Suspected spontaneous early hemorrhagic transformation of multiple ischemic strokes secondary to primary splenic torsion in a German Shepherd dog
Abstract
A 3-year-old female German Shepherd dog was presented with generalized tonic-clonic epileptic seizures, right-sided central vestibular syndrome, and right trigeminal nerve dysfunction. Acute lacunar ischemic strokes within both thalami, right side of the mesencephalon, left side of the myelencephalon, both sides of the cervical spinal cord, and acute hemorrhagic strokes within the rostral part of the right cerebellar hemisphere and right rostral colliculus were identified on magnetic resonance imaging. Additional evaluation identified multiple renal infarcts and complete splenic torsion, with entrapment of the left pancreatic lobe. Medical management, splenectomy, partial pancreatectomy, and intensive physical rehabilitation led to clinical improvement. The histology of the spleen was consistent with hemorrhagic infarction. Three months after onset, neurological examination identified only mild vestibular sequelae. The final diagnosis was multiple ischemic strokes secondary to primary splenic torsion. Spontaneous early hemorrhagic transformation, a well-known condition in human medicine, also was found in this case.
Abbreviations
-
- ADC
-
- apparent diffusion coefficient
-
- CSF
-
- cerebrospinal fluid
-
- CT
-
- computed tomography
-
- DIC
-
- disseminated intravascular coagulation
-
- DWI
-
- diffusion weighted imaging
-
- FLAIR
-
- T2-weighted fluid-attenuated inversion recovery
-
- FS
-
- fat suppression
-
- GTCS
-
- generalized tonic-clonic epileptic seizure
-
- HS
-
- hemorrhagic stroke
-
- HT
-
- hemorrhagic transformation
-
- IS
-
- ischemic stroke
-
- ICHT
-
- intracranial hypertension
-
- MRI
-
- magnetic resonance imaging
-
- RI
-
- reference interval
-
- SWAN
-
- T2 star-weighted angiography
-
- T1W
-
- T1-weighted fast spin echo
-
- T2W
-
- T2-weighted fast recovery fast spin echo
-
- TNCC
-
- total nucleated cells count
1 CASE SUMMARY
1.1 Clinical presentation
A 3-year-old female German Shepherd dog was presented with acute onset of intracranial clinical signs. The owner reported food-responsive enteropathy for 2 years associated with poor body condition. Six days before presentation, the dog was weak and had lost its appetite for 2 days without any apparent reason. The day before presentation, the dog experienced a brief generalized tonic-clonic epileptic seizure (GTCS) with hypersalivation. The next day, the owner described sudden deterioration in neurological condition with right lateral decubitus, right-sided head tilt, pathological nystagmus that changed direction depending on the position of the head, and spasticity of all 4 limbs. The left lateral decubitus position was not tolerated by the dog. There was no history of trauma or toxin exposure. Physical examination disclosed only bradycardia and low body condition score (3/9). Neurological examination identified stupor, opisthotonus, extensor rigidity of the thoracic limbs with pelvic limbs held in extension and flexed at the hips and consistent with decerebellate posture, crossed extensor reflexes in the 4 limbs, positional vertical nystagmus, absence of menace response in the right eye but normal palpebral reflex on both sides, loss of sensitivity of the right nostril, and anisocoria with left-sided myosis. Postural reactions and vision could not be assessed because of agitation. The clinical signs were consistent with acute multifocal encephalopathy, including thalamocortical sign (GTCS), right-sided rostral myelencephalon or left-sided caudal cerebellum (right-sided central vestibular syndrome), and right trigeminal nerve dysfunction or left thalamocortical sign (loss of sensitivity of the right nostril). Intracranial hypertension (ICHT) was suspected based on the presence of stupor and bradycardia. Absent menace response in the right eye was consistent with a left-sided cerebral cortical or cerebellar lesion. Left-sided myosis was considered as partial Horner's syndrome consistent with a left-sided thalamic or brainstem lesion. Differential diagnoses included an inflammatory or vascular process or, less likely, a neoplasm or metabolic or toxic encephalopathy.
1.2 Investigations
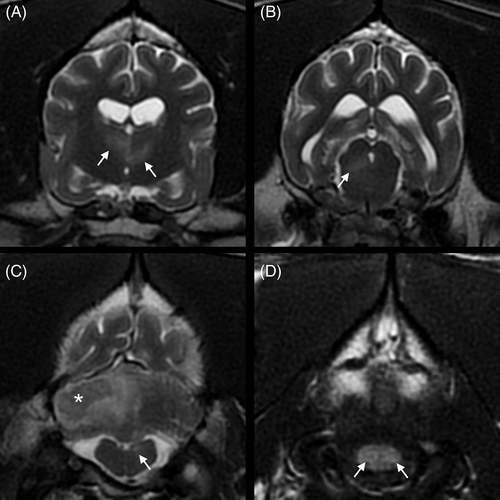
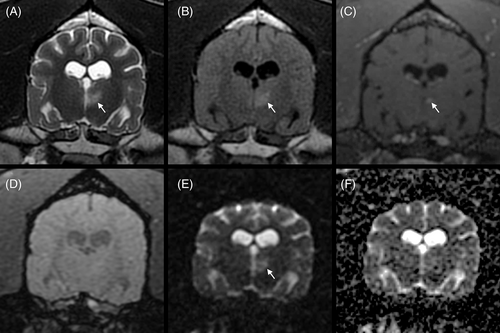
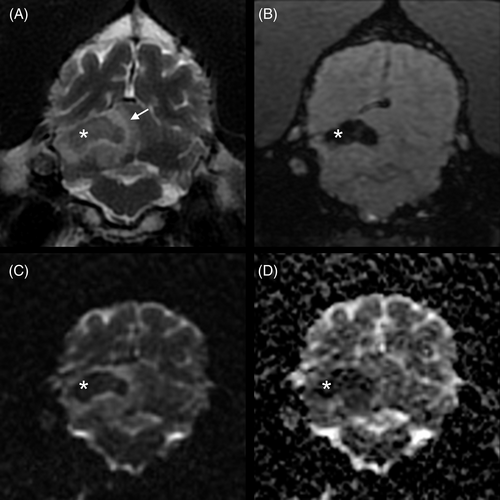
Systolic arterial blood pressure was 200 mm Hg, which was consistent with the suspicion of ICHT. Routine blood gas analysis, serum biochemistry, and urinalysis results were within reference intervals (RI). A CB disclosed mild anemia (PCV, 24%; RI, 35%-52%; hemoglobin, 8.2 g/dL; RI, 12.4-19.2 g/dL) and monocytosis (2680/μL; RI, 400-1600/μL). Platelet count was within the RI (526 K/μL; RI, 64-613 K/μL). High-field magnetic resonance imaging (MRI; Signa HD23 optima 1.5T; General Electric Healthcare) of the brain included T2-weighted fast recovery fast-spin echo (T2W), T1-weighted fast-spin echo (T1W), T2W fluid-attenuated inversion recovery (T2 FLAIR), T2W with fat suppression (T2W FS), T2 star-weighted angiography (SWAN), diffusion-weighted imaging (DWI) with b = 1000, and precontrast and postcontrast 3-dimensional fast spin echo (CUBE) T1W with fat suppression (T1W FS) sequences. Gadoteric acid was administered IV (0.1 mmol/kg). All intensities were compared with those of normal gray matter for descriptive purposes. Six small focal T2W and T2 FLAIR hyperintense lesions were observed within the left and right thalami, right side of the mesencephalon, left side of the myelencephalon, and both sides of the spinal cord at the level of C1 (Figure 1). In these areas, lesions were isointense to hypointense on T1W and hyperintense on DWI and SWAN images, and apparent diffusion coefficient (ADC) maps did not identify any abnormalities. The left thalamic lesion showed mild contrast enhancement (Figure 2). These lesions were suspected to represent acute lacunar ischemic stroke (IS) in the distribution of the proximal perforating arteries (thalamus), caudal perforating arteries (mesencephalon), and vertebral arteries (myelencephalon, spinal cord). Two T1W hypointense and T2W isointense to hyperintense lesions with T2W and T2 FLAIR hyperintense peripheral rims (perilesional edema) were observed within the right rostral cerebellar hemisphere (Figures 1 and 3) and the right rostral colliculus. These lesions were associated with strong susceptibility artifacts on SWAN and were hypointense on the DWI and ADC maps. They measured respectively 16 × 12 × 8 mm and 5 × 2 × 2 mm. These lesions were suspected to represent hemorrhagic stroke (HS) in the distribution of the right rostral cerebellar artery and perforating arteries, respectively. The differential diagnosis for multiple IS includes hypercoagulable states, systemic hypertension, neoplasia, heartworm disease, ceroid amyloid angiopathy, and septic disease.1-8 The differential diagnosis for multiple nontraumatic intraparenchymal HS includes primary causes, such as systemic hypertension and ceroid amyloid angiopathy, and secondary causes, such as vascular malformations, intracranial neoplasms, endoparasitism (Angiostrongylus vasorum), and coagulopathy.3, 5, 7, 9, 10 The right temporal muscle showed a focal area of T2W hyperintensity and increased contrast enhancement consistent with a contusion secondary to GTCS or, less likely, focal inflammation or necrosis.
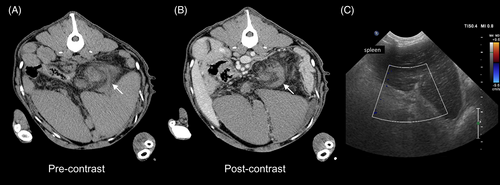
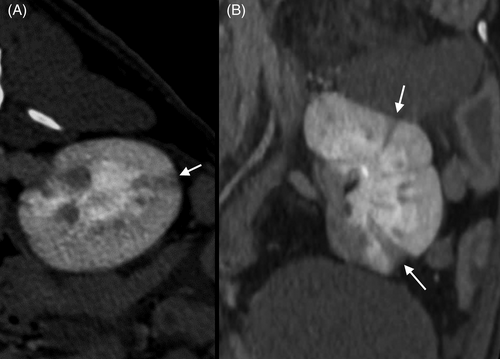
To evaluate the underlying cause of IS and HS, thoracic and abdominal computed tomography CT (BrightSpeed S 16 slices; General Electric Healthcare) scans were obtained after MRI. Thoracic imaging did not identify any abnormalities. Abdominal images showed generalized splenomegaly with a spiral-shaped pedicle with a whirl sign and lack of contrast enhancement of the splenic parenchyma. This lesion was consistent with splenic torsion, which was confirmed by ultrasonography using color Doppler examination (Affiniti 50G; Philips Medical Systems; Figure 4). The differential diagnoses included primary torsion and trauma (unobserved). Computed tomography also disclosed multiple wedge-shaped hypoattenuating and hypoenhancing areas within the renal cortex and medulla consistent with infarcts (Figure 5), right renal hypoplasia, a small volume of peritoneal effusion, and mild mesenteric lymphadenomegaly. Peritoneal fluid analysis was consistent with aseptic peritonitis, presumably secondary to splenic torsion.
Cerebrospinal fluid (CSF) analysis after cerebellomedullary cisternal collection showed a marked increase in total protein concentration (1.29 g/L; RI, 0-0.25 g/L) and total nucleated cell count (TNCC; 310/μL; RI, 0-5/μL) with neutrophilic pleocytosis (78%), erythrophagocytosis, and monocytic activation. The potential for blood contamination was low (40 erythrocytes/μL). Cerebrospinal fluid culture was negative.
Ancillary tests including prothrombin time, activated partial thromboplastin time, antithrombin III activity, and serodiagnosis (A. vasorum immunoassay; Leptospira spp. microscopic agglutination tests; Ehrlichia canis, Borrelia burgdorferi, and Anaplasma phagocytophilum/platys ELISA) were within RI. Polymerase chain reaction testing for Babesia spp., Ehrlichia spp., Anaplasma spp., and Hepatozoon canis yielded negative results.
1.3 Treatments
The dog initially was stabilized with repeated boluses of mannitol (1 g/kg IV), phenobarbital at a loading dose of 21 mg/kg IV over 24 hours and then 5 mg/kg IV q12h as well as fluid therapy. After MRI, CT, and CSF collection, corticosteroid treatment was initiated with prednisolone (0.5 mg/kg PO q24h) for brain edema. As soon as neurological condition was stable, laparotomy confirmed splenic torsion and disclosed entrapment of the left pancreatic limb by the spleen. Splenectomy, partial pancreatectomy of the left limb, and preventive incisional gastropexy also were performed. The histology of the spleen was consistent with hemorrhagic infarction. After surgery, intensive physical rehabilitation was initiated. Phenobarbital was continued PO at the same dosage. Ampicillin/sulbactam (20 mg/kg PO q12h) was initiated for 2 weeks. Corticosteroid treatment was tapered progressively over 3 weeks.
1.4 Outcome
Noninvasive blood pressure was measured daily for 1 week and remained within the RI. The PCV rapidly normalized to approximately 34%. Neurological status progressively improved, and sternal decubitus was supported after 1 week. Conscious proprioceptive testing was normal in the left limbs within a few days. Postural reactions were present in the right limbs 10 days after presentation. The dog was able to stand within 12 days and walk alone within 17 days, with severe right hemiparesis. After 6 weeks, the dog was able to walk and run with persistent ataxia in all 4 limbs, more marked in the right limbs, and had right positional ventrolateral strabismus. Three months after presentation, the clinical examination was normal, and slight ataxia was only noticed in the right limbs.
2 DISCUSSION
Initial neurological examination indicated acute progressive multifocal encephalopathy with a history of nonspecific clinical signs (weakness and loss of appetite) 5 days before GTCS. An inflammatory process initially was suspected, but HS or IS occasionally can induce multifocal signs.10, 11 Magnetic resonance imaging identified acute HS within the right rostral cerebellar hemisphere and right rostral colliculus, and multiple acute IS within both thalami, the right side of the mesencephalon, the left side of the myelencephalon, and both sides of the spinal cord at the level of C1. Intracranial hypertension was not confirmed on MRI. Cushing's reflex with arterial hypertension and bradycardia reflected brainstem ischemia. Thalamic lesions can explain the GTCS, decreased mentation, postural deficits, and absence of menace response in the right eye. Left-sided myosis was consistent with thalamic and spinal cord IS. The right-sided head tilt, asymmetric postural deficits, and pathological nystagmus were consistent with right-sided central vestibular syndrome. Some studies have indicated that paramedian thalamic, paramedian rostral mesencephalic, and rostral cerebellar IS can be associated with contralateral vestibular signs.11-13 Thus, left thalamic IS and rostral cerebellar HS can explain right vestibular syndrome. The decerebellate posture was consistent with cerebellar HS. The sensory nucleus of the trigeminal nerve extends throughout the brainstem. Thus, right mesencephalic IS and left thalamic IS could explain the loss of sensitivity of the right nostril.
High-field MRI is considered sensitive for detecting acute vascular and inflammatory brain lesions.5, 14 Recent advances in diffusion imaging with DWI, ADC maps, and perfusion-weighted imaging allow early diagnosis of brain ischemia.4, 15 Currently, restricted diffusion with hyperintensity on DWI is considered the gold standard in human medicine. This technique is nearly 100% sensitive and specific in diagnosing acute IS.15 In an induced dog model, a study found that relative DWI allowed aging of IS.16 In our case, increased DWI values could be consistent with restricted diffusion.4, 5 Nevertheless, these lesions were T2W hyperintense and associated with a normal ADC map. We therefore suspect a “T2 shine-through” effect, suggesting that the IS were too small to be assessed by DWI, or pseudonormalization of ADC after early reperfusion.4, 17 Detection of a signal void on susceptibility-weighted sequences (eg, SWAN) also has excellent sensitivity and specificity for acute hemorrhagic component and intracranial mineralization. The onset of HS can be predicted by the evolution of T1W and T2W signal intensities according to the cellular distribution and types of hemoglobin degradation products.5, 18, 19 Hemorrhagic strokes were considered clinically hyperacute (a few hours) to acute (hours to 3 days) based on hypointensity on T1W and isointense to hyperintense areas on T2W, presence of rim edema, and acute onset of clinical signs.
The first neurological event (GTCS) occurred approximately 48 hours before MRI. Considering the location of all strokes, thalamic IS is the main explanation. We hypothesize that this event was associated with other IS and was caused by multiple emboli or the breakup of an embolus within the systemic vasculature. Virchow's triad (vessel endothelial injury, venous stasis, and hypercoagulable state) associated with torsion of the spleen can explain the infarction in our case. Another possible explanation could be disseminated intravascular coagulation (DIC). However, platelet count, clotting time, and antithrombin III activity were within RI. Thus, DIC is unlikely. The neurological deterioration with vestibular signs and the decerebellate posture, which occurred 24 hours after the onset of clinical signs, can only be explained by HS. In human medicine, hemorrhage can occur as a complication of IS when cerebral blood flow is restored to damaged vasculature. Blood-brain barrier disruption is a central mechanism. This condition is referred to as hemorrhagic transformation (HT) and can cause clinical deterioration with associated poor outcomes.20 It occurs generally during the first 24 to 36 hours after stroke onset.21 Early HT (<24 hours) frequently is detected in patients treated by IV thrombolysis, but also can arise spontaneously.21, 22 Increased time from IS onset to reperfusion has been associated with an increase in HT risk with or without thrombolysis in rats.23 In addition, underlying causes of HS and IS are quite different.3, 5 Thus, the coexistence of different origins in our case seems unlikely. Considering that other causes of HS were excluded, HS in our case was most likely caused by spontaneous early HT. The limitation of this diagnosis is the absence of imaging in the first few hours after GTCS. In dogs, HT has only been described in induced models24-27 or during reperfusion of chronic ischemia.28 To the best of our knowledge, spontaneous early HT has only been suspected in veterinary medicine, but has never been documented.
In IS and HS, CSF analysis is often nonspecific and results can be normal.3, 29 In our case, erythrophagocytosis confirmed the hemorrhagic component. Cerebrospinal fluid analysis also disclosed markedly increased protein concentration and TNCC with neutrophilic pleocytosis without frank blood contamination. These results primarily reflect inflammatory disease but also can be found in vascular necrosis. Testing for infectious agents was negative. Recovery without long-term immunosuppressive treatment (corticosteroids at anti-inflammatory dosage tapered within 3 weeks) seems unlikely for noninfectious meningitis or meningoencephalitis of unknown origin. Thus, the main hypothesis was blood-brain barrier disruption associated with hemorrhagic transformation. The coexistence of a single IS and renal infarcts has been described previously in association with bacterial endocarditis30 and pancreatitis.31 Only necropsy examinations were performed in these reports. Thromboembolism was the main hypothesis in both cases. In our case, renal infarcts were suspected to be concomitant with stroke but might also be an incidental finding. It is uncommon for animals to experience multiple infarctions.11 Pancreatic entrapment was not noticed on CT and ultrasound examination, and could have occurred between imaging and surgery. Thus, the pancreatic lesions were unlikely to have caused renal infarcts or IS. After surgery, with only nonspecific treatment, the dog had almost complete recovery without relapse, and an untreated underlying etiology likely would have induced other clinical signs over time. Considering all of these factors, and in the absence of evidence of DIC, massive thromboembolic release secondary to splenic infarction was our main diagnosis.
Splenic torsion is a rare condition in dogs, accounting for 0.5% to 3.4% of all splenic conditions.32, 33 This condition often is seen in conjunction with trauma, neoplasia, and gastric dilatation-volvulus. In our case, no cause was found and primary splenic torsion was considered.34 Primary splenic torsion mostly is reported in large or giant breed, deep-chested dogs, particularly great Danes and German Shepherd dogs.34, 35 Hypotheses include weakness of the supporting ligaments of the spleen.36 Considering the episodic weakness and loss of appetite 6 days before presentation, primary splenic torsion was suspected.
In dogs with IS, the prognosis is fair to excellent, with rapid clinical improvement in many cases.8, 13, 37 Concurrent medical conditions are associated with shorter survival times.8 In dogs with HS, the presence of a single lesion seems to be associated with a better prognosis than the presence of multiple lesions, regardless of size.10 However, prognosis with concurrent ischemia and HS has not been studied. In humans, HS and early HT are associated with higher mortality than a single IS.22, 38 In our case, the outcome was excellent: the dog had an almost complete recovery within 3 months with only slight neurological sequelae.
3 CONCLUSION
To the best of our knowledge, ours is the first case of multiple IS secondary to primary splenic torsion in a dog. Evaluation suggested spontaneous early HT within the right rostral cerebellar hemisphere and right rostral colliculus. This situation is very common in human medicine with IV thrombolysis but has not been described in dogs.
ACKNOWLEDGMENT
No funding received for this study
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study




