: −12.69; upper 95%: −50.18) and between the ST and FAT group (difference: −24.28; SE: 5.87; t ratio: −4.14; P = .0003; lower 95%: −9.06; upper 95%: −39.51).
4 DISCUSSION
We characterized AD on a 12-lead ECG in a sample of dogs affected by sustained OAVRT, AFL, and FAT confirmed by EPS and compared them with a control group of dogs with ST. According to these data, AD position and features appeared to be useful in differentiating among tachycardia types in dogs, and we defined the cut-offs for AV and ventriculoatrial intervals.
Considering the sample characteristics, we confirmed that the OAVRT group had a higher prevalence of Retrievers, a higher number of male dogs,3, 4, 25 and significantly younger dogs than the other groups. As previously reported, the Bernese Mountain Dog was the most represented breed with AFL,6, 7 whereas the Boxer was the most common breed in dogs with FAT. Unsurprisingly, the control group of dogs with ST was mainly composed of small-breed, middle-aged dogs.26
The inferosuperior and right-to-left activation of AD in OAVRT was confirmed in our study,14 and this finding is consistent with the predominance of right AV accessory pathways in dogs.3, 4 The median value of MEA in FAT was characterized by a wide range of percentiles (from −72° to 76°) because of the different localizations of the ectopic foci.
Atrial flutter was characterized by the shortest AD-AD interval, followed by OAVRT, FAT, and ST. These data could be useful to differentiate between cases of atrial fibrillation with prominent fibrillatory waves (“coarse fibrillatory waves”, based on the definition given in human medicine)27 and cases of AFL with a different AV conduction ratio. Most of our dogs with AFL had an atrial rate of <120 ms (500 bpm). However, dogs with atrial fibrillation were not included in our study and therefore we cannot make assumptions about the ability of this measurement to distinguish between AFL and atrial fibrillation. Nevertheless, these can be considered preliminary data that characterize AD of AFL. If we consider the R-R interval, OAVRT was the fastest tachycardia in our sample, similar to what has been reported previously4; this result is in disagreement with our previous smaller study, wherein FAT was faster than OAVRT.14 This finding could be related to the fact that all of the intervals measured in the present study were taken during general anesthesia. The number of dogs affected by FAT included in the previous study was relatively small (9 dogs) and therefore this difference could be biased based on the different sample sizes. Furthermore, in most studies of humans, heart rate is not predictive of tachycardia type.28-30 Cycle length irregularity in our sample was a typical characteristic of FAT (45%), as reported in our previous study,14 but was also present in AFL (35%); however, it was not present in any dog with OAVRT or ST. This finding could be explained by a variation in the discharge rate of the ectopic focus or by the presence of fixed or variable-ratio AV block for FAT and AFL.31, 32 Cycle length alternans appeared instead to be a rare finding in OAVRT and possibly could be related to the presence of multiple accessory pathways or dual AV nodal physiology.33, 34
Regarding the AD position, all cases of OAVRT were characterized by a short R-AD because of the fast nondecremental retroconduction along the accessory pathway. The AD position was variable in most cases of AFL because of different AV conduction ratios. Long R-AD was present in most cases of FAT, but short R-AD was present in 12% of these cases, mainly those originating from the pulmonary veins (Figure 4). These results show that a short R-AD is not pathognomonic of OAVRT in dogs, particularly in cases of negative AD. In those cases, the fixed or variable relationship of AD to QRS complex (R-AD interval) could help differentiate OAVRT and FAT, because we demonstrated that the relative variability of R-AD interval was higher in FAT than in OAVRT.
The AD position can be numerically translated and identified with the ventriculoatrial and AV intervals, which were measured in our study. The values found in our study for the R-AD interval were similar to those reported in the previous smaller study for FAT and OAVRT and in previous studies of dogs affected by OAVRT.3, 4, 14 We also obtained values for AFL and ST. We must emphasize that this ECG interval is substantially an expression of different events. In OAVRT, it is related to the ventriculoatrial conduction velocity along the accessory pathway. In AFL, its value varies depending on the AV conduction ratio. In FAT, it is related to the rate of discharge of the automatic ectopic focus. In ST, it depends mainly on the autonomic nervous system. In human medicine, an R-AD interval ≤ 70 ms during EPS usually indicates typical AVNRT, or less commonly FAT, but also has been reported in OAVRT.35 For surface ECG measurements, a cut-off interval of 90 ms has been proposed in another study and included in the diagnostic algorithm of the 2019 European Society of Cardiology guidelines,1, 12 but data on actual R-AD measurements during various types of SVT are scarce. In our current study, a value ≤100 ms differentiated OAVRT from FAT with good sensitivity and specificity, but less accurately than in people, probably because of the presence of FAT arising from the pulmonary veins with a short R-AD in our sample. To differentiate FAT from ST, we must consider values ≤201 ms, although the diagnostic power of this cut-off is not very high.
When considering the ratio between R-AD and AD-R in our study, it was more specific than the R-AD interval alone in the differentiation of OAVRT from FAT when considering a cut-off value of 0.85, and it resulted in better differentiation of FAT from ST than did the R-AD interval when considering a cut-off value of 2.01.
QRS alternans was present in almost half of the patients with OAVRT (46%) but only in a small proportion of patients with FAT (12%). In both groups, the prevalence was lower than that in our previous study,14 which could be attributed to the larger sample of the present study. In OAVRT, the left precordial leads were the most useful for detecting QRS alternans; in FAT, it was mainly visible in the inferior leads and V3. The higher prevalence of QRS alternans in dogs with OAVRT, which was the group with the highest heart rate in the present study, is in agreement with the most widely accepted theory in human medicine, according to which QRS alternans is a rate-related phenomenon that depends on an abrupt increase to a critical rate and is independent of the tachycardia mechanism.36
The main limitation of our present study is the possible influence of the anesthesia protocol on the measured values. Isoflurane has been reported to affect AV conduction and atrial, ventricular, and accessory pathway refractory periods in dogs and children.37, 38 However, the influence of anesthesia on these intervals in OAVRT has been studied previously by comparing the heart rates of awake dogs to those under anesthesia.4 In our study, a significant prolongation of the AD-R interval (107 ± 19 ms vs 141 ± 22 ms) and no prolongation of the R-AD interval (77.6 ± 9.4 ms vs 75.7 ± 8.2 ms) in the anesthetized versus the awake state were seen, suggesting a greater effect of general anesthesia on the AV nodal conduction system than on the accessory pathway.4 Therefore, we assume that our values for the R-AD interval in dogs with OAVRT can be considered when evaluating awake dogs. For dogs with FAT and AFL, studies in humans have shown that volatile anesthetics slow the rate of sinoatrial node discharge by direct and indirect effects on sinoatrial node automaticity, may suppress automatic atrial foci, and that isoflurane prolongs AV conduction time and refractoriness.39, 40 Considering the possibility of a parallel increase in both R-AD and AD-R, the R-AD/AD-R ratio might have a negligible difference between awake dogs and dogs under anesthesia. Additional studies are needed to evaluate the effects of different ECG intervals of anesthetic agents in dogs. Another limitation of our study was that ST was not confirmed by the EPS, therefore FAT arising from the superior part of the crista terminalis could not be completely ruled out in these dogs. Furthermore, there were differences in weight and age between this group and the other groups, and dogs with ST also could have been affected by different cardiac diseases, which could have influenced the measurements. However, the inclusion of a control group of healthy dogs with ST confirmed by EPS would not have been ethically acceptable. Permanent junctional reciprocating tachycardia also was excluded from the analysis owing to the small number of cases. As it is a form of atrioventricular reciprocating tachycardia, it is important to emphasize that our proposed cut-off for the differentiation of FAT from OAVRT is not applicable in those rarer situations where PJRT is suspected. In addition, although the sample size of our present study was larger than that of our previous study, the FAT and AFL groups were considerably smaller than the OAVRT group. Finally, the exact starting or ending point of ADs was sometimes difficult to identify on the surface ECG, and measurements had to be referred to the sequence of intracardiac activation in these cases. However, this factor is also a strong point of our study because in these cases reliable identification of AD would not have been possible in the absence of a guidance intracardiac electrogram, and the minimum and maximum values of these intervals also could be considered in clinical practice during the analysis of fast narrow QRS complex tachycardia on surface ECG to identify the areas where AD are usually located.
5 CONCLUSION
We defined the ECG features of AD during fast OAVRT, AFL, FAT, and ST. The MEA and position of AD, which were defined using the R-AD interval and R-AD/AD-R ratio, appeared to be useful in differentiating among the most common types of SVT and could be added to diagnostic algorithms. Additional studies are needed to assess these variables in the ECG analysis of sustained SVT in awake dogs.
ACKNOWLEDGMENT
No funding was received for this study. Preliminary data were presented at the 32nd Annual Congress of the European College of Veterinary Internal Medicine-Companion Animals, Gothenburg, 1-3 September 2022. The authors thank Arch. Federica Farè for graphic artwork.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




