European College of Equine Internal Medicine consensus statement on equine flaviviridae infections in Europe
Consensus Statements of the European College of Equine Internal Medicine (ECEIM) provide the veterinary community with up-to-date information on the pathophysiology, diagnosis, and treatment of clinically important animal diseases. The ECEIM Board oversees selection of relevant topics, identification of panel members for each topic with the expertise to draft the statements, and other aspects of assuring the integrity of the process. The statements are derived from evidence-based medicine whenever possible and the panel offers interpretive comments when such evidence is inadequate or contradictory. A draft is prepared by the panel, followed by solicitation of input by the ECEIM membership which may be incorporated into the statement. It is then submitted to the Journal of Veterinary Internal Medicine, where it is edited prior to publication. The authors are solely responsible for the content of the statements.
Abstract
Horses and other equids can be infected with several viruses of the family Flaviviridae, belonging to the genus Flavivirus and Hepacivirus. This consensus statement focuses on viruses with known occurrence in Europe, with the objective to summarize the current literature and formulate clinically relevant evidence-based recommendations regarding clinical disease, diagnosis, treatment, and prevention. The viruses circulating in Europe include West Nile virus, tick-borne encephalitis virus, Usutu virus, Louping ill virus and the equine hepacivirus. West Nile virus and Usutu virus are mosquito-borne, while tick-borne encephalitis virus and Louping ill virus are tick-borne. The natural route of transmission for equine hepacivirus remains speculative. West Nile virus and tick-borne encephalitis virus can induce encephalitis in infected horses. In the British Isle, rare equine cases of encephalitis associated with Louping ill virus are reported. In contrast, equine hepacivirus infections are associated with mild acute hepatitis and possibly chronic hepatitis. Diagnosis of flavivirus infections is made primarily by serology, although cross-reactivity occurs. Virus neutralization testing is considered the gold standard to differentiate between flavivirus infections in horses. Hepacivirus infection is detected by serum or liver RT-PCR. No direct antiviral treatment against flavi- or hepacivirus infections in horses is currently available and thus, treatment is supportive. Three vaccines against West Nile virus are licensed in the European Union. Geographic expansion of flaviviruses pathogenic for equids should always be considered a realistic threat, and it would be beneficial if their detection was included in surveillance programs.
Abbreviations
-
- AAEP
-
- American Association of Equine Practitioners
-
- AST
-
- aspartate aminotransferase
-
- CBC
-
- complete blood cell count
-
- CSE
-
- central southern European
-
- CSF
-
- cerebrospinal fluid
-
- E protein
-
- envelope protein
-
- ECDC
-
- European Centre for Disease Prevention and Control
-
- ECEIM
-
- European College of Equine Internal Medicine
-
- EqHV
-
- equine hepacivirus
-
- EU
-
- European Union
-
- GGT
-
- gamma glutamyl transferase
-
- GLDH
-
- glutamine dehydrogenase
-
- HBLB
-
- Horserace Betting Levy Board
-
- HCV
-
- hepatitis C virus
-
- IHC
-
- immunehistochemistry
-
- ISH
-
- in situ hybridization
-
- JEV
-
- Japanese encephalitis virus
-
- LIPS
-
- luciferase immunoprecipitation system
-
- LIV
-
- louping ill virus
-
- MVEV
-
- Murray valley encephalitis virus
-
- NS3
-
- nonstructural protein 3
-
- OIE
-
- World Organization for Animal Health
-
- PRNT
-
- plaque reduction neutralization test
-
- qPCR
-
- quantitative polymerase chain reaction
-
- R/R
-
- Romanian/Russian
-
- RT-PCR
-
- reverse transcriptase-PCR
-
- SDH
-
- sorbitol dehydrogenase
-
- TBEV
-
- tick-borne encephalitis virus
-
- USUV
-
- Usutu virus
-
- UTR
-
- untranslated region
-
- VNT
-
- virus neutralization test
-
- WNND
-
- West Nile neuroinvasive disease
-
- WNV
-
- West Nile virus
1 INTRODUCTION
The family Flaviviridae includes the genus Flavivirus, Pestivirus, Hepacivirus, and Pegivirus. All Flaviviridae are enveloped single-stranded RNA viruses. Known pathogens in horses have been identified in the genera flaviviruses and hepaciviruses1-7 (Figure 1), while pestiviruses and pegiviruses do not seem to play a role as equine pathogens.10, 11 Three neurotropic flaviviruses documented in horses suffering from meningoencephalitis are enzootic in Europe: West Nile virus (WNV), tick-borne encephalitis virus (TBEV) and Louping ill virus (LIV).1 Among the many flaviviruses which can cause disease in mammals, West Nile virus probably has the most impact on equid health. The equine hepacivirus (EqHV) is a recently identified hepatotropic virus infecting equids (donkeys and horses).12-14 It is a close homologue to the HCV and is classified as hepacivirus A.
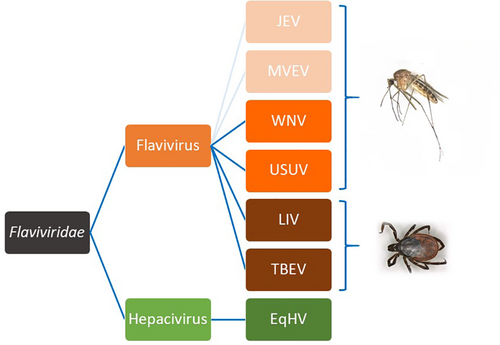
The goal of this consensus statement was to summarize recent knowledge on Flaviviridae infections relevant for equids, and to address questions of importance for the equine clinician/practitioner focusing on viruses already circulating in Europe.
2 CLINICAL DISEASE
2.1 West Nile virus
2.1.1 West Nile neuroinvasive disease—WNND
Overall, the combination, severity, and duration of clinical signs can be highly variable. Low grade fever, obtund mentation, inappetence, colic, or lameness can be among the first recognized signs in diseased animals.15 In different outbreaks the most frequent clinical signs observed are ataxia (57%-100%), weakness (30%-100%), and muscle fasciculations (42%-100%). Ataxia can be symmetrical or asymmetrical, such as weakness affecting either fore−/hindlimbs or all 4. Fewer horses present with hyper-responsiveness and cranial nerve deficits such as facial paralysis, vestibular ataxia, drooping lip and/or inability to swallow, photophobia and central blindness.16-22 Abnormal behavior such as obtund mentation, somnolence, disorientation, hyperexcitability and aggressive behavior as well as changes in personality have also been associated with WNV infection in equids.15, 20, 23, 24 Disease presentation has been observed to be similar between WNV lineage 1 and lineage 2 infected equids.22, 25
2.1.2 Risk factors of developing WNV neuroinvasive disease (WNND) and of nonsurvival of WNND
Clinical reports of case series,17, 25, 26 outbreak investigations20, 22, 27-29 and experimental infections,30-32 contribute to our current understanding of disease manifestation and risk factors for WNND. European outbreak descriptions18, 19 cover relatively low numbers of cases (80 at most by Murgue et al19) compared to outbreak evaluations in North America (up to 1698 cases in Texas outbreak by Ward et al21). Similarly, data about lineage 2 outbreaks22, 25-27 is limited compared to lineage 1.18-20, 28, 29, 33
In line with the epidemiological evidence that the majority (>80%) of equine WNV-infections are subclinical or resulting in mild clinical signs, it has also proven difficult to experimentally replicate neurological disease in otherwise healthy horses.16, 27, 30, 34 Risk is multifactorial and involves viral, host, and environmental factors. There seems to be no age, sex, or breed predisposition in horses. Mean age in different outbreaks is between 6 and 14 years (3 months-38 years).18-20, 23, 25, 28, 29, 33, 35-37
Viral lineage: There does not seem to be a difference in the risk for clinical manifestations due to either lineage 1 or lineage 2 infections in naturally infected horses.22, 25 Similarly, case fatality rate among WNV lineage 2-infected horses is similar to lineage 1.22, 25, 29 Based on a rodent model,38 differences in pathogenicity among WNV strains do not correlate with the phylogenetic lineage, geographic origin or year of isolation. Rather, virulence appears to be an evolving phenotype acquired independently of the genetic background during virus adaptation to varying ecological niches.
Vaccination status and previous natural infection: In experimental infections vaccinated horses challenged either by the intradermal or by the intrathecal route appear to be protected against severe illness.30-32 Large scale studies in North America have noted that equids that are not vaccinated more likely show clinical signs or die than do vaccinated equids. However, some horses respond poorly and WNND can occur in vaccinated animals.28, 35 Natural infection theoretically results in long lasting protection, but the humoral and cellular immunity have not been assessed in a longitudinal study.
2.2 TBEV clinical disease
Horses are prone to TBEV infection; however, they remain mostly asymptomatic. Although TBE in humans has been studied extensively, there are only a limited number of reports on TBEV infections in animals, especially in horses. Neurological signs after TBEV infection are nonspecific and can range from peracute to acute or mild subacute courses. Signs described are: reduced general condition, inappetence, hyperreactivity, ataxia, seizures, and paralysis of neck and shoulder muscles.39-43
2.3 USUV clinical disease
Reports on clinically apparent USUV infections are scarce and few human cases are described in the literature.8, 44-50 Infection of humans is usually asymptomatic, but rarely serious neurological disease is observed. Pathogenicity of USUV is unclear and sometimes a case of USUV infection is wrongly identified as being caused by WNV.51 Equine USUV-related clinical disease has not been described, but horses in Poland and Croatia can be seropositive.52, 53 Anti-USUV antibodies were detected in 2017 in horses in Austria (unpublished data).
2.4 LIV clinical disease
Louping ill virus derives its name from an old Scottish term describing the leaping motions of encephalitic sheep. Louping ill virus causes a febrile illness in sheep, cattle, grouse and occasionally in horses and some other species, that can progress to fatal encephalitis.54 Affected horses frequently have muscle tremors of the neck and facial area, or even generalized tremors. Paralysis, paresis of a single or all extremities, obtunded mentation, and abnormal behavior occur. Some horses are pyrexic (up to 40.5°C) and inappetent.55, 56
2.5 EqHV clinical disease
Equine hepaciviraus infections are mainly subclinical with mild clinicopathologic abnormalities indicating hepatopathy.13, 14 Overt clinical hepatopathy in association with the infection is rarely described.4, 57 Equine hepacivirus infections are usually self-limiting and resolve within weeks or months. Infected horses showed a delayed seroconversion against the nonstructural protein NS3 starting from 3 to 8 weeks after infection, which often precedes viral clearance.3, 13, 14, 58, 59 Additionally, successful seroconversion seems to provide a nonsterilizing immunity against reinfection, leading to lower level of viremia and shorter viremia in subsequent infections.13, 14 Liver enzyme activities (GLDH, SDH, GGT, AST) increase around the time of viral clearance; although hepatitis lasts 1 to 4 months, peak liver enzyme activity in serum frequently remain within the reference interval.13 Histopathological changes are subtle, including scattered individual hepatocyte necrosis and mononuclear cell infiltrate.3, 13, 14
Equine hepacivirus can also cause persistent infections. Experimental inoculation of naïve horses showed that adult horses were more likely to be able to clear the infection than young horses (8 months or younger) which implies an important role for the adaptive immune system in clearing the infection.2, 59 Two case reports document persistent EqHV infection with chronic hepatitis, indicating horses might develop a similar condition of chronic hepaciviral hepatitis as is observed in humans with HCV.4, 57 Histopathologic changes include fibrosis, hepatocyte necrosis, mononuclear inflammation, and biliary reaction.
3 TRANSMISSION
3.1 General
The 4 genera of the Flaviviridae family have diverse mechanisms of transmission. The flaviviruses are also known as arboviruses because they are arthropod-vectored. In contrast, the pestiviruses are primarily transmitted through contact, such as fecal-oral transmission, or vertically transmitted. Method of transmission of the hepaciviruses and pegiviruses is unclear. Both are transmitted iatrogenically by blood or a blood-product transfer, but modes of natural horizontal transmission are not known.
3.2 WNV transmission
Like many other flaviviruses, WNV is mosquito vectored. It is primarily an avian virus, where birds serve as amplifying hosts (Figure 2). Over 150 species of birds can be infected with WNV.60, 61 More than 65 mosquito species transmit WNV, with Culex spp. being the primary vectors worldwide.62, 63 Mosquitoes are capable of vertical transmission and can therefore sustain the virus for a period of time in the absence of infected hosts.63 While mosquitoes often have a narrow host range, those that “host-switch” or feed on both birds and mammals, such as Culex tarsalis in the western United States and Culex pipiens in the eastern United States and Europe, are responsible for the occasional transmission to horses and humans.63, 64 Many species of birds are susceptible to infection, some with high case fatality rate.61 There however, is variation in the reservoir/amplifying species based on geographic location.65-67 Both changing bird and mosquito habitats and ecology can contribute to the spread of WNV into new geographic regions.
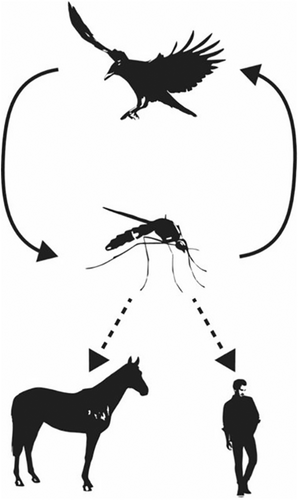
Unlike birds, horses and humans are both typically dead-end hosts. While they can develop clinical disease, they do not develop high enough viremia to transmit back to mosquitoes.61, 64 In humans, there is limited horizontal transmission via blood transfusion or organ transplantation, and vertical transmission through the placenta or breast milk.63 These routes are not documented in horses to date.
Because WNV is arthropod-borne, there is a seasonal transmission pattern, with cases starting in the summer and continuing through the autumn, although clustering of cases within those seasons depends on the local climate.68 In Europe, WNV outbreaks occur during the summer and autumn seasons (July-November) when Culex mosquitoes are abundant. Overwintering likely occurs through winter diapause, or hibernation, of infected mosquito females, or through transovarial transmission in mosquitoes.69, 70
3.3 USUV transmission
Usutu virus ecology is remarkably similar to WNV (Figure 2), and the 2 viruses cocirculate in Europe.71 Like WNV, USUV is mosquito vectored, with birds as amplifying hosts. Culex spp., particularly C. pipiens, are the major vectors, although other genera, including Aedes, contain competent species.62, 71 At least 93 bird species are susceptible to infection, with blackbirds, gray owls, and house sparrows particularly susceptible to lethal infection.71 Usutu virus infects many Passeriformes that are also susceptible to WNV. Susceptible mammals, including horses and humans, are suspected to be dead end hosts, as is observed with WNV.71
3.4 TBEV transmission
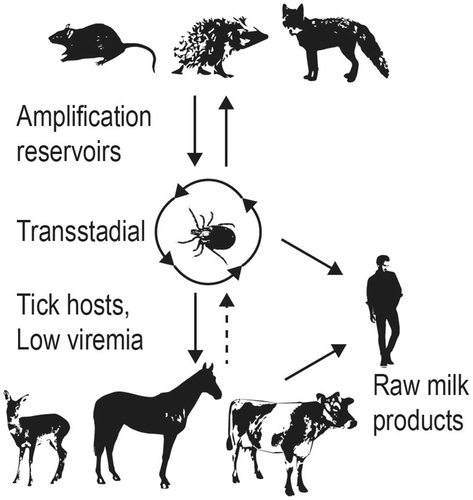
In contrast to WNV and USUV, TBEV is tick-borne (Figure 3). Although more than 14 species of ticks can be infected with TBEV, Ixodes spp. are the primary vectors. Ixodes ricinus have a broad pattern of feeding on over 300 different species including mammals, birds, and reptiles. Ticks are active while temperatures exceed 5 to 7°C, which determines the seasonal transmission to mammals.72 In more temperate regions such as central Europe, there are 2 peaks of activity: first in May-June and then in September-October. However, in colder regions there is only 1 peak in the middle of summer. The first clinical cases are typically observed 2 to 4 weeks after the onset of tick activity.72
3.5 LIV transmission
Louping ill virus is transmitted to sheep, deer, mountain hares, and grouse by feeding I. ricinus73; LIV, similarly to the other TBEV subtypes, is also transmissible to offspring by goat and sheep milk and thus has a potential to be transmitted to humans.74, 75 Naturally acquired equine infections have occurred in enzootic areas.
3.6 EqHV transmission
Just like the closely related Hepatitis C Virus, EqHV infects hepatocytes and causes both acute and chronic infection.58, 59, 76 The virus can be transferred via blood, plasma, or serum,14, 59 including vertical transmission to the foal.77, 78 In contrast to other flaviviruses, hepaciviruses are not known to be insect vectored. EqHV was not detected in mosquito pools in Austria; however, this does not rule out mechanical or biological insect vectoring.79
3.7 Geographical distribution
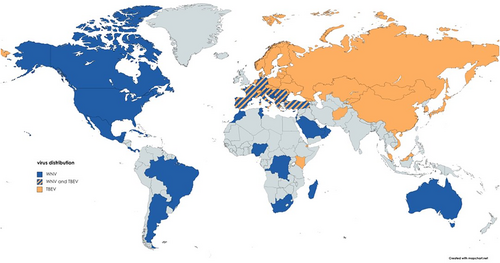
West Nile virus is the most widely distributed arbovirus that induces equine encephalitis (Figure 4). It was first isolated in 1937 in Uganda,81 then detected in a few European countries between 1958 and 1970,82-84 reemerging in the late 1990's.
West Nile virus strikingly exemplifies how unpredictably flaviviruses can emerge in naïve populations. In the United States, WNV lineage 1 was introduced in New York City in 1999, producing large and dramatic outbreaks in humans and horses. It rapidly spread throughout the country, causing more than 30 000 cases and 1200 deaths in humans and more than 24 000 cases in horses within a 10-year period.82 In contrast, Europe epidemics were irregular and limited in time and space from 1996 to 2010.
Until 2004, only lineages 1 and 3 WNV strains had been found in Europe. A first WNV lineage 2 strain was initially detected in Hungary in 2004,85, 86 with a noticeable increase in WNV transmission and outbreaks in Europe since the 2010 s associated with the spread of these WNV lineage 2 strains (Table 1).1, 88 Another WNV lineage 2 strain, first detected in 2004 in Southern Russia,98 has also been occasionally reported in Europe.
| Country | First detection of WNV | Strain detected | Hosts infected | References |
|---|---|---|---|---|
| Hungary | 2004-2008 | CSE WNV-L2 clade | Wild birds, sheep, horses, human | 22, 85, 88 |
| Russia | 2004-2007 | R/R WNV-L2 clade | Human | 89 |
| Eastern Austria | 2008 | CSE WNV-L2 clade | Wild birds | 26, 85, 90 |
| Balkan Peninsula, including Greece | 2010 | CSE WNV-L2 clade | Human, wild birds, mosquitoes | 27, 86 |
| Italy | 2010 | CSE WNV-L2 clade | Human, wild birds, mosquitoes | 91 |
| Romania | 2010 | R/R WNV-L2 clade | Human | 92 |
| Serbia, Croatia, Bulgaria | 2012 | CSE WNV-L2 clade | Human | 68, 93 |
| France | 2017-2018 | CSE WNV-L2 clade | Human, horses | 87, 94 |
| Spain | 2017 | CSE WNV-L2 clade | Wild birds | 87 |
| Germany | 2018 | CSE WNV-L2 clade | Horses | 95 |
| Netherlands | 2020 | NR | Birds, mosquitoes, humans | 96, 97 |
In Europe the most important transmission wave occurred in 2018. At the end of 2018, a total of 1503 human confirmed cases were reported in 11 countries of the EU, of which some experienced the first autochthonous infections.95 Reports from the ECDC also indicated a high transmission among horses with 285 outbreaks99 (Table 2). Independently of the spread of the dominant lineage 2 strains, lineage 1 strains can be still responsible for local outbreaks like the 1 in Spain in 2020 (77 human and 137 equine cases).101, 102
| Country | Number of equine cases | Number of human cases |
|---|---|---|
| Italy | 238 | 610 |
| Hungary | 93 | 215 |
| Greece | 19 | 315 |
| France | 13 | 27 |
| Austria | 3 | 21 |
| Bulgaria | 0 | 15 |
| Croatia | NR | 58 |
| Czech Republic | 0 | 5 |
| Romania | 18 | 277 |
| Slovenia | 1 | 4 |
| Germany | 2 | NR |
Usutu virus is thought to have been introduced into Europe multiple times through bird migration, starting in the 1950s.71, 103 In Europe, the first cases of USUV-associated large-scale deaths of wild birds were described in Italy in 1996 and in Austria in 2001.89 Within 2 decades, USUV has spread all over Europe, except for the Baltic countries and Scandinavia, and in 2020, USUV emerged in the United Kingdom.104 Viruses from African lineages were recently detected in Germany105 and the Camargue area of France.106
Tick-borne encephalitis virus is the most important human tick-borne pathogen in Europe and Asia. Temporal and geographical distribution of seropositivity in horse populations is highly variable in Europe9, 39, 107, 108 (Figure 4). Horses seem to be less sensitive to the pathogen; even with an unexpectedly high infection rate between 20% and 30% of TBEV in Austria, Germany and Lithuania,40, 109-111 equine clinical disease is rarely identified.42, 43, 111
Based on epidemiological investigations, LIV distribution area was initially limited to the British Isles (particularly in Scotland, Cumbria, Wales, Devon and Ireland), but seroconversion or clinical cases in sheep due to LIV-like viruses have been reported during the last decade in Norway, Denmark, and Spain.9, 54, 56, 112 A limited serological survey in Ireland disclosed a positive rate of 10.6%, indicating a moderate infection rate in animals of mixed breed and of different environmental backgrounds.56 Suspected equine neuroinvasive cases have been reported from clusters in the British Isles.113
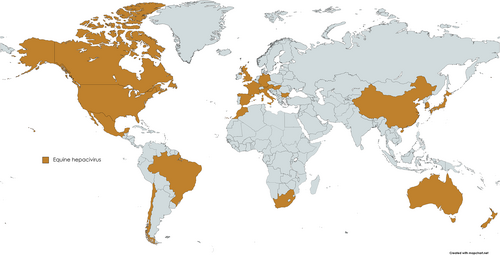
Equine hepacivirus is globally distributed with reported cases in all continents except for Antarctica (Figure 5).3, 58, 114-118 Its prevalence was reported to be between 2% and 40% on the nucleic acid level while the seroprevalence ranged between 22% and 84% in different equine populations.12, 115, 116, 118-120 Noteworthy, EqHV, so far, does not cluster in different geographical genotypes, and all deposited sequences show high similarity, in contrast to the high diversity observed in HCV.121
4 DIAGNOSIS
4.1 DIAGNOSIS OF WEST NILE VIRUS INFECTION IN EQUIDS
4.1.1 Antemortem diagnosis of WNV infection
In Europe the following diseases should be included in differential diagnosis: other flaviviruses (USUV, TBEV, LIV) which have similar seasonal (although TBE usually diagnosed early summer) and clinical presentations and cross-react in most of the diagnostic tests. Apart from equine herpesvirus myeloencephalopathy, which is endemic in Europe, rabies and Borna-virus infection can occur in certain geographical regions. Verminous meningoencephalomyelitis, bacterial meningitis, botulism, toxicosis and trauma may lead to similar acute neurologic signs as WNND. Horses with cervical vertebral malformation or equine degenerative myeloencephalopathy usually show more age-related and chronic disease progression. Autochthonous cases caused by Alphaviruses or equine protozoal myeloencephalitis have not been diagnosed so far in Europe.
A suspected diagnosis of WNV infection is generally considered based on origin of the patient (horse residing in an endemic area for WNV, Figure 4), time of year, clinical presentation of the horse and vaccination history (ie, no or inadequate vaccination history against WNV).17, 20, 122 Ancillary diagnostic tests such as complete blood count (CBC), biochemical analysis and cerebrospinal fluid (CSF) analysis could help rule in or rule out other neurological or systemic causes. Similar to other viral infections, horses can display a mild, absolute lymphopenia. Biochemical abnormalities might suggest organ-related conditions causing neurological deficits such as liver disease. Muscle enzymes can be elevated in horses experiencing trauma or prolonged periods of recumbency. CSF analysis is often nonspecific and variable with elevated nucleated cell count (mononuclear or neutrophilic pleocytosis) and/or protein concentration and mild xanthochromia.123, 124 The antemortem detection of WNV in blood and/or CSF via culture and qPCR is rarely achieved because of the short-lived and low-level viremia reported in equids.34, 125
Neutralizing antibodies have a critical role in the long-term protection from disease and, presently, their measurement provides the best correlate of flavivirus immunity and can be used for WNV field surveillance and for clinical diagnostics.126 The flavivirus envelope (E) protein is the major target of virus neutralizing antibodies. The flaviviruses are antigenically related and broad serological cross-reactions in general and cross-neutralizations in particular are observed. Their extent and duration are strongly dependent on amino acid similarity in the E-protein which ranges from 40% to 44% for unrelated flaviviruses and 60% to 70% within closely related flaviviruses.127
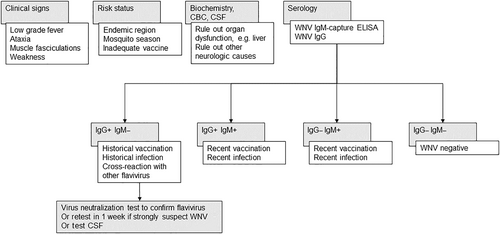
Even though there is a problem of cross-reactions with most of the serological tests, serology still provides the mainstay of presumptive WNV antemortem diagnosis (Figure 6). West Nile virus IgM-specific antibodies are often targeted to document recent infection since these antibodies rise sharply during the first few days of clinical disease and can be detected for about 4 to 6 weeks. However, because IgM antibodies can be detected up to 52 days after vaccination with either recombinant or inactivated vaccines, the serological results always need to be interpreted in the context of recent immunization.128, 129 From a diagnostic standpoint, the detection of intrathecally-derived IgM antibodies to WNV in CSF is even more conclusive to support a WNV infection, if there is no evidence of blood contamination or loss of integrity of the blood-brain barrier.130 The serological test of choice offered by many veterinary diagnostic laboratories is the IgM WNV capture ELISA (MAC). The detection of anti-WNV IgM via MAC-ELISA is more specific and sensitive than the IgG assays for currently circulating European viruses, so the diagnosis of WNV infection often only requires a single test on serum, CSF or both.131, 132 However, cross-reactivity with other viruses of the JEV group like JEV is possible and such the reliability in areas with cocirculating cross-reacting viruses is questionable.133, 134 In Europe, circulating closely related flaviviruses like TBEV, USUV, and possibly LIV, induce antibodies that generate positive results in rapid serological diagnostic tests detecting IgG.135 The gold standard confirmatory serological test in a nonvaccinated horse is thus based on the detection of serum neutralizing antibodies. All positive serological results using rapid assays should be confirmed by the more specific virus neutralization test (VNT) with the viruses known to circulate in the area (Figure 6). In nonvaccinated horses, a 4-fold change in paired neutralizing antibody titers is confirmatory of a diagnosis of WNND. Serum neutralizing antibodies can be measured by 1 of 2 formats, the plaque reduction neutralization test (PRNT) or the microwell (SN) neutralization test. The PRNT is the most commonly used neutralizing antibody test because the results are less influenced by individual differences between samples.126 Since vaccination induces formation of neutralizing antibody to the E protein of the virus, the interpretation of serological results using PRNT can be more challenging. Additional tests, such as the microsphere immunoassay, hemagglutination inhibition test and indirect fluorescent antibody test, have been developed and are often used for the surveillance of WNV in various animal species.136-138
4.1.2 Postmortem confirmation of WNV infection
West Nile virus can be isolated or detected after death in brain material of diseased horses by the use of cell cultures, through intracerebral inoculation of suckling mice with infected brain material, and by detection of specific nucleotide sequences using reverse transcriptase-polymerase chain reaction (RT-PCR) technology.126 West Nile virus has been detected in the thalamus, hypothalamus, pons/medulla and spinal cord of infected horses.139 The medulla contains the highest mean concentration of viral RNA, and WNV RNA could be detected by RT-PCR in fresh tissues and in formalin-fixed neural tissue.140 Immunohistochemistry (IHC) and in situ hybridization (ISH) have been used to detect WNV within formalin-fixed, paraffin-embedded brain tissue of horses. While these techniques are excellent for the detection of equine eastern encephalitis virus in the brain of affected horses, they are less reliable for the detection of WNV, mainly because of the low viral load in brain tissue of affected horses.141, 142 It is always advised that the laboratory performing such detection methods uses several sections of brain and spinal cord in order to increase the chances to detect WNV.
4.2 Diagnosis of TBEV and USUV
Diagnosis of infection and disease caused by TBEV and USUV follows the same algorithm as previously described in the WNV section.
4.3 Diagnosis of LIV
In the field, serology plays a key role in confirming infection. The hemagglutination inhibition test can be used as a simple means of monitoring seroconversion in man and livestock.143, 144 Because LIV is cytolytic in tissue culture, a plaque reduction neutralization test using LIV can also be used to measure antiviral titers.54 There is no data available in horses whether neutralizing antibodies against LIV can cross-react with other flaviviruses but it must be assumed based on some limited data in humans.145
4.4 Diagnosis of EqHV
EqHV RNA can be isolated from serum or liver specimens and virus copy number can subsequently be quantified via quantitative RT-PCR. For reliable virus detection, highly conserved regions such as the 5′ untranslated region (UTR) are targeted.146 Moreover, the Luciferase Immunoprecipitation System (LIPS) allows semiquantitative serologic detection of anti-EqHV specific IgG antibodies, although this is available on a research basis only at this time. For the detection of previous hepacivirus infection, the NS3 protease is a suitable target, since it is highly conserved.146 Horses are seropositive during acute hepatitis and persistent infection.13, 14
4.4.1 Panel opinion
Because horses can be EqHV viremic for months-to-years without hepatitis, a single positive serum or liver PCR does not confirm that EqHV is the cause of hepatitis. In acute EqHV infections, viremia should decline or clear within a few weeks after the onset of hepatitis, and hepatitis (especially elevated GGT) might continue past viral clearance.13, 14 In chronic hepatitis, persistent infection should be confirmed by repeated serum RT-PCR, and other causes should be ruled out by anamnesis, hematology, liver biopsy with culture, and investigation of the diet.
5 TREATMENT
5.1 Flaviviruses—WNV, USUV, TBEV, LIV
In horses, survival rate for WNV encephalitis is high compared with other infectious encephalitides (55%-70%).21, 22, 33, 35 Treatment of WNND (and neuroinvasive diseases caused by TBEV) is mainly supportive. Nonsteroidal anti-inflammatory agents (flunixin-meglumine: 1.1 mg / kg, q12h IV) are often used for 3 to 7 days after onset of clinical signs to reduce inflammation and relieve pain. The use of corticosteroids is controversial because of their immunosuppressive effect that might increase viremia.147 On the other hand, it can be hypothesized that the immunomodulatory effects of corticosteroids might hold benefits by reducing the amount of immune-mediated inflammation of the CNS. There is no scientific evidence supporting the use of corticosteroids, but also there are no contraindications and short-acting glucocorticoids (dexamethasone sodium: 0.05-0.1 mg/kg q24h IV) are often administered in cases of quickly deteriorating or recumbent animals. Antiviral agents and immunoglobulin therapy have been tested in studies mainly in humans and other species but did not gain widespread use due to limited data on their efficacy.148
There is currently no treatment available against EqHV infections in equids. There are many direct-acting antiviral treatments for the closely related HCV, at least 1 of which, sofosbuvir, has been predicted to also bind EqHV by computer modeling.149 Although it is possible that these treatments could be adapted for equine use, important hurdles remain, among these ethical concerns in sourcing such medications for equine use when they are not easily obtained for human patients.
6 PREVENTION
All 3 vaccines induced protective antibody levels for a minimum of 6 to 12 months, and protected animals against the severe neurologic form of the disease in field and clinical trials.153-155
After intrathecal challenge, some vaccinated horses (in the inactivated and the modified-live vaccine groups) developed mild/moderate clinical signs, but none required euthanasia.32 Studies comparing the antibody response duration in horses vaccinated with either whole inactivated vaccines or the live canarypox-based vaccine have reported contradictory results. The neutralizing antibody response induced by the live canarypox-based vaccine was higher and lasted longer than did that induced by whole inactivated vaccines.128 However, an earlier publication found that the IgG response induced by the live canarypox-based vaccine had a lower magnitude and lasted for a shorter duration than did that induced by the killed WNV vaccine.156
It is important to note that neutralizing antibodies are not the only factors involved in the protection against WNV infection and disease. Current evidence does not suggest a clear benefit of 1 vaccine over another.
6.1 Vaccination schedule and recommendations
West Nile virus vaccines are usually recommended from 5 to 6 months of age, depending on the WNV vaccines used, with annual boost immunization (usually recommended in spring before the mosquito season). The frequency of boost immunization could be adjusted based on the epidemiological situation (eg, semiannual or more frequently in case of active circulation, at the start of the mosquito season). Vaccination of pregnant mares is recommended before the breeding season, with a boost-immunization a few weeks before foaling to provide passive immunity to foals through colostrum.
The impact of preexisting immunity (either maternal derived immunity or previous immunization) on subsequent immunization has not been fully determined.155 Horses that have been subclinically infected or recovered from clinical disease might have long-term immunity, but there is only limited, nonpublished data about the topic and thus it is recommended to vaccinate these horses.
Recommendation for WNV vaccination will vary from country to country. For example, the HBLB Code of conduct recommend WNV vaccination for horses traveling to countries with active WNV circulation157 while the AAEP recommends immunization for all horses in North America.158 In general, vaccination is recommended in endemic areas.
There are no vaccines licensed for horses against TBEV, USUV, LIV or EqHV. To date, there is no available scientific data that reports potential cross-protection induced by a flavivirus vaccine against infection with heterologous flaviviruses in horses.
7 ONE HEALTH
7.1 Syndromic surveillance of West Nile virus activity
Horses as nonhuman vertebrate hosts are particularly sensitive to WNV, and among clinically affected horses, approximately 10% present with neurological disorders as compared to 1% of humans159, 160 rendering its detection in equids highly pertinent in a public health perspective.161 Considering that horses are spatially dispersed within human populations, and equine WNV infections typically precede human infections,160, 162, 163 a syndromic surveillance system based on the analysis of these data could be especially useful in serving as early warning for possible human viral infections.164 However, temporal distribution of previous outbreaks does not always support the hypothesis that clusters of equine cases precede human cases.27, 33, 165, 166 This situation raises concerns about the suitability of passive WNV surveillance strategies in horses.
It is suspected that, by now, large numbers of horses have been exposed to WNV in many parts of Europe and have not developed clinical signs, while developing protective immunity. This, in combination with widespread vaccination, should eventually decrease the pool of susceptible horses and decrease their role in syndromic surveillance systems. On the other hand, clinical findings in horses might be of help for syndromic surveillance for WNV activity in countries so far considered to be free of WNV.167
7.2 EqHV as a model virus
EqHV is the closest known relative to HCV, an important human pathogen. Since HCV and EqHV share common features, EqHV is also an interesting surrogate model to study hepacivirus immune responses and pathogenesis.
8 CONCLUSION
The consensus statement covers information on flaviviruses currently circulating in Europe known to infect horses and induce clinical disease. The propensity of West Nile virus to emerge to new geographic areas has already led to a spread of the virus to formerly unaffected areas like Germany, potentially leading to emergence of the infection to other countries currently considered nonendemic for WNV. Similarly, related flaviviruses like JEV are currently not endemic to Europe but show a similar pattern of emergence. Recently, JEV has spread in Australia to new geographic regions.168 Geographic expansion of flaviviruses pathogenic for equids should thus always be considered a realistic threat, and their detection should be included in surveillance programs.
ACKNOWLEDGMENT
No funding was received for this study.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




