Peripheral biomarkers of oxidative stress in dogs with acute pancreatitis
Funding information: Comparative Gastroenterology Society in Conjunction with Royal Canin
Abstract
Background
Oxidative stress is considered a pathomechanism of acute pancreatitis (AP), but no studies have extensively characterized oxidant status in dogs with naturally-occurring AP.
Hypothesis or Objectives
Evaluate measures of oxidant status in dogs with AP and explore whether these measures correlate with AP severity.
Animals
Fifteen dogs with AP and 9 control dogs.
Methods
Prospective, controlled observational study. Plasma reactive metabolite (RM) concentrations, antioxidant potential (AOP), and urinary F2 isoprostane concentrations were measured in AP dogs and healthy controls. Severity of AP was assessed by length of hospitalization and 3 disease severity indices: canine acute pancreatitis severity (CAPS), modified canine activity index (M-CAI), and the acute patient physiologic and laboratory evaluation score (APPLEfull).
Results
Reactive metabolite (RM) concentrations (median, 65 relative fluorescent units [RFU]/μL; range, 20-331 RFU/μL) and RM:AOP (median, 7; range, 4-109) were higher in AP dogs than healthy controls (median RM, 25 RFU/μL; range, 16-41 RFU/μL; median RM:AOP, 4; range, 2-7; P < .001 for both comparisons). Reactive metabolite (rS = 0.603, P = .08) and RM:AOP (rS = 0.491, P = .06) were not correlated with the duration of hospitalization or disease severity indices evaluated. However, disease severity indices did not predict mortality in our study. Normalized urine 2,3-dinor-8-iso-prostaglandin F2α concentrations were correlated with C-reactive protein (CRP; rS = 0.491, P = .03), canine specific pancreatic lipase (Spec cPL; rS = 0.746, P = .002), and CAPS (rS = 0.603, P = .02).
Conclusions and Clinical Importance
Oxidant status is altered in dogs with naturally occurring AP, but the clinical relevance of this finding is unknown.
Abbreviations
-
- AOP
-
- antioxidant potential
-
- AP
-
- acute pancreatitis
-
- APPLEfull
-
- acute patient physiologic and laboratory evaluation score
-
- CAPS
-
- canine acute pancreatitis severity
-
- CIBDAI
-
- canine inflammatory bowel disease activity index
-
- CRP
-
- C-reactive protein
-
- M-CAI
-
- modified canine activity index
-
- NF-kB
-
- nuclear factor kappa B
-
- RFU
-
- relative fluorescent units
-
- RM
-
- reactive metabolites
-
- Spec cPL
-
- specific canine pancreatic lipase
-
- TE
-
- trolox equivalents
1 INTRODUCTION
Over 100 years ago, it was proposed that pancreatitis was caused by autodigestion of pancreatic acinar cells after premature activation of digestive zymogens.1 Despite this longstanding concept, limited information is available regarding potential inciting mechanisms for premature trypsinogen activation, particularly in naturally-occurring disease. Additionally, experimental models have provided strong evidence that trypsin independent pathways of acinar cell injury and pancreatic inflammation exist, likely mediated via nuclear factor kappa B (NF-kB).2-4 Studies to further elucidate the inciting mechanisms of pancreatic inflammation may be useful in advancing our knowledge of this challenging disease process and may provide an opportunity for identification of prognostic factors and therapeutic targets.
Multiple potential initiating events have been proposed to explain trypsinogen and NF-kB activation during the early stages of pancreatitis. One proposed central mechanism is oxidative stress.5-9 Specifically, oxidative stress in acute pancreatitis (AP) is proposed to lead to pathological calcium signaling and mitochondrial dysfunction, which causes pancreatic acinar cell necrosis and AP.10 Additionally, oxidative stress may amplify the inflammatory cytokine response once AP has developed.11 Thus, oxidative stress is suspected to be involved in both disease initiation and maintenance. Markers of oxidative status therefore have garnered considerable attention in human medicine, not only as potential prognostic indicators, but also as potential targets for treatment.12-16
Oxidative stress can be defined as an imbalance between the production and elimination of free radical species. Therefore, to comprehensively evaluate oxidant status, measures of oxidative stressors and antioxidant potential (AOP) are required. Reactive metabolites (RM) include both reactive oxygen and nitrogen species and are key molecules of oxidant injury. Antioxidant potential is an overall assessment of reduction potential and is often a preferred assessment over individual antioxidant measures because it considers the collective influence of all of these molecules. Isoprostanes are chemically stable end-products of RM-associated lipid peroxidation, and the F2 isoprostanes have been evaluated as potential markers of oxidative injury in various disease processes in both human and veterinary medicine.17, 18 Collectively, these measures provide a detailed and direct assessment of oxidant status and oxidative stress.
Therefore, the primary objective of our study was to evaluate plasma RM concentrations, plasma AOP concentrations, and urinary F2 isoprostane concentrations in dogs with naturally occurring AP and in healthy control dogs. We hypothesized that dogs with AP would have increased RM concentrations, RM:AOP ratio, and urinary F2 isoprostane concentrations relative to control dogs. Additionally, we hypothesized that dogs with AP would have lower AOP concentrations relative to control dogs. An exploratory aim also was to determine whether these biomarkers would correlate with disease severity indices, duration of hospitalization, and patient outcome.
2 MATERIALS AND METHODS
2.1 Study overview and AP case definition
Dogs presented to a university teaching hospital between January 2021 and January 2022 with suspected AP were eligible for prospective enrollment in the study. The owners of all known eligible cases were contacted by study investigators for potential enrollment. The study protocol was approved by the Institutional Animal Care and Use Committee at Michigan State University. Informed consent was obtained from all owners. Sample size calculations were performed assuming power of 0.8 and α of 0.05. These calculations suggested studying 14 experimental subjects (AP dogs) and 7 control dogs to be able to detect approximate differences of 4.5 to 5.5 in RM:AOP ratio (representing a 2-fold increase from the mean value observed in a prior healthy control population studied in our laboratory). Sixteen AP dogs and 9 control dogs were enrolled to account for potential exclusions.
The diagnosis of AP was based on integration of clinical signs, pancreatic lipase concentrations, and abdominal ultrasound findings.19 Dogs were required to have ≥2 of the following clinical signs: hyporexia/dysrexia, vomiting, diarrhea, lethargy, and abdominal discomfort. In addition, dogs were required to have an abnormal patient-side pancreatic lipase assay result (SNAP cPL, IDEXX Laboratories Inc, Westbrook, Maine) and ≥2 sonographic features of AP, which included pancreatic enlargement, hypoechoic pancreatic parenchyma, and abnormal peripancreatic mesenteric echogenicity.20 All ultrasound examinations were performed by a board-certified radiologist or a trained sonographer with extensive experience in veterinary ultrasonography. When ultrasound examinations were performed by the sonographer, images were reviewed by a board-certified internal medicine specialist or radiologist to confirm study eligibility. All ultrasound scans were performed using a Canon Aplio i700 (Canon Medical Systems USA Inc, Tustin, California). An abnormal patient-side pancreatic lipase assay is indicative of pancreatic lipase concentrations ≥200 μg/L (reference interval [RI], <200 μg/L), and has moderate specificity for AP diagnosis.21-23 The presence of ≥2 of the above sonographic abnormalities is approximately 69% specific for AP diagnosis.20 Dogs also had quantitative pancreatic lipase concentrations determined using a validated immunoassay (Spec cPL, Texas A&M Gastrointestinal Laboratory, College Station, Texas) that has been shown to be highly sensitive and specific for the diagnosis of AP.21-24 It is unaffected by extrapancreatic lipases.25, 26 Although quantitative Spec cPL results were not immediately available, any dog with a normal Spec cPL concentration (RI, <200 μg/L) subsequently was excluded from statistical analyses. Other inclusion criteria included a CBC, serum biochemistry, and venous blood gas analysis within 2 days of enrollment to evaluate for concurrent disease. The diagnostic criteria utilized in the study are consistent with clinically probable or clinically suspect AP based on recently published guidelines.27 A matched control population was selected based on age, sex, and weight grouping from wellness visits and staff dogs. Control dogs were deemed healthy based on history, physical examination, CBC, and serum biochemistry assessment. The Spec cPL assays and abdominal ultrasound examinations were not performed in the control group. Dogs receiving antioxidant therapy, fatty acid supplementation, or any other nutraceuticals within 3 months of study enrollment and dogs with body conditions score ≥8/9 were excluded. Obese animals were excluded given the associations between obesity and oxidative stress.28
2.2 Sample collection and analysis
Blood and urine samples were collected from all dogs at the time of study enrollment. Blood was collected into both EDTA and plain tubes for collection of plasma and serum, respectively. Serum samples were stored at −80°C until being submitted on ice for measurement of C-reactive protein (CRP) concentration (Gentian canine-specific immunoturbidimetric CRP assay, Cornell University Animal Health Diagnostic enter, Ithaca, New York) and Spec cPL concentration (Spec cPL, Texas A&M Gastrointestinal Laboratory). The CRP and Spec cPL assays utilized in the study have been previously validated for use in dogs.24, 29 Plasma samples were flash frozen in liquid nitrogen and then stored at −80°C until RM and AOP analysis. Urine samples were placed into cryovials, 1 of which was submitted to the laboratory for measurement of urine creatinine concentration, and the second vial was treated with 4 μL/mL of an antioxidant/reducing agent (50% methanol, 25% ethanol, and 25% water, with 0.9 mM butylated hydroxy toluene, 0.54 mM EDTA, 3.2 nM triphenylphosphine, and 5.6 nM indomethacin) to prevent degradation of preformed isoprostanes and prevent ex vivo lipid peroxidation, flash frozen in liquid nitrogen and then stored at −80°C until urinary F2 isoprostane analysis.30 Markers of oxidative stress were evaluated at the authors' institution.
Oxidant status was assessed using RM and AOP concentrations in plasma. Because oxidant status is determined by the balance between RM and antioxidants, the RM:AOP ratio was calculated as a measure of overall oxidant status. Plasma RM concentrations were measured by counting the relative fluorescent units per μL (RFU/μL) using a commercial kit (OxiSelect In Vitro ROS/RNS Assay Kit, Cell Biolabs Inc, San Diego, California).31, 32 Plasma AOP was quantified by units of Trolox equivalents (TE), a synthetic analogue of α-tocopherol, measured using a photometric plate reader.31, 32 For lipid extraction from urine, samples were thawed on ice and 400 μL of urine were combined with 20 μL of the antioxidant reducing agent and 355 μL of 6 M KOH to facilitate hydrolysis. Subsequently, solid phase extraction and urinary F2 isoprostane quantification using liquid chromatography tandem mass spectrometry were performed.32 The targeted F2 series isoprostanes were 8-iso-15R-PGF2α, 5-iPF2α-VI, 8,12-iso-iPF2α-VI, and 2,3-dinor-8-iso-PGF2α. Urinary F2 isoprostane concentrations were normalized to urine creatinine concentration, to account for differences in creatinine clearance and urine flow.
2.3 Disease severity indices
Disease severity was determined on the day of enrollment by the following indices: canine acute pancreatitis severity score (CAPS), modified canine activity index (M-CAI), and the acute patient physiologic and laboratory evaluation score (APPLEfull), as utilized in previous studies.33-35 Duration of hospitalization also was used as a surrogate marker of disease severity. Mortality also was recorded.
2.4 Statistical analysis
Baseline characteristics of the AP and control groups were compared using Mann-Whitney U tests and Fisher's exact tests. Continuous data sets were assessed for normality by Shapiro-Wilk testing. Mann-Whitney U tests subsequently were used to compare concentrations of RM, AOP, RM:AOP, and normalized urinary isoprostane concentrations between dogs with AP and healthy controls. Spearman's rank correlation coefficients (rs) were used to investigate potential associations among oxidant status, CRP, CRP: albumin, pancreatic lipase concentration, disease severity indices, and duration of hospitalization. For Spearman testing, a significant correlation score of ±0.3 to 0.5 was considered a weak correlation, ±0.5 to 0.7 was considered a moderate correlation, and ±0.7 to 1.0 was considered a strong correlation.36 Statistical analyses were performed using commercially available software (GraphPad Prism Version 9.0, GraphPad Software Inc, San Diego, California). Significance for all statistical comparisons was set at P < .05.
3 RESULTS
3.1 Animals
Sixteen AP dogs and 9 control dogs met initial inclusion criteria, 1 dog with AP subsequently was excluded because of a Spec cPL concentration within the reference interval, leaving 15 dogs for analysis. Three of these 15 dogs had Spec cPL concentrations within the equivocal range. No control dogs were excluded. Baseline characteristics of the AP and control groups were similar and summarized in Table 1. Of the 15 dogs with AP, there were 6 mixed breed dogs. The remaining 9 dogs represented a variety of breeds including Golden Retriever (n = 2), Labrador Retriever (n = 2), and 1 each of: Beagle, Miniature Schnauzer, Pomeranian, Shih Tzu, and Yorkshire Terrier. The control population comprised of 7 mixed breed dogs, 1 Portuguese Water Dog, and 1 Golden Retriever.
| Variable | AP dogs | Healthy controls | P value |
|---|---|---|---|
| Age (years) | 9 (4-15) | 7 (3-11) | .10 |
| Sex (male/female) | 11/4 | 6/3 | >.99 |
| Weight (kg) | 17.6 (5.45-50.0) | 27.7 (11.0-48.0) | .38 |
- Note: Median, (range) reported. The table summarizes the baseline characteristics of the AP and healthy control groups. No significant differences were noted.
3.2 Peripheral biomarkers of oxidative stress
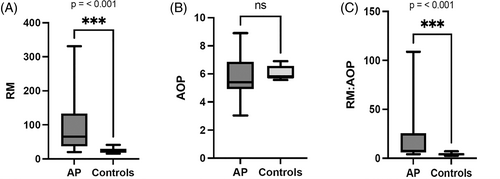
Oxidant status was altered in dogs with AP as compared to control dogs. Plasma RM concentrations were significantly higher in the AP dogs (median, 65 RFU/μL; range, 20-331 RFU/μL) than in healthy control dogs (median, 25 RFU/μL; range, 16-41 RFU/μL; P < .001; Figure 1A). One dog was euthanized because of disease severity and failure to respond to treatment and had an RM concentration of 331 RFU/μL. Plasma AOP was not significantly different between AP dogs (median, 5.40 TE/μL; range, 3.04-5.86 TE/μL) and healthy control dogs (median, 5.76 TE/μL; range, 5.57-6.90 TE/μL; P = .19; Figure 1B). The dog that was euthanized had an AOP of 3.04 TE/μL. Overall oxidant status, as measured by RM:AOP, was significantly higher in the AP dogs (median, 7; range, 4-109) than the healthy control dogs (median, 4; range, 2-7; P < .001; Figure 1C). The RM:AOP of the dog that was euthanized was 109.
Total urinary F2 isoprostane concentrations normalized to urine creatinine concentration were not significantly different between AP dogs and healthy controls (P = .82). Additionally, the concentrations of individual urinary isoprostanes normalized to urine creatinine concentration were not significantly different between the AP group and the healthy control group (Table 2).
| AP dogs | Healthy controls | P value | |
|---|---|---|---|
| Total isoprostanes/urine creatinine | 0.19 (0.05-0.63) | 0.18 (0.02-0.57) | .82 |
| 8-iso-15R-PGF2α/urine creatinine | 0.02 (0.00-0.15) | 0.01 (0.00-0.06) | .91 |
| 5-iPF2α-VI/urine creatinine | 0.03 (0.00-0.38) | 0.05 (0.00-0.14) | .95 |
| 8,12-iso-iPF2α-VI/urine creatinine | 0.10 (0.00-0.32) | 0.10 (0.01-0.38) | .82 |
| 2,3-dinor-8-iso-PGF2α/urine creatinine | 0.01 (0.00-0.05) | 0.01 (0.00-0.03) | .82 |
- Note: Median, (range) reported. The table represents urinary isoprostane concentrations normalized to urine creatinine in AP dogs and healthy controls. No significant correlations were detected.
3.3 Associations of oxidative stress markers with disease severity indices, and duration of hospitalization
The median CAPS was 8, with a range of 0 to 18. Three dogs had a CAPS ≥11 and did not die. The median M-CAI was 9, with a range of 0 to 10. The median APPLEfull was 25.5, with a range of 9 to 37. Median duration of hospitalization was 3 days (range, 1-7). Plasma RM concentrations, AOP concentrations, and RM:AOP were not correlated with any disease severity index (Table 3). Total urinary isoprostane concentrations and individual urinary isoprostane concentrations normalized to urine creatinine concentration were not correlated with M-CAI, CAPS, APPLEfull, or duration of hospitalization, with the exception of urine 2,3-dinor-8-iso-PGF2α concentrations normalized to urine creatinine concentration which were moderately correlated with CAPS (rs = 0.603, P = .02; Table 4).
| M-CAI | CAPS | APPLEfull | Length of hospitalization | |
|---|---|---|---|---|
| Plasma RM concentration | −0.060 (P = .83) | 0.228 (P = .41) | −0.237 (P = .51) | 0.473 (p = .08) |
| Plasma AOP concentration | 0.205 (P = .46) | 0.457 (P = .09) | 0.268 (P = .45) | −0.217 (P = .44) |
| Plasma RM:AOP | −0.221 (P = .43) | 0.002 (P = .99) | −0.243 (P = .50) | 0.491 (P = .06) |
- Note: The table represents correlations between biomarkers of oxidant status, 3 disease severity indices, and length of hospitalization. No significant relationships were noted.
| M-CAI | CAPS | APPLEfull | |
|---|---|---|---|
| Total isoprostanes/urine creatinine | −0.196 (P = .58) | −0.137 (P = .62) | −0.596 (P = .07) |
| 8-iso-15R-PGF2α/urine creatinine | 0.203 (P = .57) | −0.234 (P = .40) | −0.274 (P = .44) |
| 5-iPF2α-VI/urine creatinine | −0.196 (P = .58) | −0.251 (P = .36) | −0.596 (P = .07) |
| 8,12-iso-iPF2α-VI/urine creatinine | −0.222 (P = .53) | −0.145 (P = .61) | −0.602 (P = .07) |
| 2,3-dinor-8-iso-PGF2α/urine creatinine | −0.209 (P = .56) | 0.603 (P = .02)* | −0.067 (P = .86) |
- Note: The table represents correlations between urinary isoprostane concentrations and 3 disease severity indices. A moderate positive correlation was found between urine 2,3-dinor-8-iso-PGF2α concentration normalized to urine creatinine and CAPS.
- *Statistically significant.
3.4 Associations between biomarkers of oxidative stress and inflammation (CRP, CRP:albumin, and Spec cPL)
Plasma RM concentrations, AOP concentrations, and RM:AOP were not correlated with Spec cPL, CRP, or CRP:albumin (Table 5). Total urinary isoprostane concentrations and individual isoprostane concentrations normalized to urine creatinine concentration were not correlated with Spec cPL concentration, CRP concentration, or CRP:albumin, with the exception of urine-2,3-dinor-8-iso-PGF2α. Urine-2,3-dinor-8-iso-PGF2α isoprostane concentrations normalized to urine creatinine concentration were moderately correlated with CRP concentration (rs = 0.561, P = .03) and CRP:albumin (rs = 0.575, P = .03), and strongly correlated with Spec cPL concentration (rs = 0.746, P = .002).
| Spec cPL | CRP | CRP:albumin | |
|---|---|---|---|
| Plasma RM concentration | 0.022 (P = .94) | −0.204 (P = .47) | −0.093 (P = .74) |
| Plasma AOP concentration | 0.459 (P = .09) | 0.032 (P = .91) | −0.104 (P = .71) |
| Plasma RM:AOP | −0.086 (P = .76) | −0.279 (P = .31) | −0.150 (P = .59) |
- Note: The table represents correlations between biomarkers of oxidant status and biomarkers of pancreatic or systemic inflammation. No significant relationships were noted.
4 DISCUSSION
We characterized plasma and urinary biomarkers of oxidant status and oxidative stress in dogs with AP compared to a healthy control population. Plasma RM and RM:AOP were higher in dogs with AP suggesting a state of oxidative stress, but AOP and normalized urinary F2 isoprostanes were not significantly different between the 2 groups. Our results provide supportive evidence for the hypothesis of altered oxidative status in dogs with naturally-occurring AP. Based on these exploratory analyses, the clinical relevance of these abnormalities remains unknown.
We measured RM concentrations using a commercially available assay that has been utilized previously in dogs.32 The RM assay is a broad assessment of oxidative stressors and detects both reactive oxygen and reactive nitrogen species. Because oxidative stress results from an imbalance of RM and antioxidants, we also measured plasma AOP, which is a direct assessment of total antioxidant capacity. This approach was chosen in preference to indirect biomarkers such as vitamin E and glutathione which have been studied in dogs with other diseases.37, 38 Damage to tissue components such as lipids is also a defining feature of oxidative stress and results in the formation of several compounds including isoprostanes.18 Urinary F2 isoprostanes are chemically stable end products of lipid peroxidation.18 The AP dogs in our study had increased RM concentrations and RM:AOP ratio, as hypothesized. Additionally, the dog that was euthanized had the highest RM and RM:AOP of the study group. However, normalized urinary F2 isoprostane concentrations were not significantly different between the groups. Two studies involving dogs with anemia and copper-associated hepatitis also failed to identify significant differences in urinary isoprostane concentrations between test populations, despite other evidence of oxidative stress.32, 39 However, despite lack of a significant difference in concentrations between AP dogs and healthy control dogs, normalized urine 2,3-dinor-8-iso-PGF2α isoprostane concentrations were moderately positively correlated with CAPS (rs = 0.603, P = .02). Given the increased variation in the AP group, it is plausible that differences in urine 2,3-dinor-8-iso-PGF2α isoprostane concentrations or other isoprostanes may have been detected if a larger number of dogs and dogs with more severe AP had been included in the study, given the increased variation in the AP group. Alternatively, it could be argued that if more severe AP is needed to detect a significant difference in this test, then it is of limited clinical value. This result also may be a consequence of type I error. Additional large-scale studies are needed to further investigate this hypothesis.
We also aimed to determine if measures of oxidant status correlated with disease severity and patient outcomes. However, we encountered challenges in this regard. First, only 1 dog died (was euthanized), and the disease severity indices utilized were unable to predict outcome in our study. The M-CAI is a recently proposed disease severity index, which was adapted from the canine inflammatory bowel disease activity index (CIBDAI).33 The M-CAI has been shown to be significantly different between survivors and nonsurvivors of AP, although only 2 dogs died in the study reporting use of M-CAI.33 The median M-CAI values for survivors and nonsurvivors in that study were 3 and 6, respectively. The median M-CAI in our study was 9 (range, 0-10). The CAPS index is a weighted score based on serum creatinine and ionized calcium concentrations, and the presence of coagulation disorders with or without systemic inflammatory response syndrome. It has been validated for use in dogs and a CAPS ≥11 is reported to have 86% sensitivity and 92% specificity for prediction of short-term mortality.34 Three dogs in our study had CAPS ≥11, but none of these dogs died or were euthanized. Finally, APPLEfull is a nondisease specific clinical severity index used in hospitalized dogs. An APPLEfull score >30/80 is reported to have 81.2% sensitivity and 89.4% specificity for predicting mortality.35 Only 1 dog in our study had a APPLEfull score >30, and it survived to discharge. Overall, oxidant status was not correlated with the disease severity indices evaluated.
Reactive metabolite and AOP concentrations, and the RM:AOP ratio also were not correlated with CRP or Spec cPL concentration at enrollment. C-reactive protein is a positive acute phase protein that is increased in dogs with AP.40, 41 Two potential factors could explain the lack of association between CRP and RM, AOP, and RM:AOP. First RM, AOP, and RM:AOP may not be correlated with the degree of inflammatory response or disease severity and, second, CRP at presentation is a poor predictor of outcome in dogs with AP.42-44 Instead, sequential monitoring of CRP during hospitalization is recommended to offer prognostic information in dogs with AP.33, 44, 45 To further investigate this hypothesis and to overcome the challenge of the lack of sequential CRP data in our patient population, correlations of RM, AOP, and RM:AOP, to the CRP:albumin ratio were evaluated. The CRP:albumin ratio has been shown to be prognostic at presentation in both humans and dogs with AP.46, 47 The CRP:albumin ratio also was found not to correlate with RM, AOP, and RM:AOP. The lack of a significant correlation of RM, AOP, and RM:AOP with Spec cPL also may be a result of lack of correlation with pancreatic inflammation. The Spec cPL concentration typically is restricted to diagnosis rather than prognosis, although markedly increased Spec cPL concentrations (>1000 μg/L) have been identified as a negative prognostic indicator in 1 study.44 Normalized total urinary isoprostane concentrations and individual normalized urine isoprostane concentrations were not correlated with CRP, CRP:albumin, or Spec cPL concentrations, with the exception of urine-2,3-dinor-8-iso-PGF2α. Urine-2,3-dinor-8-iso-PGF2α isoprostane concentrations normalized to urine creatinine concentration were moderately correlated with CRP (rs = 0.561, P = .03) and CRP:albumin (rs = 0.575, P = .03) and strongly positively correlated with Spec cPL concentration (rs = 0.746, P = .002). Thus, pancreatic lipase concentration, as measured by the Spec cPL assay, is strongly correlated to a biomarker of oxidative stress, which is a suspected mechanism of AP pathogenesis. The specific isoprostane correlated with Spec cPL, CRP, CRP:albumin, and CAPS is the major urinary metabolite of 1 of the most abundant F2 isoprostanes produced during lipid peroxidation.48 This result also may represent a type I error because of multiple comparisons.
Our study had some limitations, predominantly related to the low mortality rate in the study population which precluded assessment of a correlation between oxidative stress biomarkers and mortality. Additionally, study population size was determined based on identifying differences in RM:AOP. Power calculations based on RM concentration or AOP concentration were not performed because oxidative stress is a result of an imbalance between the concentrations of oxidants (ie, reactive metabolites) and anti-oxidants, rather than the concentration of each considered in isolation. A larger study group may have been required to evaluate for significant differences in isoprostane concentrations between study groups. Duration of hospitalization also likely was influenced by several factors other than disease severity including cage-space limitations, owner preference, and financial considerations. Measures of oxidant status also may have been affected by other nonstudied factors such as differences in genetics, medical treatments, or nutritional intake among dogs.49, 50 Although enrolled dogs were screened for concurrent disease by blood testing and abdominal imaging (full abdominal ultrasound examination required as part of the AP diagnosis) it was not possible to fully exclude occult disease that may affect biomarkers of oxidant status. Three dogs enrolled in the study had equivocal Spec cPL concentrations but were included because of supportive clinical signs and imaging findings. Thus, our diagnosis was based on a complete clinical assessment rather than using the results of a single diagnostic assay alone to diagnose pancreatitis. Diagnostic discrepancies between ultrasound findings and pancreatic lipase concentration or activity are commonly reported and may relate to temporal factors or other causes.20, 51, 52 Nevertheless these discrepancies may have limited diagnostic specificity. Healthy dogs also were selected as the control group in our study. Studies comparing levels of oxidative stress in dogs with AP compared to other sick but non-AP dogs also may be of value. The control population was matched based on age, sex, and weight groupings, but breed matching was not possible.
In conclusion, dogs with AP have altered oxidant status, but the clinical relevance of these abnormalities cannot be definitively determined based on the exploratory analyses performed in our study.
ACKNOWLEDGMENT
This study was funded by a competitive grant from the Comparative Gastroenterology Society in conjunction with Royal Canin. The authors acknowledge Deborah Paperd for technical support. We also thank the Michigan State University Mass Spectrometry and Metabolomics Core for their assistance in liquid chromatography.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Michigan State University IACUC.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




