Neurological disease suspected to be caused by tick-borne encephalitis virus infection in 6 horses in Switzerland
Abstract
Background
Reports on acute tick-borne encephalitis virus (TBEV) infections with signs of neurologic disease in horses are limited.
Objectives
To describe the epidemiological, clinical, and laboratory findings of suspected acute TBEV infections in 6 horses.
Animals
Six horses originating from TBEV endemic regions of Switzerland were presented to equine hospitals with acute onset of neurologic disease between 2011 and 2019.
Methods
Retrospective case series. Horses with acute onset of signs of neurologic disease that were subjected to clinical and microbiological examinations to rule out infectious diseases affecting the central nervous system.
Results
All horses exhibited acute signs of neurologic disease including ataxia and proprioceptive deficits. Horses tested positive for TBEV using virus neutralization test and samples were further tested for TBEV-specific IgM. The presence of TBEV-specific IgM antibodies was confirmed in 5 horses (cases 1-5, Laboratory Unit [LU] values ranging from 30 to 56). One horse (case no. 6) with an LU value just below the test threshold (LU = 22.3) was also included under the hypothesis that the horse was transitioning from acute to chronic infection. All horses originated from areas where humans with confirmed tick-borne encephalitis reported to have been bitten by ticks.
Conclusions and Clinical Importance
Acute TBEV infection should be a differential diagnosis in horses with signs of neurologic disease and originating from TBEV endemic areas. The establishment of harmonized diagnostic criteria would help to overcome the diagnostic challenges associated with TBEV and other Flavivirus infections in horses.
Abbreviations
-
- BDV
-
- Borna disease virus
-
- C
-
- cervical
-
- CNS
-
- central nervous system
-
- CSF
-
- cerebrospinal fluid
-
- DMSO
-
- dimethyl sulfoxide
-
- ECDC
-
- European Centre for Disease Prevention and Control
-
- EHV
-
- equine herpesvirus
-
- ELISA
-
- enzyme-linked immunosorbent assays
-
- FLI
-
- Friedrich-Loeffler-Institute
-
- IFAT
-
- immunofluorescence antibody test
-
- IHC
-
- immunohistochemistry
-
- IVI
-
- Swiss Institute of Virology and Immunology
-
- LU
-
- laboratory unit
-
- NSAIDs
-
- nonsteroidal anti-inflammatory drugs
-
- SAA
-
- serum amyloid A protein
-
- TBE
-
- tick-borne encephalitis
-
- TBEV
-
- tick-borne encephalitis virus
-
- USUV
-
- Usutu virus
-
- VNT
-
- virus neutralization test
-
- WBCs
-
- white blood cells
-
- WNV
-
- west Nile virus
1 INTRODUCTION
Tick-borne encephalitis (TBE) is an arboviral zoonosis that is distributed throughout Eurasia and is considered to be the most important tick-borne viral disease in Europe.1, 2 Tick-borne encephalitis is caused by the single-stranded RNA TBE virus (TBEV), a member of the genus Flavivirus within the Flaviviridae family. The principal vectors are hard ticks within the genus Ixodes (I), in Western Europe particularly the castor bean tick, I. ricinus. The virus is maintained in several vertebrate reservoirs, which serve as virus amplifying hosts because of the development of high viremia.3
In Switzerland, TBEV occurs endemically in certain risk areas which are defined as those with reported tick bites in humans with confirmed TBEV infection.4, 5 Tick-borne encephalitis in humans is notifiable and about 110 to 120 cases are reported every year.4, 6 There are no data on the seroprevalence of TBEV in humans in Switzerland and data for domestic animals is also limited and only regional.7 Seroprevalence of TBEV in horses in Switzerland is unknown, but it is reported to be as high as 30 and 26.1% in the neighboring countries of Germany and Austria.8, 9
In humans, aside from subclinical infection and flu-like symptoms, the virus can also cause central nervous system (CNS) infections with meningoencephalitis and potentially serious outcomes such as persistent neurological deficits or even death.2 In animals, infection can vary from asymptomatic cases to severe disease similar to that observed in humans. In most domestic animals (such as ruminants and pigs) however, TBEV infections take a subclinical course and neurological manifestations are rare but observed in dogs and horses.3, 10-12
The sparse reports of clinical TBEV infections in horses describe signs such as ataxia, seizures, and paralysis of the neck and shoulder muscles.8, 10, 12, 13 These signs are also observed with West Nile virus (WNV) infections14, 15 which are often suspected in horses because of the recent emergence and ongoing expansion of this virus in Europe.16 Also, other viral causes of encephalitis/encephalomyelitis, such as equine herpesvirus type 1 (EHV-1) and other Flavivirus infections can induce similar clinical signs and are therefore important differential diagnoses.17, 18 Laboratory testing is essential to rule out TBEV infections in horses, however diagnosis can be challenging because of cross-reactivities observed between Flaviviruses.15, 19, 20 Furthermore, diagnosis can be complicated by the lack of clear criteria to establish a causal relationship between positive TBEV laboratory findings and compatible clinical signs in horses, similar to those used in human medicine.10, 21
The aims of this retrospective case series study were (a) to expand the limited knowledge on acute TBEV infections in horses, (b) to highlight the diagnostic challenges associated with TBEV infections in horses, and (c) to raise awareness among horse owners and veterinarians to improve detection of cases, especially in TBEV endemic areas.
2 MATERIALS AND METHODS
2.1 Case selection
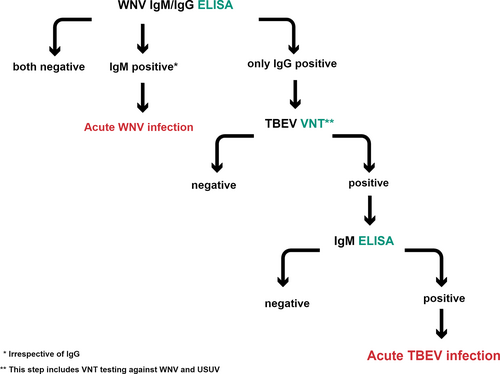
Horses (N = 74) presented to the equine hospitals of the University of Bern and Zurich, and private equine hospitals with acute onset of neurologic disease between November 2011 and May 2020 were subjected to WNV testing because of an ongoing surveillance system for equine emerging diseases. All horses had serum submitted, some also EDTA-blood (N = 5) and cerebrospinal fluid (CSF, N = 20). The samples were sent to the Swiss Institute of Virology and Immunology (IVI). Laboratory diagnosis performed at the IVI was based on 2 serological assays to detect anti-WNV antibodies in serum and CSF, and on molecular methods to detect the viral genome in blood and CSF if sampling material was available. Commercially available enzyme-linked immunosorbent assays (ELISA) were used for the detection of either WNV-IgG (IDScreen West Nile Competition Multi-species, IDvet, Grabels, France) or WNV-IgM (IDScreen West Nile IgM Capture, IDvet, Grabels, France) antibodies according to the manufacturer's protocol. The WNV-IgG ELISA yields an unspecific result because of cross-reactions with antibodies against other Flaviviruses such as TBEV, Japanese encephalitis virus (JEV), and Usutu virus (USUV). Molecular methods applied to detect WNV genome included 2 slightly modified real-time reverse transcription-polymerase chain reaction (RT-qPCR) assays.22 Because of the cross-reactivity among Flaviviruses, serum samples that tested positive or doubtful for WNV-IgG in the ELISA where forwarded to the Institute of Novel and Emerging diseases at the Friedrich-Loeffler-Institute (FLI), Insel Riems, Germany, which serves as a national reference laboratory for WNV. Diagnostic tests conducted at the FLI consisted of virus neutralization tests (VNT) to detect neutralizing antibodies against WNV, TBEV, and USUV23 as well as RT-qPCR methods to detect RNA of these viruses.22, 24, 25 Samples that tested positive for TBEV in the VNT and RT-qPCR alone or using both tests were sent for TBEV-specific IgM testing by a private laboratory that uses a modified version based on a human ELISA. This test has been validated using samples from horses with signs of neurologic disease from TBEV endemic areas in Germany (Novatec Immunodiagnostica GmbH, Dietzenbach, Germany). Samples with values >25 laboratory units (LU) were defined as positive. Horses that tested positive for TBEV-IgM were considered to be acute TBEV infection cases and were included in this case series. An overview of the serological tests conducted in the horses is presented in Figure 1. Additional samples such as nasal swabs and blood samples were analyzed with PCR and antibody testing from these horses to rule out other important differential diagnoses such as EHV-1/4 infection, Borna disease virus (BDV), and anaplasmosis (Table 1).
| Cases | WNV | TBEV | EHV1 | EHV4 | USUV | BDV | Anaplasma phagocytophilum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | VNT (ND50) | PCR | VNT (ND50) | PCR | IgM (LU) | PCR | PCR | VNT (ND50) | Blood serology | CSF serology | CSF PCR | Blood smear | PCR | IFAT | |
| 1 | − | + | − | − | 2560 | − | 56.1 | − | − | − | i | − | − | − | nt | nt |
| 2 | − | + | − | − | 1920 | − | 30 | − | − | − | nt | nt | nt | − | − | nt |
| 3 | − | + | − | − | 1280 | − | 43.9 | − | − | − | nt | nt | nt | − | − | − |
| 4 | − | i | − | − | 80 | − | 34.4 | nt | nt | − | nt | nt | nt | nt | nt | nt |
| 5 | − | + | − | − | 60 | − | 32.2 | − | − | − | − | − | − | − | − | − |
| 6 | − | + | − | − | 640 | nt | 22.6 | − | − | − | − | − | − | − | − | − |
- Note: EHV1/4 tested from nose swabs and blood except for cases 1 to 3 where only blood was tested. Threshold: ND50 > 10 positive; LU > 25 positive.
- Abbreviations: BDV, Borna disease virus; CSF, cerebrospinal fluid; EHV, equine herpes virus; i, inconclusive; IFAT, immunofluorescence antibody test; LU, laboratory units; ND50, the 50% neutralization dose; nt, not tested; TBEV, tick-borne encephalitis virus; USUV, Usutu virus; VNT, virus neutralization test; WNV, West Nile virus; +, positive; −, negative.
2.2 Medical records
Medical records from horses with positive TBEV IgM titers were obtained and signalment and clinical signs as well as treatment and outcome were extracted. The degree of ataxia was graded as described.26, 27
2.3 Data analysis and spatial epidemiology
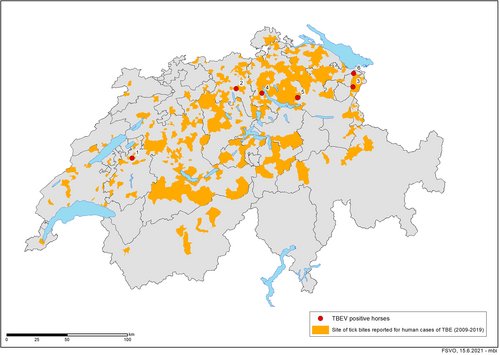
Laboratory, clinical, and geographic data were entered and summarized in excel (Microsoft Corporation, Redmond, Washington). The map was created using ArcGIS 9, ArcMap Version 10.8 by ESRI, Inc (Redlands, California). The Swiss community identification numbers for the TBE risk areas were extracted from the website of the Swiss Federal Office of Topography.5 The communities depicted as risk areas represent those regions where at least 1 human tick-bite associated TBEV case has been reported between 2009 and 2019.
3 RESULTS
3.1 Laboratory findings
Between November 2011 and May 2020, 74 horses that displayed acute signs of neurologic disease compatible with WNV infection were sampled. Serological testing with a WNV ELISA performed at the IVI revealed 17 positive and 2 doubtful results. All positive samples were forwarded to the FLI and tested negative for WNV in the VNT, except for 4 horses that had been vaccinated against WNV. However, 9 samples had tested positive for TBEV using the VNT of which 6 were further sent for TBEV-specific IgM testing. In the remaining cases, no further material was available for IgM testing. The presence of TBEV-specific IgM antibodies against TBEV was confirmed in 5 samples (cases 1-5, LU values ranging from 30 to 56). One horse (case no. 6) with a LU value just below the test threshold (LU = 22.3) was also included under the assumption that the horse was transitioning from acute to chronic infection (Table 1).
3.2 Demographic characteristics
The ages of the horses ranged from 5 to 18 years (mean and median of 11 years) and the horses were presented to the clinics between May and December. The horses belonged to a variety of breeds (4 Warmblood, 1 Haflinger, and 1 Criollo) and included 2 geldings and 2 mares (Table S1, Supporting Information). All 6 horses originated from areas where humans with confirmed TBE were reported to have been bitten by ticks. Specifically, all equine TBEV infection cases were located within the central plateau of Switzerland (Figure 2).
3.3 Clinical findings
All 6 horses exhibited signs of acute neurologic disease including ataxia (grade 2-5) and proprioceptive deficits that varied between individuals (Table 2). The neuroanatomical lesions were localized to the central nervous system in all horses, 4/6 showed signs of cerebral involvement while 2 had normal mentation. Cranial nerve abnormalities were present in 2/6 horses and abnormal skin sensitivity in 5/6. Anal tonus and tail tonus were normal in all horses as was bladder function and defecation. Common causes of neurologic diseases in horses were ruled out using microbiological tests (Table 1). There were no signs of trauma, nor was any visible leaking of blood or CSF. Radiographs of the neck were taken in 4/6 horses and did not reveal abnormalities in 2/4 and mild signs of arthritis of facet joints in the other 2 horses (cases no. 4 and 5). On blood examination, 6/6 horses had leukocytosis, 2/4 hyperfibrinogenemia, and 4/6 increased serum amyloid A concentration. The CSF was analyzed in 5/6 horses of which 2 had an increased white blood cell count (WBC) when correcting for the number of erythrocytes. An increased CSF protein concentration was seen in 2/5 horses (Table S2).
| Case | Mentation | Cranial nerves | Ataxia | Mayhew scale | Proprioceptive tests | Skin sensitivity | Tremors | Other | Localization of neuroanatomical lesion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Normal | Normal | Present | 2-3 | Abnormal | Hyperaesthesia/hyperresponsiveness | No | Twitching of nostrils and lips | CNS (cerebrum, spinal cord C1-C7) |
| 2 | Anxious, stressed, aggressive | Reduced pupillary reflexes and tongue tone, left facial nerve paralysis | All limbs, falling over, unable to get up | 5 | Abnormal | Hyperaesthesia/Hyperresponsiveness | No | Photophobia, reduced vision | CNS (cerebrum) |
| 3 | Normal | Normal | All limbs, worse on left | 4 | Abnormal | Hypoaesthesia/hyporesponsiveness | No | None | CNS (spinal cord C1-C7) |
| 4 | Severe agitation | Normal | All limbs | 4 | Abnormal | Hyperaesthesia/hyperresponsiveness | Yes, worsened by stimulation | Stringhalt like gait | CNS (cerebrum, spinal cord C1-C7) |
| 5 | Obtundation | Left head tilt | All limbs | 3 | Abnormal | Normal | No | None | CNS (cerebrum, spinal cord C1-C7) |
| 6 | Obtundation | Normal | All limbs | 2 | Abnormal | Hyperaesthesia/hyperresponsiveness | Yes | Increased muscle tone/spasms | CNS (cerebrum, spinal cord C1-C7) |
- Abbreviations: C, cervical; CNS, central nervous system.
3.4 Treatment and outcome
Five horses (cases no. 1-4 and 6) survived with intensive treatment including administration of nonsteroidal anti-inflammatory drugs, corticosteroids, dimethyl sulfoxide (DMSO), antimicrobials, valacyclovir, and vitamin E. Duration of hospitalization for surviving horses was 12 to 20 days. Three horses had fully recovered at a 3 to 4 months follow-up examination, and 2 showed residual signs of ataxia at a follow-up examination after 4 weeks and 12 months, respectively. Case no. 5 was euthanized after 5 days because of clinical deterioration (Table S3). On postmortem examination no gross abnormalities were observed. On histologic examination mild to moderate nonpurulent meningitis and encephalitis were present. A TBEV immunohistochemistry (IHC) staining performed at the Institute for pathology at the University of Bern was negative.
4 DISCUSSION
Here we describe 6 cases of acute TBEV infection in horses with neurologic signs that were diagnosed in Switzerland between 2015 and 2019. All horses originated from TBE risk areas (Figure 2) and all had tested negative for other common known infectious causes of encephalitis (Table 1). All horses had a positive TBEV VNT result and all but 1 a positive TBEV-specific IgM ELISA result. Tick-borne encephalitis virus is endemic in most parts of Europe, but only few symptomatic cases have been reported in horses so far, including the first case ever described, a horse from Switzerland.8, 10, 12, 13 Most studies on TBEV infections in horses have addressed the seroprevalence in horse populations and the exposure to TBEV seems to be consistently present in endemic parts of Europe.8, 9, 28-32 These prevalence studies suggest that horses—similarly to other grazing animals—could be regarded as a sentinel species for monitoring the TBEV status of an area.7, 29, 33 This is also supported by the results of the current study, which demonstrates that all 6 horses with acute TBEV infection originated from areas that have been associated with human TBEV infections (Figure 2). Assuming that the majority of TBEV infections in horses remain asymptomatic,9 it is expected that the clinical cases from our study only represent the tip of the iceberg of TBEV infections in horses in Switzerland. Indeed, TBEV seroprevalence in horses can be as high as 30% in endemic areas of neighboring countries, such as Germany and Austria,8, 9 and even higher (37.5%) in countries such as Lithuania.29 However, prevalence data should be compared carefully as studies were carried out in different years and TBEV infection rates can fluctuate over time.34 Annual fluctuations of human TBE cases have been observed in Switzerland, neighboring Germany and other European countries4, 35, 36 and could be related to variations in rodent populations and climatic conditions.10, 37
In Switzerland, TBEV epidemiology has been described extensively in recent years through different studies looking at TBEV prevalence in ticks, accompanied by phylogenetic and virulence analyses of the virus.6, 38, 39 Furthermore, TBEV has been detected in ticks collected from migratory birds40 as well as from samples taken from European hedgehogs (Erinaceus europaeus).41 Serological evidence has been described in blood collected from goats and rodents.7, 42 The year 2018 marked an all-time record high of 377 human TBE cases, up from an average number of 100 to 250 per year since 2015. This led the Swiss public health authorities to recommend TBEV vaccination for almost the entire Swiss population, with the exception of individuals living in the cantons of Geneva and Ticino.43 The 2018 record has already been surpassed by the number of cases in 2020.44 It was suggested that the increase in human TBE cases in recent years could have resulted from a combination of different factors, such as favorable climatic conditions for tick survival and development, as well as mild weather that led to an increase in peoples' outdoor activities.43 We expect that the increasing trend in human infections will be reflected in other affected species, including horses. However, the small, nonrepresentative number of cases in this study and the absence of any recent prevalence studies in horses or other sentinel animals do not allow any conclusions. Longitudinal studies in horses including both representative sampling to estimate seroprevalence and data from syndromic surveillance systems45 could be used to address this hypothesis.
Laboratory and clinical diagnosis of TBEV infections in horses can be challenging because of the cross-reactivity with related Flaviviruses,19, 23 the similarity in clinical signs caused by other neurologic diseases, and finally the often asymptomatic nature of TBEV infections.9 The emergence and expansion of other Flaviviruses such as USUV and WNV46-49 in TBEV endemic areas of Europe and the resulting cocirculation is further complicating TBEV diagnostics in horses and other species. Therefore, only a combination of diagnostic methods can provide an accurate laboratory diagnosis. Indeed, all horses presented in our study where initially tested with a capture WNV IgM ELISA to exclude an acute WNV infection. In the second step all animals were tested with a competition WNV ELISA which reveals virtually all Flavivirus infections because of a high degree of cross-reactivity. Therefore, positive ELISA results must essentially be deciphered by using individual Flavivirus-specific virus neutralization assays (Table 1). This underlines the need to further improve the laboratory diagnostic resolution for the detection of the different Flaviviruses which can infect the same vertebrate species.15, 20 Furthermore, the rise of WNV infections in both humans and horses in Europe50 might have also resulted in an increased disease awareness and therefore higher chances that other endemic vector-borne infections like TBEV will be diagnosed more frequently. Therefore, TBEV infections, in addition to WNV infections, should be regarded as differential diagnoses in horses exhibiting signs of neurologic disease. This is especially relevant for countries like Switzerland, where WNV infections have never been reported in animals or humans and therefore other etiologies, such as TBEV infection are more likely to be the underlying viral cause of neurologic diseases. Recently, a first case report about the clinical presentation and laboratory diagnostic work-up of a horse with TBEV from Switzerland was published.10
Information on signs of neurologic disease in humans with TBE is limited, and neuropathological findings are rather nonspecific. Lesions are seen in the cerebral and spinal meninges which exhibit diffuse infiltration with inflammatory cells, resulting in meningitis, meningoencephalitis, or meningoencephalomyelitis.51 Histopathological examination of 2 recent equine cases revealed a lymphocytic/histiocytic encephalomyelitis.12 Clinical signs can therefore vary and mimic other diseases, depending on the affected regions of the brain.12 This variation was also observed in the equine cases described in the current study. Therefore, several noninfectious causes and a number of other infectious diseases (in addition to WNV) should also be considered in horses with signs of neurologic disease in Europe, such as rabies (terrestrial rabies has been eradicated in Switzerland and most European countries), EHV-1, BDV, USUV, and in rare cases equine infectious anemia.52-54
No specific diagnostic criteria exist for establishing causal relationship between TBEV infection and signs of neurologic disease in horses. Because of the often asymptomatic nature of TBEV infection in horses and other species, it is challenging to prove that signs of neurologic disease and other clinical signs present in a TBEV seropositive horse are caused by this virus. However, the evidence of recent TBEV infection as supported by the presence of IgM antibodies, combined with the lack of additional diagnoses and the presence of signs of neurologic disease in horses from TBEV endemic areas, provides strong evidence for a causal relationship between TBEV infection status and clinical signs. The detection of IgM antibodies and concurrent CNS signs is also in accordance with the criteria used in human medicine to establish causality between TBEV infection and neurologic disease. In the European Union, a confirmed human case of TBE is defined by the European Centre for Disease Prevention and Control (ECDC) based on the following criteria: that are indicative of meningitis or meningoencephalitis combined with 1 of 5 laboratory confirmations: (a) TBEV-specific IgM and IgG antibodies in blood, (b) TBEV specific IgM antibodies in CSF, (c) seroconversion or 4-fold increase of TBEV-specific antibodies in paired serum samples, (d) detection of TBEV viral nucleic acid in a clinical specimen, or (e) isolation of TBEV from clinical specimen.36 The short duration of viremia in the beginning of the infection55 however poses an additional challenge related to criteria 4 and 5, especially when testing blood and CSF samples collected at later clinical stages. A short- and low-level viremia is also typical for humans and horses infected with the closely related WNV, therefore considered as dead-end hosts in the transmission cycle.56, 57 Case no. 6 with a negative (but just below the threshold) IgM result was included in the case definition under the assumption that the horse was transitioning from acute to chronic infection and IgM levels started declining while IgG levels were increasing. This hypothesis however could not be verified and therefore case no. 6 must be interpreted with caution. A demonstration of this IgM to IgG transition in all 6 cases would have been valuable, but unfortunately serial sampling was not performed. This antibody class switching has been observed in another case diagnosed recently in Switzerland.10 Finally, the negative TBEV IHC result in case no. 5 does not rule out TBEV infection as a cause for the signs of neurologic disease, as IHC negative cases have been reported in humans, sheep, and dogs.58-60 In Humans, at the time when neurological manifestations are present, TBEV is no longer present in blood and is only exceptionally detected in cerebrospinal fluid.51
There are currently no validated antiviral treatments available against TBEV infection and treatment of horses with signs of neurologic disease is initially often broad (anti-inflammatory, antimicrobial, antioxidant, etc) until diagnostic results become available.
In conclusion, our cases indicate that horses with acute TBEV infection exhibit signs of neurologic disease therefore it is important to raise awareness among horse owners and veterinarians to improve detection of cases, especially in TBEV endemic areas. The establishment of harmonized laboratory diagnostic criteria would help to address the issue of diagnostic cross-reactions, considering the expansion (and therefore overlap) of other vector-borne Flaviviruses that can cause signs of neurologic disease. Furthermore, horses could be routinely tested for the presence of antibodies against TBEV to better understand the epidemiology and expansion of TBEV endemic areas. The concept of using horses as sentinel species has the advantage that horses share the same environment with humans throughout the year, in contrast to other grazing animals such as sheep and cattle which are usually kept in remote alpine pastures during summer months.
ACKNOWLEDGMENT
No funding was received for this study. We thank Michael Binggeli from the Swiss Food Safety and Veterinary Office (FSVO) for creating the map shown in Figure 2.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




