Idiopathic functional urinary outflow tract obstruction in dogs, a retrospective case series (2010-2021): 31 cases
Abstract
Background
Idiopathic functional urinary outflow tract obstruction (iFUOTO) is an uncommon but life-limiting disease whose etiology and clinical course of disease remain poorly understood.
Objective
Characterize signalment, clinical signs, clinicopathologic findings, treatments, and propose a standardized response score for dogs with iFUOTO.
Animals
Thirty-one client-owned dogs diagnosed with iFUOTO.
Methods
Retrospective case series. Medical records from 2010 to 2021 were reviewed and findings recorded. Dogs were categorized based on the presence or absence of overt signs of neurological disease. Response to treatment was scored.
Results
Thirty-one dogs were included. All dogs had stranguria and 14 (45%) had overflow urinary incontinence. Mean age of onset for signs was 6.9 years±8 months. Twenty-four dogs (77%) were castrated males, 5 (16%) intact males, and 2 (6%) spayed females. Eight (26%) of dogs had overt neurological deficits. Follow-up data were available for 29 dogs (median 38 days, range: 2-1277). Final outcome scores were not significantly different among dogs with overt signs of neurological disease (median score 2.5; range: 0-3) and those without (median score 1; range; 0-3; P = .35). Treatments included alpha antagonists, skeletal muscle relaxants, parasympathomimetics, anti-inflammatories, castration, temporary placement of a urethral catheter, or a combination of these.
Conclusions and Clinical Relevance
Multimodal treatment was frequently prescribed, but a standard outcome score is needed to evaluate the effectiveness of these therapies. Serial neurological examinations and monitoring of the dogs' dysuria are warranted.
Abbreviations
-
- CNS
-
- central nervous system
-
- DSD
-
- detrusor sphincter dyssynergia
-
- DUD
-
- detrusor urethral dyssynergia
-
- iFUOTO
-
- idiopathic functional urinary obstruction
-
- LUT
-
- lower urinary tract
-
- LUTS
-
- lower urinary tract signs
-
- NNS
-
- no neurological signs P
-
- NS
-
- neurological signs
-
- UTI
-
- urinary tract infection
-
- VRV
-
- postvoid residual volume
1 INTRODUCTION
Urethral obstructions in dogs can occur because of mechanical or functional diseases and can be life-threatening. Mechanical urethral obstructions can be caused by urolithiasis, urethral or prostatic masses, urethral strictures, or blood clots. The etiology of functional micturition disorders remains poorly understood. Whereas thoracolumbar intervertebral disc disease and other neurological disorders might lead to a lack of inhibition of the pudendal and hypogastric nerves and result in functional urinary outflow tract obstruction, these dogs generally have overt neurological abnormalities noted on physical examination that coincide with the micturition disorder.1 In some dogs, functional urethral obstruction occurs without concurrent neurological deficits and these dogs are often diagnosed with reflex dyssynergia (also known as detrusor urethral dyssynergia [DUD]). In humans, detrusor sphincter dyssynergia (DSD) is a disease with similarities to idiopathic functional urinary outflow tract obstruction in dogs, but is specifically associated with central nervous system (CNS) disease. For example, spinal cord injury and multiple sclerosis are common causes of functional urinary outflow tract obstruction in humans, resulting from dyscoordination between the detrusor muscle and the urethral sphincter.2
Definitive diagnosis of DSD requires urodynamic testing.2 Urodynamic studies can be challenging in dogs, requiring specialized equipment and staff training. Most dogs require sedation or general anesthesia for successful performance of urodynamic testing and many of the prescribed medications will alter urethral pressures and bladder compliance, which can limit their clinical utility.3 In dogs, high-pressure spikes might be documented during a urethral pressure profilometry, suggestive of a functional obstruction;4 however, this finding is not always reliable. As such, the etiology of functional voiding disorders, assumed to be similar to that in people, are not definitively diagnosed in dogs. Therefore, this disease in dogs, often characterized by stranguria with or without overflow urinary incontinence and a large postvoid residual volume (PVRV) for which no underlying mechanical or overt neurological abnormality is identified, might be more appropriately termed idiopathic functional urinary outflow tract obstruction (iFUOTO) and is the term used in this study.
In prior studies, large-breed male dogs have been overrepresented for idiopathic functional voiding disorders,5, 6 but comparison populations were not reported. Various therapeutic approaches have been implemented for iFUOTO, but response to treatment has never been standardized, which limits comparisons among studies.
The objectives of this retrospective study were to characterize the signalment, clinical signs, pertinent clinicopathologic data, treatments prescribed to dogs with a clinical diagnosis of iFUOTO, and response to treatment. Some dogs diagnosed with iFUOTO in the medical record during the study period at our institution had chronic neurological deficits. Therefore, we divided dogs into 2 groups: those with No overt Neurological Signs (NNS) and those with overt Neurological Signs (NS) in order to better understand whether iFUOTO was an appropriate diagnosis for these dogs with preexisting neurological disease. Additionally, we propose a standardized therapeutic outcome score that might be utilized in future studies and clinical settings.
2 MATERIALS AND METHODS
Medical records from the UC Davis School of Veterinary Medicine electronic medical record system were reviewed from 2010 to 2021. Search terms utilized to identify dogs included “reflex dyssynergia,” “reflex dyssynergy,” “detrusor urethral dyssynergia,” “functional urinary obstruction,” and “neurogenic bladder” to capture as many functional voiding disorders as possible.
Dogs were included if their medical record had a final clinical diagnosis of iFUOTO (or previous terms used to describe this disorder), presented with stranguria and a large bladder or overflow urinary incontinence, and the absence of abnormalities noted on cystourethroscopy, contrast cystourethrogram, or combination of the 2. Overflow urinary incontinence was diagnosed by the attending clinician usually based on a large bladder after the dog attempted to void. Objective and subjective PVRV were recorded. Dogs with mechanical (eg, urethrolith, neoplasia) urethral obstructions, prostatic disease other than benign prostatic hypertrophy, resolution of lower urinary tract signs with antimicrobial treatment for UTI, and a primary acute neurological disease with concurrent onset of urinary signs, were excluded from the study.
Data collected from the medical records included signalment, presence or absence of stranguria, duration of the observed signs, and the need for intermittent or indwelling urethral catheterization. Additionally, pertinent clinicopathologic data were recorded, including serum BUN, creatinine (Cr), and potassium, as well as the urinalysis and urine culture results if available. Treatments prescribed, including pharmacologic and interventional, and response to treatment also were recorded. Response to treatment was documented utilizing the following therapeutic scoring system: 0—normal void, 1—stranguria with no need for bladder expression/catheterization, 2—stranguria, bladder expression prescribed, and 3—stranguria, bladder catheterization prescribed. These scores were assigned retrospectively based on data obtained from follow-up visits at UC Davis, referral veterinary records, client phone calls, communication logs, or a combination of these. Final outcome scores were provided for all dogs that had at least 1 reevaluation. Final clinical outcome scores were provided for each dog based on the score assigned at the last available recheck or client communication. Response to treatment over time was also recorded for individuals with 2 or more follow-up time points. Therapies prescribed at each recheck were recorded. Responders were defined as dogs with a decrease (improvement) in the clinical outcome score over time. Nonresponders were dogs with the same or an increased clinical outcome score at subsequent evaluations. Relapses were noted for dogs that had an initial decrease in their outcome score, but their score increased at subsequent reevaluations.
Neurological examinations were conducted by the UC Davis Neurology service, the Internal Medicine service, or both. Dogs with a normal neurological examination were categorized as having no overt signs of neurological disease (NNS). Dogs with abnormalities identified on examination, such as pelvic limb postural reaction deficits, were categorized as having overt signs of neurological disease (NS) group. Dogs were excluded from analysis if the neurological abnormalities noted on the examination were acute and coincided with onset of stranguria.
2.1 Statistical analysis
Descriptive statistics were utilized to analyze the data. Normality was assessed by the Shapiro-Wilk test. Parametric data are reported as the mean with SE of the mean; nonparametric data are reported as the median and range. Body weight, age of onset of urinary signs, and final outcome scores were normally distributed; an unpaired, 2-tailed t-test was used to compare each variable between dogs in the NS and NNS groups. The duration of LUTSs in months, prior to presentation was not normally distributed across groups and these data were analyzed using the Mann-Whitney test.
3 RESULTS
3.1 Clinical features
Thirty-one dogs were included in this study, and approximately 1 to 5 dogs were diagnosed with iFUOTO per year. Twenty-three were castrated males (age at castration could not be determined from the records), 6 intact males, and 2 spayed females. Identified breeds included Labrador retriever (n = 8), German shepherd (n = 4), golden retriever (n = 2), mastiff (n = 2), Great Dane, Weimaraner, wirehaired pointing griffon, border collie, Rottweiler, giant schnauzer, Rhodesian ridgeback, borzoi, bulldog, Doberman pinscher, Yorkshire terrier, Tibetan terrier, Boerboel, and mixed breed (n = 2). Labradors, retrievers, and German shepherd dogs had an increased risk of iFUOTO when comparing dog breeds examined at the VMTH at the University of California-Davis during the same period as the study (OR = 3.3; 95% CI: 1.5-7.2, P = .002 and OR = 3.5; 95% CI: 1.2-9.9, P = .02), respectively). The mean age at evaluation at our hospital was 6.9 years ±7.6 months. Intact male dogs ranged in age from 2 to 12 years of age. Bladder size noted at referral presentation was large in 25 dogs, moderate in size in 1 dog, and not reported in the medical record for 3 dogs. Two dogs presented with a urinary catheter in place. The median body weight of all dogs was 37.7 (range -3.0 kg- 72.8kg. Other physical examination findings included a body condition score > 5 (n = 16), abdominal pain (n = 4), urine staining of perineal region (n = 3), and prostatomegaly (n = 3). Two of the 3 dogs with prostatomegaly were intact males and the third was a castrated male for which the age of castration was not known.
Twenty-three dogs were classified into the NNS group and 8 into the NS group. All signs of neurological disease noted in the NS dogs preceded signs of the micturition disorder (0.5-5 months prior to stranguria). All NS group dogs were male (2 MI, 6 MN). Median body weights were not significantly different between NS dogs (39.2 kg, range: 28-72.8 kg) and NNS dogs (37.4 kg, range: 3-72.5 kg; P = .21). Neurological deficits noted included altered proprioception (n = 5), pelvic limb weakness (n = 1), mildly decreased segmental reflexes in all limbs (n = 1), and a mild, intermittent left-sided head tilt with generalized ataxia (n = 1). Magnetic resonance imaging of the brain was evaluated for only 1 dog in the NS group. This dog had a diagnosis of inflammatory brain disease based on multiple focal noncontrast enhancing regions of increased intensity on FLAIR and T2 weighted images in the brainstem, cerebellum, deep gray matter, and cortical white matter noted on the MRI and lymphocytic inflammation on CSF analysis.
3.2 Lower urinary tract signs
All dogs had a history of stranguria with a mean age of onset at 83 ± 8 months. The mean age of onset of stranguria was not significantly different between NS dogs (86 ± 36 months) and NNS dogs (76 ± 40 months; P = .63; Figure 1). The median duration of stranguria prior to presentation was 1 month (range: 0-60 months). The median duration of stranguria was longer for NS dogs (14.5 months [range: 0-36 months]) compared to NNS dogs (1 month, range: 0-60 months; P < .05). In addition to stranguria, 14 of 31 (45%) dogs also presented with overflow urinary incontinence. Twenty-eight of 31 (90%) dogs required bladder catheterization prior to or at presentation to our hospital to alleviate their urinary obstruction. Twenty of 23 (87%) of NNS dogs required bladder catheterization at, or prior to, presentation and 9 of them (39%) had concurrent overflow urinary incontinence. Five of the 8 NS dogs (63%) had overflow incontinence, all of which required bladder catheterization for management. No dogs had gross hematuria.
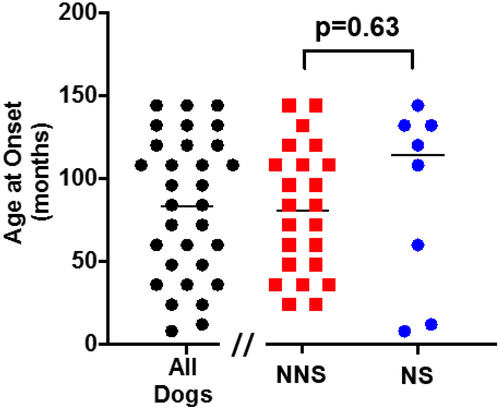
Post void residual volumes (PVRV) were estimated based on point-of-care ultrasound in 17 dogs and measured via catheterization in 2 dogs. For catheterized dogs, PVRV was 41.8 and 10 mL/kg, respectively. Estimations of PVRV were determined to be increased based on an enlarged bladder noted on ultrasound examination.
3.3 Clinicopathological data
Twenty-seven dogs had urinalysis results available for review. Nine of 27 (33%) dogs had pyuria (5 NNS, 4 NS) and 3/27 (11%) had bacteriuria (2 NNS, 1 NS) noted on urine sediment. Twenty-nine dogs had aerobic bacterial urine culture results available and 5/29 (17%) were positive for growth [102 CFU/mL (n = 2), 103 CFU/mL (n = 1), and ≥105 CFU/mL (n = 1) Escherichia coli, and 1 each had 103 CFU/mL Staphylococcus pseudintermedius, and ≥105 CFU/mL Enterococcus spp.]. These dogs were administered antimicrobials based on susceptibility [enrofloxacin (n = 1), amoxicillin-clavulanic acid (n = 3), and chloramphenicol (n = 1)] but remained stranguric and clinical for their voiding disorder. Eight of the dogs with negative urine cultures were administered antimicrobials at the time urine specimen was submitted for culture.
Serum biochemistry was available in 29 dogs. The median (range) for the following serum biochemistry values that were obtained at presentation were: BUN 16 mg/dL (7-54 mg/dL), Cr 1 mg/dL (0.6-2.1 mg/dL), K 4.4 (3.8-6 mmol/L), CK 206 (42-1501 IU/L). Eleven dogs had 1 or 2 serum biochemistry values that were considered abnormal based on laboratory reference range. One had an increased serum creatinine concentration (2.1 mg/dL) and 10 dogs had increased creatine kinase concentrations (290-1501 mg/dL). Complete blood counts were available in 27 dogs. Six dogs had mild changes on CBC including a mild, mature neutrophilia (n = 2), mild anemia (n = 3), mild lymphopenia (n = 2), and mild polycythemia (n = 2).
3.4 Directed therapies
The most common medication classes prescribed and the number of dogs that were administered these drugs are provided in Table 1. The median doses of the most commonly administered medications were: prazosin 0.067 mg/kg q8-12h (range: 0.06-0.27), tamsulosin 0.01 mg/kg q8-12h (range: 0.005-0.2), bethanechol 0.2 mg/kg q8-12h (range: 0.09-0.4), and diazepam 0.14 mg/kg q6-8h (range: 0.09-0.64). All dogs in the NS group and 21 dogs in the NNS group were prescribed alpha-antagonists at the time of evaluation to our institution. Five dogs in this study were prescribed an alpha-1 antagonist alone at the time of discharge. Other treatment modalities included intermittent bladder catheterization (9/31), indwelling red rubber urinary catheters (3/31), or a combination of these 2 interventions. Urethral stents were not placed in any dogs.
| Medication classification | Medication | # Dogs | ||
|---|---|---|---|---|
| All dogs | NNS | NS | ||
| Alpha-antagonists | Prazosin | 16 | 13 | 3 |
| Tamsulosin | 14 | 10 | 4 | |
| Phenoxybenzamine | 2 | 1 | 1 | |
| Skeletal muscle relaxants | Diazepam | 13 | 11 | 2 |
| Methocarbamol | 2 | 0 | 2 | |
| Parasympathomimetics | Bethanechol | 9 | 5 | 4 |
| Cisapride | 1 | 0 | 1 | |
| Anti-inflammatories | NSAIDs | 9 | 6 | 3 |
| Glucocorticoids | 2 | 2 | 0 | |
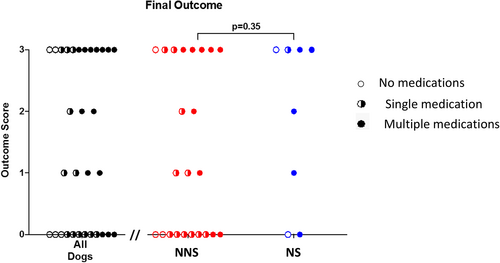
3.5 Final outcome scores
The median final outcome score for all dogs was 1 (range: 0-3). Final outcome scores were not significantly different between NS (2.5; range: 0-3) and NNS dogs (1; range; 0-3; P = .35; Figure 2). Ten of the 31 dogs were not receiving any medication at the time the final outcome score was assessed. One of these dogs was being intermittently catheterized by the owners. For dogs in the NS group, 2 dogs had improvement in their neurological function, 1 of these dogs had a slow improvement in their final outcome score, and the other dogs scores remained the same. One dog had static neurological deficits and no change in final outcome score. The 4th dog had progressive neurological deficits with an improved final outcome score (0 = normal voiding). Data regarding the progression or improvement of neurological function was not apparent from the medical records in the other 4 NS dogs.
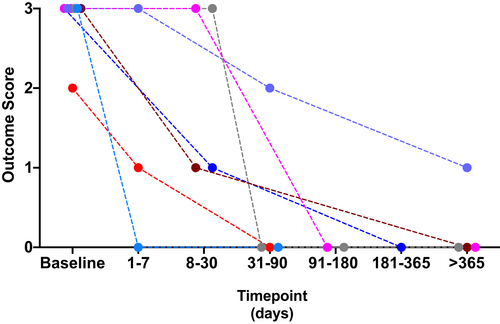
Outcomes scores for those dogs with 2 or more documented reevaluations (either at referral or communication with the owner) were available for 3 dogs in the NS group and 12 dogs in the NNS group. All 3 dogs in the NS group were nonresponders or relapsed. Seven dogs in the NNS groups were responders (Figure 3), while 5 were nonresponders or relapsed (Figure 4). Follow-up times varied for dogs in this study and we were unable to document exact times to recovery or relapse of their clinical signs. For the 2 female dogs in this study, 1 had only a single follow-up data point available with an outcome score of 0 on single-agent treatment (tamsulosin 0.008 mg/kg PO SID). The other female was euthanized 2 weeks following presentation for persistent clinical signs (stranguria with inability to void) and acute decline after a presumed vagal episode when attempting to void.
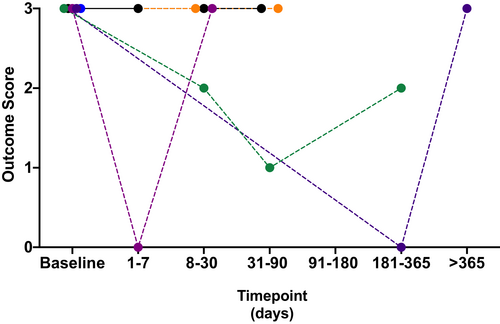
Five of 6 dogs that were castrated had a decrease in their outcome score. In these dogs, initial scores were 3 and final outcome scores were 0 in 4 dogs and 1 in 1 dog. Two of these 5 dogs were castrated with no additional medications prescribed. Three of these dogs had medications prescribed at the time of castration and had improvement in their outcome score. Two of the 3 dogs had all medications discontinued following castration and dogs had a clinical score of 0 at their last reevaluation (3 and 6 months). These medications included bethanechol, diazepam or methocarbamol, and tamsulosin in 2 dogs.
Three dogs had temporary urethral catheters placed. For 2 of these dogs, catheters migrated distally out of the urethra and prepuce and needed to be re-sutured in place. No ascending urinary tract infections were documented in these dogs. Two of these dogs either died or were euthanized with the red rubber catheters in place for 3 and 5 weeks, respectively. The third dog had the catheter removed approximately 12 weeks after placement due to improvement in clinical signs.
Three of 31 (10%) dogs were euthanized due to persistent clinical signs. All euthanized dogs had an outcome score of 3. All 3 of these dogs were administered an alpha-1 antagonist (prazosin, tamsulosin) and diazepam, 2 received concurrent anti-inflammatories (prednisone, carprofen), and 1 also had a red rubber catheter. There was no apparent dosing difference in any medication for responders vs nonresponders. Time to euthanasia ranged from 0.5 to 18.2 months from diagnosis. One dog with an outcome score of 3 died due to unknown causes. Nine dogs with outcome scores of 3 were still alive at the end of the study.
4 DISCUSSION
Idiopathic functional urinary outflow tract obstruction is an uncommon disease, with only 1 to 5 cases seen at our hospital yearly, yet has considerable morbidity and even death associated with it. This study documents the clinicopathologic features of iFUOTO, and also highlights the additional need for a tiered therapeutic clinical outcome scoring system to characterize response to treatment for dogs with iFUOTO. Longer-term evaluation of dogs with and without overt signs of neurological disease to better characterize this disorder is needed and determine if the clinical diagnosis, iFUOTO is actually appropriate or not.
Similar to previous studies,5, 6 iFUOTO primarily occurred in large breed, male dogs in our study, however, a dog as small as 3 kg was included in our study. Additionally, 2 spayed females were included in this study, compared to 4 female dogs in a prior study.6 For all dogs included in this study, cystoscopy or contrast cystourethrograms were performed to exclude mechanical obstructions, including proliferative urethritis, a condition more commonly diagnosed in female dogs.7 Therefore, iFUOTO appears to occur in female dogs, albeit less commonly. Urodynamic studies are needed in both sexes to evaluate for similarities or differences in their maximal urethral closure pressures to identify if this micturition disorder is the same in males and females. Although these female dogs had a similar clinical history as the male dogs, too few females are represented to make meaningful comparisons.
Labrador retrievers and German shepherd dogs were at increased risk for iFUOTO when compared to the distribution of breeds presenting to our hospital during the study period, comprising 26% and 13% of dogs in this study, respectively. This is similar to a previous publication where Labrador retrievers were also overrepresented,6 but odds ratios were not provided. Genetics have not been evaluated to document if this disease is heritable. The mean age of dogs at the time of evaluation in our study was almost twice as old as those in previous publications, 86 months compared to 48 and 64 months.5, 6 Therefore, iFUOTO should be considered in both younger and middle-aged dogs that present with functional voiding disorders. While some of these dogs might have been managed by their primary care veterinarians for a longer time period prior to referral, the mean age of onset of LUTS in our study was also older than previous studies, suggesting dogs in our study truly presented at an older age.
All dogs in the NS group had chronic neurological deficits. The attending clinician determined these deficits were unrelated to their voiding disorder, and ultimately diagnosed them with iFUOTO. As iFUOTO is currently reported to be “idiopathic,” we elected to categorize dogs as NS and NNS to investigate if their micturition disorder progressed or improved in association with their neurological disease and if iFUOTO appeared to be an appropriate diagnosis for these dogs. Only 4/8 dogs in the NS group had long-term data available for review. One of the dogs' neurological diseases did not appear to be associated with functional urinary obstruction because their LUTS resolved but developed progressive neurological deficits. For the other 3 dogs, it was difficult to ascertain if the voiding disorder was related to the neurological problem or not. In humans, inflammation in the CNS can result in urine retention.8 However, while urinary retention can be the only outward clinical sign of myelitis, it is generally followed by progressive and more profound neurological deficits.8 Additionally, in humans with either traumatic or atraumatic spinal cord injury resulting in neurogenic bladder and concurrent DSD, no significant correlation between detrusor activity (based on urodynamic studies) and neurological recovery has been found.9 These data imply urinary status in patients with neurological abnormalities is dynamic and recovery of neurological function, or lack thereof, cannot be used as a tool to predict improvement of the micturition disorder. There were no statistically significant differences between the NS and NNS groups with signalment, presenting complaint, medications prescribed, or response to treatment. While the attending clinicians presumed the neurological abnormalities were not associated with the micturition problem, clinicians should be cautious with this assumption, and serial neurological examinations and continual monitoring of the dogs' urinary signs are warranted. Urodynamic studies and EMGs could be considered to evaluate if obvious differences in urethral pressures are present in dogs with and without NS. Furthermore, monitoring these dogs closely over time will help determine if a primary underlying neurological problem is associated with the voiding disorder. If that is determined, the term iFUOTO is not appropriate for these dogs.
Urine cultures were available from 29/31 dogs, and 17% were positive for bacterial growth; however, 8 dogs were administered antimicrobials at the time of the negative urine culture, which could have reduced the number of positive urine culture results. Although dogs with bacteriuria can have LUTS and a clinical urinary tract infection, no dogs in this study had resolution of their stranguria with appropriate antimicrobial treatment. Therefore, these dogs likely had subclinical bacteriuria secondary to large PVRV and bladder catheterization, and bacteriuria was a consequence of iFUOTO rather than a cause. However, antimicrobials should be considered at the initial presentation if bacteriuria is noted, and the clinician should monitor for clinical improvement of the voiding disorder. If no improvement is noted, long-term antimicrobials and microbiologic cure is likely not warranted.
Response to treatment is difficult to compare across studies given a lack of standardized clinical outcome scores and variable treatment protocols. Thirty-nine percent of dogs in our study had complete resolution of clinical signs and normal micturition (score of 0), but differed in their time line to reach this score. Previous studies have documented that 68% and 59% of dogs have a “good” response to treatment, however, only subjective data were used to evaluate responses. Three dogs were euthanized with outcome scores of 3, compared to the 9 dogs still alive at the end of our study with outcome scores of 3, suggesting clients' perception of “good” or tolerable varies widely. Like previous studies,5, 6 assessment of response to treatment is still difficult to interpret due to multiple prescribed therapies and lack of standardized approach. Due to the retrospective nature of this study and tailored therapeutic medication alterations that were discussed with clients via phone calls and their primary care veterinarian, the therapies prescribed were not standardized. A score should be recorded after each medication is prescribed, to allow for better assessment of the efficacy of each medication in the dog's protocol.
We did not have objective PVRV documented often, but all dogs were assessed to have stranguria and increased PVRV based on point-of-care ultrasound. Unfortunately, PVRV was not recorded at recheck examinations, so it is possible that some dogs that were perceived to void normally, might have had an increased PVRV, despite the absence of clinical signs noted by their owners. However, owners' perceptions of their dogs' clinical signs were the primary outcome variable of this study.
Alpha-1 antagonists were the most commonly prescribed medication class, and prazosin (16) was the most common medication within this drug class, followed by tamsulosin (14) and phenoxybenzamine (1). This class of medications should decrease the urethral closure pressure of the smooth muscle of the urethra, thereby facilitating voiding during bladder contraction. Medications prescribed in this study are similar to previous publications,5, 6 however, tamsulosin was prescribed more often in our study compared to the other 2, despite 1 of these studies occurring over a similar period of time. Tamsulosin, an alpha-1 adrenergic antagonist, is more selective for the alpha-1a subtype, the primary receptor subtype located in the prostate, bladder neck, and proximal urethra.10, 11 Prospective studies regarding its efficacy in iFUOTO have not been reported, but due to anecdotal success at our institution, it is prescribed more frequently. Studies evaluating anesthetized dogs report the maximal urethral pressure decreased in a dose-dependent manner after tamsulosin administration.12 Additionally, both oral and intravenous administration of tamsulosin have had negligible effects on systemic blood pressure in dogs minutes to hours after administration.8, 10 As in both prior studies,5, 6 skeletal muscle relaxants, most commonly diazepam and methocarbamol, were the second most frequently prescribed medication class in our study. These drugs might help decrease urethral closure pressure in the skeletal muscle of the urethra (ie, the external urethral sphincter). In female dogs, the urethra is comprised largely of collagen and elastic fibers with smooth muscle extending along its length and striated muscle at the external urethral orifice.13 Conversely, greater than 50% of the urethral wall in male dogs is comprised of type I, IIa an IIc muscle fibers.14 It is unknown if iFUOTO is a disease of the sympathetic or somatic system, or a combination of both. The improvement in clinical signs for some dogs that were administered diazepam suggests abnormalities in both systems could be present. Botulinum toxin can be effective in people with detrusor external urethral dyssyngergia.15
In our study, as well as another publication,6 parasympathomimetic and anti-inflammatory medications were also prescribed, although less frequently. Parasympathomimetics are often prescribed for suspected bladder atony, but clinical studies evaluating its efficacy are lacking. It is possible NSAIDs were prescribed for analgesia or for potential LUT inflammation. No major medication adverse events were noted in any of the medical records for dogs included in our study.
While indwelling urinary catheters are not considered standard of care, and were rarely placed (3 dogs), urethral catheters were placed solely to provide a noninvasive, short-term, cost-effective treatment for urine evacuation in dogs refractory to medical management. These dogs could be managed at home and the response to medical management assessed periodically. No major adverse events were noted in these dogs. Tube cystostomies, another salvage procedure for urine evacuation can also be considered, but complications in up to 50% of dogs and cats have been described and 17/76 animals had major complications.16
Five of the 6 dogs that were castrated as part of their treatment regimen had an improved outcome score. Three of these dogs required no medications to maintain a score of 0. Our results support those found in 1 prior study in which all intact males with functional voiding that underwent castration (n = 7) demonstrated partial (n = 1) or good (n = 6) response to treatment.6 However, all castrated male dogs in that study required additional medications and the outcomes were subjective. In our study, benign prostatic hypertrophy was noted in 6 dogs. This benign change rarely causes stranguria in dogs, making it unlikely to be the cause of the functional urinary obstruction. In dogs unlike humans, the prostate is not fixed and hyperplasia is diffuse throughout the peripheral terminal glands expanding outwardly leading to tenesmus, while in humans the hypertrophy of the prostate is concentric, which often results in stranguria.17 However, due to the response in this small subset of dogs, castration should be discussed as an initial treatment option when tailoring the management strategy for the dog. Mild prostatitis or the effects of hypertrophy on surrounding nerves, can be difficult to diagnose, which could lead to an increased urethral pressure.4
Urodynamic studies were not performed on these dogs. If the increase in urethral pressures occurs during urine voiding, capturing this information is difficult to do for dogs. Unless telemetric devices are used,18 urodynamics are often performed while the dog is sedated or anesthetized,19, 20 but can be performed in male dogs without sedation, depending on their temperament. However, dogs are still positioned in lateral recumbency and pressures obtained do not reflect normal voiding patterns. Furthermore, reliable cystometrogram results are difficult to obtain in dogs that are not sedated. All medications and previous catheterizations should also be considered when interpreting urethral closure pressure results.
There were a number of limitations of this retrospective study including lack of long-term follow-up in many cases. Only 15 dogs had 2 or more evaluations after the initial referral, so longer-term data regarding outcome is lacking. Dogs were not randomized to treatments, treatments were not standardized, and PVRV was only estimated for most dogs. We did aim to standardize the clinical response to treatment.
In conclusion, our study demonstrates that dogs with iFUOTO have numerous interventions prescribed and variable responses to these therapies, however, 39% of dogs in this study had complete resolution of clinical signs with normal voiding perceived by owners and clinicians. This study also demonstrates the importance of implementing a standardized therapeutic outcome score so that clinicians can compare therapies across studies. Finally, until the etiology of this disease is understood, reflex dyssynergia and detrusor urethral dyssynergia are not appropriate terms for this condition and iFUOTO or dysfunctional voiding should be used instead.
ACKNOWLEDGMENT
No funding was received for this study.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




