Effects of an intravenous infusion of diltiazem on glomerular filtration rate, electrolyte excretion, and urine output in healthy dogs
Funding information: American College of Veterinary Internal Medicine, Grant/Award Number: 958079; Kansas State University; Morris Animal Foundation, Grant/Award Number: D21CA-613
Abstract
Background
Acute kidney injury (AKI) in dogs has a high case fatality rate. Diltiazem might improve renal function, but effect of intravenous infusion has not been adequately studied in dogs.
Hypothesis/Objectives
To determine if an intravenous infusion of diltiazem improves renal function through changes in glomerular filtration rate (GFR), fractional excretion of sodium (FENa), and urine output (UOP) in healthy dogs.
Animals
Ten healthy adult dogs.
Methods
Prospective, unmasked, crossover study. Dogs were randomized to receive diltiazem (loading dose of 240 μg/kg followed by 6 μg/kg/min for 300 min) or the same volume of 5% dextrose in water (D5W). The opposite treatment was given after a 7-day washout period. GFR and FENa were obtained at baseline and after infusion. UOP was measured starting 1 hour before diltiazem administration.
Results
GFR did not significantly increase from baseline with diltiazem (before diltiazem median = 2.371 mL/min/kg, range = 1.605-4.359; after diltiazem median = 2.305 mL/min/kg, range = 1.629-4.387; median difference = 0.080 mL/min/kg, 95% confidence interval [CI] = −0.417 to 0.757; P = .85), and there was no difference in D5W GFR before and after diltiazem (median = 2.389 mL/min/kg, range = 1.600-3.557; median difference = 0.036 mL/min/kg, 95% CI = −0.241 to 1.112; P = .69). FENa did not increase from baseline after administration of diltiazem (median difference = 0%, 95% CI = −0.1 to 0.1; P = .81), and there was no difference in D5W FENa (median difference = 0.1%, 95% CI = −0.1 to 0.2; P = .26). UOP did not increase with diltiazem (P = .06).
Conclusion and Clinical Importance
Intravenous administration of diltiazem does not improve markers of renal function in healthy dogs. Further studies are needed in dogs with AKI.
Abbreviations
-
- AKI
-
- acute kidney injury
-
- CRI
-
- constant rate infusion
-
- D5W
-
- 5% dextrose in water
-
- FENa
-
- fractional excretion of sodium
-
- GFR
-
- glomerular filtration rate
-
- RPF
-
- renal plasma flow
-
- UOP
-
- urine output
1 INTRODUCTION
Acute kidney injury (AKI) is a common, albeit broad clinical syndrome that encompasses both structural damage and loss of renal function. Prognosis varies depending on etiology, but the all-cause case fatality rate for dogs and cats is high at approximately 50%.1-4 Furthermore, oliguria and anuria disease states worsen the prognosis.1, 3 Management strategies vary depending on the underlying cause; however, general goals in the medical treatment of AKI are to increase or maintain glomerular filtration rate (GFR) and urine output (UOP).
Historically, diuretics such as furosemide and mannitol have been utilized to improve UOP, but they do not have any positive effects on GFR in dogs.5, 6 Other medical therapies that have been implemented to increase GFR include administration of both dopamine and fenoldopam. Negative adverse effects and conflicting data on the renal benefits have ended the use of dopamine.7-9 Fenoldopam has shown promise in increasing GFR and fractional excretion of sodium (FENa) in healthy dogs,10 but there is a lack of benefit with AKI and its use might be cost-prohibitive.11, 12
Diltiazem is a calcium channel blocker that is commonly used to treat supraventricular tachyarrhythmias in dogs. It blocks calcium entry into systemic vascular smooth muscle which causes vasodilatation.13 There might be a benefit to patients with AKI with proposed mechanisms being direct preglomerular dilatation, inhibition of tubuloglomerular feedback vasoconstriction, and natriuriesis independent of GFR.14, 15 Experimental studies in rodents15-17 and dogs14, 18-20 show positive effects on renal function, but the doses and routes previously studied in dogs are not clinically applicable. Most recently, the efficacy of a diltiazem infusion as a treatment for AKI secondary to leptospirosis in dogs was retrospectively investigated.21 Benefits of the administration of diltiazem were not found in this study, but there are multiple limitations which might have precluded detecting them.
A study investigating the renal effects of a clinically used dose and route of diltiazem in healthy dogs would be beneficial in determining the feasibility of this therapy. The primary objective of this study was to determine if a clinically used infusion rate of diltiazem administered intravenously improves renal function in healthy dogs through assessment of changes in GFR. Secondary objectives were to evaluate if natriuresis occurs through changes in FENa and UOP. Our hypothesis is that diltiazem will increase GFR, FENa, and UOP.
2 MATERIALS AND METHODS
2.1 Animals
A prospective, randomized, unmasked, crossover study was used. This protocol was approved by the Institutional Animal Care and Use Committee at Kansas State University. Informed consent was obtained from all clients before enrollment. The target cohort was apparently healthy, client-owned dogs >1 year of age and weighing >10 kg. Dogs were enrolled between April 2021 and August 2021. Dogs were deemed to be normal based on history provided by the client and physical examination findings performed by 1 of the investigators. Those determined to be normal were further screened for underlying renal disease with a CBC, serum biochemical profile, urinalysis, indirect systemic blood pressure measurement, and GFR measurement.
2.2 Screening diagnostics
Screening took place approximately 30 days before study interventions were initiated. Study participants were fasted for 10 to 12 hours before obtaining biosamples, but access to water was not restricted. Briefly, the CBC, biochemical profile, and urinalysis were performed inhouse by the Kansas State University Veterinary Health Center clinical pathology laboratory. GFR was measured by iohexol clearance reported in mL/min/kg. Iohexol at a dose of 300 mg/kg was administered IV to each patient, and blood was collected at timepoints 120, 180, and 240 min after the injection. Serum was submitted to the Michigan State University Veterinary Diagnostic Laboratory for determination of iohexol clearance using standard methods (inductively coupled plasma-mass spectrometry).22 This GFR measurement also served as the study participant's baseline. Indirect systolic blood pressure was measured by Doppler sphygmomanometry in accordance with the American College of Veterinary Internal Medicine guidelines to determine if blood pressure was normal (systolic blood pressure between 90 and 160 mm Hg).23 Echocardiographic and electrocardiographic data was also collected on this cohort and is reported separately. Dogs diagnosed with underlying disease based on these screening diagnostics were excluded.
2.3 CRI administration
Dogs were randomized to receive either diltiazem (diltiazem hydrochloride injection, 0.5%, Akorn, Inc., Lake Forest, Illinois, USA) or the same volume of 5% dextrose in water (D5W) (5% dextrose injection USP, B. Braun Medical Inc., Bethlehem, Pennsylvania, USA). Half of the dogs received diltiazem first which was determined by simple randomization (first half of names pulled out of a container). Diltiazem was diluted with D5W to a concentration of 1 mg/mL according to the manufacturer's instructions and administered as an IV bolus of 240 μg/kg followed by a CRI at 6 μg/kg/min for 300 min. An IV loading dose of 240 μg/kg was chosen as this dose should quickly achieve steady state concentrations of 120 ng/mL based on the volume of distribution of diltiazem in dogs.24 A diltiazem CRI of 6 μg/kg/min should maintain this steady state concentration and was not expected to cause hypotension.25, 26 Since hypotension can be seen with a diltiazem IV bolus,26 the loading dose was administered slowly over 10 min. For the dogs randomized to receive diltiazem, blood pressure was monitored every other minute during the loading dose to monitor for diltiazem-induced hypotension. Hypotension was defined as a systolic blood pressure <90 mm Hg.27 If hypotension developed but blood pressure remained >80 mm Hg, the rescue protocol was to discontinue the diltiazem for 5 min and recheck a blood pressure. If blood pressure was >90 mm Hg, diltiazem administration was resumed at a rate 50% lower than the original rate. If blood pressure was still <90 mm Hg or if the original measurement is <80 mm Hg, a crystalloid fluid at a dose of 10 mL/kg IV was to be administered as needed until systolic blood pressure is >90 mm Hg, and the experiment would be discontinued. After the loading dose, diltiazem was infused at a rate of 6 μg/kg/min for a total of 300 min. Blood pressure and heart rate was continually monitored every 15 min during this CRI whereas those patients in the D5W infusion treatment group had blood pressure and heart rate monitored every 60 min.
2.4 Data collection
Dogs were fasted for 10 to 12 hours before administration of each treatment. Access to water was not restricted before or during the CRI. A sterile IV peripheral catheter and urinary catheter (5 or 6 French Foley catheter) attached to a closed collection system were placed in each dog. If needed, butorphanol at a dose of 0.2 to 0.4 mg/kg IV was given to facilitate placement of the urinary catheter since this medication has minimal effects on renal function.28 The urinary bladder was emptied at the time of urinary catheter placement. UOP was quantified for 1 hour before administering the diltiazem or D5W bolus. After starting each CRI, urine production was quantified hourly until completion.
At the time of placement of the catheters, blood and urine were collected and submitted to the Kansas State University Veterinary Health Center clinical pathology laboratory in order to obtain values for calculation of FENa. Immediately after completion of the diltiazem and D5W CRIs, blood and urine were collected again for after infusion evaluation of FENa. FENa was calculated based on the following formula: FENa = 100 × ([Naurine × creatinineserum]/[Naserum × creatinineurine]).
GFR was measured again by iohexol clearance from serum samples submitted to the Michigan State University Veterinary Diagnostic Laboratory. After diltiazem had reached steady state for 60 min, iohexol was administered IV at a dose of 300 mg/kg to all dogs followed by blood sample collection at 120, 180, and 240 min after the injection. Both diltiazem and D5W infusions were continued during sample collection for GFR measurement.
After a 7-day washout period, dogs returned and received the opposite treatment, allowing for each of them to serve as their own control. This timeframe allowed for elimination of both diltiazem24 and iohexol29 given during the first treatment. Data collection was similar to the first treatment.
2.5 Statistical analysis
Sample size calculation was conducted using commercially available software (G*Power 3.1, Düsseldorf, Germany). Based on healthy dogs receiving a CRI of D5W, a total of 10 dogs with each serving as their own control provided 80% power to detect, as significant, a 30% change in GFR.10 Variables were checked for normal distribution with the D'Agostino-Pearson normality test (Prism 8, GraphPad Software, Inc. San Diego, California, USA). Since most results were not normally distributed, nonparametric tests were used. The Wilcoxon matched-pairs signed rank test was used to compare before diltiazem infusion and before D5W infusion GFR and FENa, to those taken after diltiazem infusion and after D5W infusion, respectively. The after diltiazem infusion GFR and FENa, were also be compared to their respective after D5W infusion variables with a Wilcoxon matched-pairs signed rank test. UOP measurements were compared using the Freidman test. Statistical significance was set at P < .05.
3 RESULTS
3.1 Study cohort
Ten client-owned dogs were originally screened for the study including 6 spayed females and 4 castrated males. Five of the females were excluded because of an inability to place an indwelling urinary catheter with sedation with butorphanol alone. Consequently, 5 additional male dogs were recruited for this study. A total of 10 dogs completed the study which included 9 castrated males and 1 spayed female. None of the dogs that completed the study received any sedation. The median age was 3 years (range, 1-6). There were 6 breeds represented, including Border Collie (n = 2), Goldendoodle (n = 2), Pembroke Welsh Corgi (n = 1), and Golden Retriever (n = 1). The remaining 4 dogs were of mixed breeding. Median weight was 25.3 kg (range, 14.8-31.6). Screening diagnostics were within normal limits for all enrolled dogs. None of the dogs were hypotensive at any point of the study.
3.2 Glomerular filtration rate
Three GFR values were obtained for each patient during the study which consisted of a baseline, GFR after administration of diltiazem, and GFR after administration of D5W. Results are presented in Figure 1. GFR did not significantly increase from baseline (median = 2.371 mL/min/kg, range = 1.605-4.359) with either administration of diltiazem (median = 2.305 mL/min/kg, range = 1.629-4.387; median difference = 0.080 mL/min/kg, 95% confidence interval [CI] = −0.417 to 0.757; P = .85) or D5W (median = 2.389 mL/min/kg, range = 1.600-3.557; median difference = 0.014 mL/min/kg, 95% CI = −0.426 to 0.189; P = .49). Furthermore, there was no statistical difference found between GFR after administration of diltiazem and GFR after administration of D5W (median difference = −0.036 mL/min/kg, 95% CI = −0.241 to 1.112; P = .69).
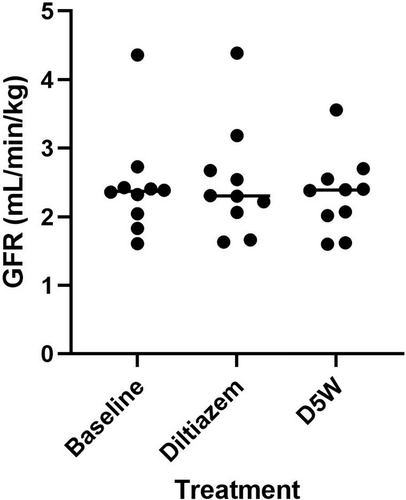
3.3 Fractional excretion of sodium
FENa did not significantly increase from baseline with diltiazem (baseline median = 0.038%, range = 0-0.5; diltiazem median = 0.045%, range = 0-0.9; median difference = 0%, 95% CI = −0.1 to 0.1; P = .81) or with D5W (baseline median = 0.1%, range = 0-0.3; D5W median = 0.17%, range = 0.1-0.4; median difference = 0%, 95% CI = −0.1 to 0.2; P = .44) (Figure 2). Additionally, FENa after diltiazem did not differ significantly from FENa after D5W (median difference = 0.1%, 95% CI = −0.1 to 0.2; P = .26). The baseline FENa results obtained before each CRI were not significantly different from each other (P > .99).
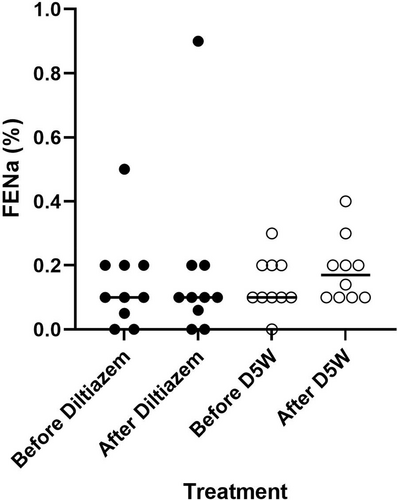
3.4 Urine output
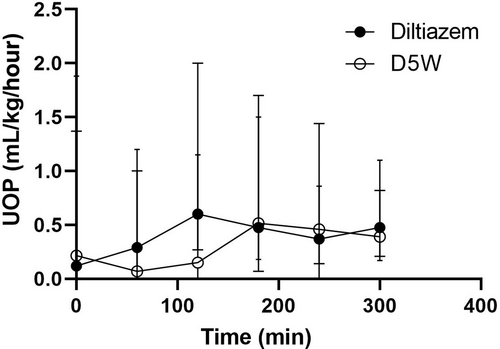
Median baseline UOP before diltiazem administration was 0.12 mL/kg/hour (range, 0-1.37), and median baseline UOP before D5W administration was 0.22 mL/kg/hour (range, 0-1.88). Baseline UOP between the 2 groups was not significantly different (P = .74). UOP did not vary significantly over time with either diltiazem (P = .06) or D5W (P = .06) (Figure 3).
4 DISCUSSION
The dose of diltiazem studied did not result in detectable increases in GFR, FENa, and UOP in healthy adult dogs. The mechanisms through which diltiazem exerts effects on the kidneys remains incompletely understood. There is generally a coordinate rise in RPF and GFR.30 However, diltiazem at a single dose of 5 μg/kg administered IV caused an increase in GFR by 24% but did not significantly change renal blood flow (RBF) in a rodents in 1 study.16
Intravenous drug delivery is standard practice in the clinical setting, so we aimed to investigate the effects of an IV infusion of diltiazem. There is a paucity of information of the renal effects with this route in dogs. When given an IV bolus of diltiazem at a dose of 100 μg/kg, there was an increase in RBF31 and mild induction of natriuresis and diuresis20 in healthy dogs, but GFR was not evaluated. This is a report of the effects on GFR in healthy dogs from a standard, clinically used IV infusion of diltiazem. Our findings of no effect might be partially because of the selected dose and route used in this study compared to previous studies, but we suspect that the prevailing reason might be the lack of renal vasoconstriction present in our study cohort. It is proposed that diltiazem's dominant effect is to counteract renal vasoconstriction since the renal vasodilatory effects of IV diltiazem might be greater in the presence of increased vascular tone, which should be absent in healthy dogs.20, 31
Both FENa and UOP did not significantly change with diltiazem in this study. Many of the dogs in this study would be considered oliguric based on their measured UOP. Although dogs were considered euhydrated on physical examination and had access to water throughout the study, it is possible that many of them were subclinically dehydrated to some degree causing a physiologic oliguria since the infusions were started early in the morning and the study participants may not have drank adequately before. We also recognize that urine might have been retained in the urinary bladder and consequently not measured. While attempts were made to move the participants and ensure there was no kinking of the urine collection system, the urinary bladders were not evaluated with sonography for evidence of urine retention during the study.
There are several other limitations of this study, 1 of which was that the study cohort consisted exclusively of healthy dogs which inherently limits the extrapolation of this data to dogs with AKI. Another limitation was that the study participants were mostly male dogs. Our initial goal was to have a study cohort of 50% females and 50% males; however, the overall difficulty of placing urinary catheters in female dogs with minimal sedation led to their exclusion. We elected to forgo more extensive sedation since our outcomes are affected by sedatives and anesthesia.32 None of the dogs that completed the study received sedation at any timepoint. Nevertheless, sex might have an influence on GFR, but it appears to be of concern with creatinine clearance techniques and not iohexol clearance.33 Although renal clearance of inulin is considered to be the gold standard for assessing GFR, iohexol plasma clearance is a valid and commonly accepted technique to measure GFR in clinical practice.33 Additionally, the sample size of this study was small which might have limited our ability to detect significant changes with some of the outcomes.
ACKNOWLEDGMENT
Funding provided by the American College of Veterinary Internal Medicine Resident Research Grant Program and the Kansas State University Department of Clinical Sciences Mark Derrick Canine Research Fund. Student funding support provided by the Morris Animal Foundation Veterinary Student Scholars Program and the Kansas State University College of Veterinary Medicine Office of Research.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Kansas State University IACUC (protocol # 4496).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




