A 50-step walking test for analysis of recovery after decompressive surgery for thoracolumbar disc herniation in dogs
Suzanne Rosen and Jessica Lynn Grzegorzewski contributed equally to this study.
Abstract
Background
Despite its importance, there is no agreed definition of recovery of ambulation in dogs with spinal cord injury.
Objectives
To validate a new walking test in dogs recovering from thoracolumbar spinal cord injury.
Animals
Two hundred twenty-four dogs weighing <20 kg: 120 normally ambulatory dogs, plus 104 dogs undergoing decompressive surgery for acute thoracolumbar intervertebral disc herniation.
Methods
Prospective cohort studies. The distance each freely-ambulatory dog walked during 50 step cycles was regressed on ulna length. For each postsurgical dog, we recorded when the calculated 50-step distance was completed without falling, or their inability to complete this distance by 4 months or more after surgery. Bayesian analysis compared outcomes for presurgical neurologic categories; association of recovery with several preoperative variables was explored using logistic and time-to-event regression.
Results
For control dogs, 50-step distance (m) = 1.384 × ulnar length (cm) + 2.773. In postsurgical dogs, the 50-step test provided decisive evidence that deep pain-negative dogs were less likely to recover ambulation than dogs with intact pain perception (12/29 recovered vs 71/75; Bayes factor [BF] = 5.9 × 106) and, if they did recover, it took much longer (median 91 days vs median 14 days; BF = 1.5 × 103). Exploratory analysis suggested that presurgical neurologic status (subhazard ratio [SHR] = 0.022; P < .001) and duration of presurgical anesthesia (SHR = 0.740; P = .04) were associated with rapidity of recovery.
Conclusions and Clinical Importance
This straightforward 50-step walking test provides robust data on ambulatory recovery well-suited to large scale pragmatic trials on treatment of thoracolumbar spinal cord injury in dogs.
Abbreviations
-
- ANOVA
-
- analysis of variance
-
- BF
-
- Bayes factor
-
- BIC
-
- Bayesian information criterion
-
- CT
-
- computed tomography
-
- DPN
-
- deep pain negative
-
- IQR
-
- interquartile range
-
- MRI
-
- magnetic resonance imaging
-
- PPR
-
- paraparetic
-
- PPL
-
- paraplegic
-
- WISCI
-
- walking index for spinal cord injury
1 INTRODUCTION
Acute spinal cord injury is common in dogs, largely because of the high prevalence of intervertebral disc herniation, especially in chondrodystrophic breeds.1-3 Fortunately, although the initial presentation is often dramatic, most affected dogs recover satisfactory function following current standard therapy.4, 5 However, there remain many unanswered questions about the treatment of disc-associated spinal cord injury in dogs, such as whether there is a need for emergency treatment6-8 and whether durotomy provides benefit in severe injuries.
To answer these and other questions, reliable and generalizable methods are needed to assess the outcome of new or traditional therapies. Surprisingly, there is no universally recognized method of defining recovery of ambulation after thoracolumbar spinal cord injury. Traditional scoring schemes, such as the modified Frankel score,9, 10 and a more recent gait score,11 describe “ambulatory” as “walking without assistance,” without further definition. Although a number of scales for quantifying various aspects of gait have more recently been developed,12-16 these are focused on defining quality of ambulation rather than whether a dog “can walk or not.” Perhaps for this reason, such scales have not become widely adopted and many, but not all,6 published reports on recovery after decompressive thoracolumbar spinal surgery continue to describe “restoration of ambulation” without a specific definition.17-19
In comparison studies, differences between therapies in recovery of ambulation after thoracolumbar spinal surgery are often modest. Therefore, to achieve adequate power, large numbers (hundreds) of cases are required,20 for both observational or more formal study designs. Therefore, useful outcome measures must be reliable, quick to perform and easy to apply so that data can rapidly be gathered on large numbers of affected dogs, often from many different geographical sites. There is a similar need for large-scale studies in human medicine that is often filled by “pragmatic” trials.21 Pragmatic trials are characterized by inclusion of large numbers of patients, simple, real-life measures of outcome and little restriction on the type of patients that are included.21-23 Thoracolumbar spinal cord injury in dogs constitutes a good target for this type of trial and pragmatic outcome measures would facilitate collection of plentiful data. Pragmatic outcomes have already been identified for humans with spinal cord injuries, such as the 10-m and 6-minute walking tests,24 WISCI (walking index for spinal cord injury)25 and 50-ft walking speed,26 and having similar measures available for dogs would also expedite interspecies translation.
In this study we investigated the value of a new 50-step outcome measure in defining and differentiating recovery of ambulation after thoracolumbar spinal cord injury in dogs. Our primary aim was to provide a pragmatic definition of “ambulatory” in dogs recovering from thoracolumbar spinal cord injury that is meaningful in everyday life, can be widely applied in veterinary clinics and clinical trials and, through close analogy with human spinal cord injury outcomes, facilitate translational relevance. As a secondary aim we also wished to use this outcome to reexplore the effects of various preoperative variables that have previously been highlighted as being of possible prognostic importance.
2 MATERIALS AND METHODS
Client-owned dogs that presented to the Neurology Clinic of the Small Animal Hospital at Texas A&M University College of Veterinary Medicine and Biomedical Sciences from September 2020 to December 2021 were included in this study. Of these, some served as control dogs to derive a regression equation relating dog size to distance walked in 50 step cycles, and some served as test dogs for which we determined the time taken to recover (and if they recovered) to walk the 50-step distance unaided (see below).
Puppies (<1 year of age), dogs that weighed >20 kg and those with symptomatic systemic disease, generalized neuropathy or that had serious medical conditions that meant they were unlikely to live longer than 1 year from presentation were excluded from both parts of the study. We restricted this investigation to dogs weighing <20 kg because these are the most commonly affected by acute spinal cord injury (because acute intervertebral disc herniation is most common in small chondrodystrophic dogs) and limiting the weight range would enhance the precision of our regression equation (see below). In the test group we included only dogs with acute (less than 3 weeks from first clinical signs to functional nadir) injury to the spinal cord between T9 and L4 vertebrae caused by herniation of an intervertebral disc (ie, the most caudal included lesion was at the L3/4 disc).
The test of ambulation was based on the ability of a dog to recover to walk unassisted for 50 step cycles without falling. This distance was selected for 2 main reasons: (i) analogy with the human 50-ft walking test26—this distance is ~15 m, over which distance a miniature dachshund takes about 50 steps (see below); and, (ii) we considered that the 50-step distance was appropriate for an owner to designate a dog as “functionally ambulatory” because it implies that the dog can walk sufficiently far to carry out routine elimination behavior without assistance.
2.1 Testing of control dogs
Because dogs with thoracolumbar spinal cord injury vary in size, we first needed to determine the relationship between dog size and distance walked in 50 steps. For this first group, we set out to collect walking data on 120 dogs that weighed <20 kg that presented to the neurology clinic for reasons other than thoracolumbar spinal cord disease (eg, dogs presenting for seizures, movement disorders, endocrine disease, those recovering from vestibular lesions, etc) and were able to walk indefinite distances without falling. We recruited 120 dogs for this part of the study because it is the number commonly used to establish reference intervals for laboratory measurements.27
Dogs presented to the neurology clinic and meeting the inclusion criteria were led along an outdoor concrete walkway, while an observer counted out 50 step cycles (a “step cycle” was defined as 1 step being made by each leg, implying that it was necessary to count 50 steps made by any 1 limb). The walking speed was adjusted to each individual so as to be sufficiently slow to allow step counting but not so slow to encourage the dog to stop and start; counting was commenced from a “flying start” (each dog was already walking and then crossed a start line where the count was started). The distance over which each dog completed 50 step cycles was measured, using a steel measuring rule, and recorded. Then, each dog's ulna was measured (as a proxy for the size of the dog), to the nearest 0.5 cm, from the externally-palpable landmarks of the proximal tip of the olecranon to the distalmost point of the styloid process of the ulna, using a tape measure. We also recorded the breed and weight of each participating dog.
At the completion of this part of the study, when the designated number of dogs had been walked and measured, the 50-step-cycle distance was plotted against ulna length and the corresponding regression equation calculated. This equation was used to determine the 50-step-cycle-distance for test dogs in the second part of the study, over which to measure the time to recover ambulation after surgery.
2.2 Dogs undergoing decompressive surgery
Test dogs were those that were undergoing routine surgical treatment of nonambulatory paraparesis or paraplegia caused by thoracolumbar disc herniation. Each dog underwent routine physical and neurological examination. Ambulatory function was categorized as: (i) nonambulatory paraparetic (unable to walk but with detectable voluntary motor function—“PPR”); (ii) nonambulatory paraplegic (no voluntary motor function but “deep pain” sensation intact—“PPL”); and, (iii) paraplegic with loss of deep pain perception in the hindquarters—“DPN.” For these 3 categories, we defined “nonambulatory” according to our clinic's rule-of-thumb for considering dogs for decompressive spinal surgery as being unable to take 10 consecutive pelvic limb steps without falling. During reflex testing the ability to detect a noxious stimulus to the pelvic limb paws was assessed by pinching with fingers. If a cranial response to this stimulus was not observed then “deep pain” sensation was assessed by crushing the nails beds of at least 1 digit on each pelvic limb with metal implements (ranging from artery forceps to pliers). If a conscious response to these stimuli was not observed then the tail and individual phalanges of the pelvic limbs were also tested in the same way.8, 28 Care was taken not to damage the skin (eg, by wrapping bandage material around the jaws of the metal implements).
After initial physical and neurological examination, consent for diagnostics and surgical decompression of the spinal cord was given by the owners and lesion localization was confirmed using MRI (Siemens 3T Magnetom Verio, Siemens Medical Solutions, Malvern, Pennsylvania), CT (Siemens Somatom Definition, Siemens Medical Solutions, Malvern, Pennsylvania), or both, with the dogs under general anesthesia (or, in some cases, deep sedation induced by dexmedetomidine and methadone, for CT). Immediately following diagnosis of acute disc herniation, each dog underwent decompressive spinal surgery and, upon recovery from anesthesia, received standard analgesic and bladder care appropriate for their neurologic status. While hospitalized, each dog underwent daily or twice-daily neurological examination with emphasis on gait, motor function and cutaneous trunci reflex cutoff.29
At discharge, the time of which was dependent upon the dog's attitude and neurologic status, especially bladder function and appropriateness for home-management, each owner was given a length of string (twisted sisal rope 0.06 in., Blue Hawk item #1289804, Lowe's Home Improvement), corresponding to the distance that their dog was predicted to complete 50 step cycles (based on the previously developed regression equation) along with instructions about how to use it (see Supplementary Material A). Owners were instructed to measure out the taut length of the string on any convenient surface or area that they were able to walk the dog (such as the yard or sidewalk, etc) and then inform us when the dog was able to walk the entire distance without any part of the limbs above the hock or above the carpus touching the ground at any time. A photograph of a dog was provided to aid with these anatomical instructions. The owners were instructed that the full distance did not have to be completed without stopping, but that if the dog sat or lay down then the distance needed to start again from zero at that point. In addition to asking the owners to contact us and to send videos to confirm dog walking abilities, we made repeated contact with the owners in person at recheck appointments (routinely scheduled for 4-6 weeks postoperatively), or by phone calls at intervals determined by each dog's progress: for those that were “deep pain-negative” and not walking by 4 to 6 weeks we checked every 2 to 4 weeks, for those that had been paraplegic or paraparetic at presentation we checked in at weekly intervals until they recovered to walk or, if not recovered by 6 weeks, at the same intervals as deep pain-negative dogs. For analysis of time to recover, dogs were grouped according to their neurologic status at presentation.
2.3 Exploratory analysis of pre- and intraoperative factors that might influence recovery
The data on recovery collected in this study presented an opportunity to reexplore some of the variables that have previously been associated with whether and how soon recovery of ambulation occurs following decompressive surgery in nonambulatory dogs. Besides routine demographic data we also noted, from the electronic medical records, the duration of clinical signs before each dog presented to our clinic (timed from when the dog was first observed to be unable to walk on the pelvic limbs) and the delay between presentation at our hospital and commencement of surgery. Total time under anesthetic (from injection of induction agent [propofol in all cases] until termination of gaseous anesthesia) and duration of surgery were also recorded from the electronic records, allowing calculation of “presurgical anesthetic time” and surgery time for each dog. Presurgical anesthetic time therefore included 2 components: (i) the time during which animals are prepared for surgery, such as clipping, placement of arterial catheters, and surgical scrubbing, which was relatively invariable; plus, (ii) the time needed to acquire diagnostic images of the lesioned area. Time allocated to diagnostic imaging was more variable because, for the majority of dogs, MR or CT images, (or in 1 dog, both) were acquired under general anesthesia directly before proceeding to surgery, while in others, CT scans were acquired under sedation prior to anesthetic induction.
2.4 Sample size and statistical analysis
- For development of the regression equation (in GraphPad Prism 7, GraphPad Software, San Diego, California) we followed the recognized rule for reference interval calculation of acquiring data on 120 dogs.27 We aimed to include dogs that would represent the entire range of sizes that might be included in future studies on spinal cord injury in dogs under 20 kg.
- We used Bayesian analysis in JASP (JASP Version 0.14.1, University of Amsterdam, Netherlands) during data capture for the second phase of the study because we were uncertain of how large a sample size would be needed and the Bayesian approach allows monitoring as data accumulate and avoids the hazards of repeated analysis associated with null hypothesis testing.30, 31 Using a noninformative prior (ie, although possible in the Bayesian framework, we did not incorporate information from previous studies) we determined the Bayes factors (BFs) for comparisons made between recovery for different severities of injury (BFs of >30 or <0.033 are regarded as providing strong evidence in favor of the tested [alternative or null] hypothesis).32, 33 The final sample size for this second part of the study was determined by observing that there was progress toward a highly convincing difference between the deep pain-negative group and the other 2 groups that would be completed at our initial target number (of 100 cases). We did not intend to collect sufficient data to convincingly differentiate time to recovery between paraplegic and paraparetic dogs because that is less clinically important and there was little a priori reason to consider that their recovery times would substantially differ.5 However, we also wished to achieve narrow credible intervals (Bayesian equivalent of confidence intervals) for the time to recover for each category of severity.
To reexplore the relationship of various pre- and intraoperative variables with recovery of independent ambulation and its rapidity we used logistic and competing risk regression in Stata 17 (StataCorp, College Station, Texas). Competing risk regression was selected rather than Cox proportional hazards regression because some dogs do not survive beyond a specific time after surgery (and death “competes” with recovery rather than constituting a censoring event). Competing risk regression computes a model for the “subhazard” of the event of interest—that is, ambulatory recovery (because it is competing with another “subhazard”—that of death). Because of the competing risk the subhazard represents the observed data more accurately than the (global) hazard derived from standard Cox regression but is interpreted similarly. Following univariable analysis of association with outcome, those with a P < .2 were included in a multivariable model that was modified by sequential elimination of nonsignificant variables until the Bayesian Information Criterion (BIC) was minimized.
3 RESULTS
3.1 Relationship between ulnar length and 50-step distance
For the first part of the study, we collected data on 120 dogs of a variety of breeds (see Supplementary Material B), ranging from 2.7 to 19.9 kg (none exceeded 20 kg, as stipulated in the study protocol). The median and interquartile range (IQR) for weight and ulnar length were 9 (6.1-12.5) kg and 12 (11-16) cm respectively. The regression equation between ulnar length and 50-step distance was: y = 1.384x + 2.773, where y = 50-step distance (m) and x = ulnar length (cm) (see Figure 1).
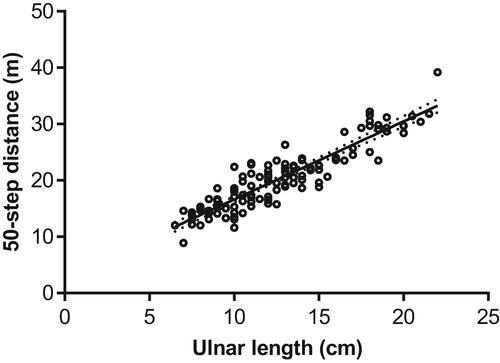
3.2 Recovery of 50-step ambulation after thoracolumbar disc herniation and decompressive surgery
The second part of the study generated data on 50-step walking ability in 104 dogs that had undergone decompressive thoracolumbar spinal surgery, of which 60 of were dachshunds and 8 were French bulldogs. Demographic and presenting data on these cases is summarized in Table 1. For in-clinic variables, median presurgical delay in the hospital was 11.5 (IQR: 3.6-17) hours, mean presurgery anesthetic time was 2.2 (SD: 0.7) hours and mean surgical time was 2.2 (SD: 0.7) hours.
| Variable | Number (%) | Median (IQR) |
|---|---|---|
| Duration of clinical signs (h) | 18.5 (8-36) | |
| Age (y) | 5 (4-8) | |
| Weight (kg) | 6.9 (5.3-10) | |
| Male | 46 (44) | |
| Neutered | 88 (85) | |
| Neurologic severity | ||
| Deep pain negative | 29 (28) | |
| Paraplegic | 25 (24) | |
| Paraparetic | 50 (48) |
Out of 104 total cases, 83 dogs recovered to walk again; 82 within 4 months of surgery. These were distributed as 12/29 (41%) deep pain-negative dogs (1 recovered at 232 days after surgery), 25/25 (100%) paraplegic dogs and 46/50 (92%) paraparetic dogs recovered (Figure 2; Table 2).
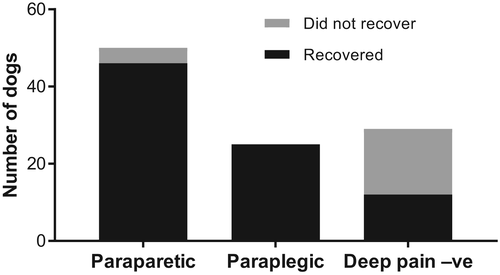
| Deep pain +ve | Deep pain −ve | Total | |
|---|---|---|---|
| Recovered | 71 | 12 | 83 |
| Did not recover | 4 | 17 | 21 |
| Total | 75 | 29 | 104 |
- Two dogs (14 year-old intact male dachshund; 12 year-old spayed female corgi) were paraparetic upon arrival and remained in that status after surgery but developed respiratory distress for clinically unknown reasons and both were euthanized. Pulmonary edema was diagnosed at necropsy in both cases and the cause was suspected to be pulmonary thromboembolism.
- One dog (11 year-old spayed female pug) was paraparetic upon arrival and remained at that status after surgery, but did not recover 50-step ambulation by 6 months after surgery. Although the breed and lack of recovery suggests the possibility of alternative diagnoses, such as arachnoid diverticulum or scarring, detailed reevaluation of the history, images and surgical report were consistent with acute disc herniation. This dog was presented for acute paraparesis after being dropped from the owners' hands about 15 hours before presentation to our clinic.
- One dog (3 year-old spayed female Boston terrier) was categorized paraparetic at presentation but deteriorated to deep pain-negative by the time of surgery (8 hours later) and did not recover 50-step ambulation by 5 months after surgery. This dog was classified according to status at presentation in the summary data and statistical analysis.
- One dog (2 year-old spayed female French bulldog) presented paraparetic but deteriorated after surgery to deep pain-negative and failed to recover ambulation by 4 months after surgery.
Of the 83 dogs that did recover, the time to recover varied between neurologic categories (Figure 3), notably there was a considerable difference between dogs that were deep pain-negative at presentation (median time to recover: 91 days) and those that were paraplegic or paraparetic (median for both: 14 days). Bayesian analysis of variance (ANOVA) in JASP indicates decisive evidence (BF > 100) in favor of concluding that the recovery times differ between groups (model BF = 3.70 × 108). More specifically, the deep pain-negative group differs from the paraplegic group (BF = 1.48 × 103) and the paraparetic group (BF = 4.48 × 107) but there is negligible evidence of difference in rapidity of recovery between the paraparetic and paraplegic groups (BF = 0.43). Bayesian analysis of the contingency table (Table 2) confirms that there is decisive evidence in favor of concluding that the probability of recovery is much lower in dogs that are “deep pain negative” than if they have intact pain sensation (BF = 5.94 × 106; median log odds ratio 3.11 [95% credible interval: 1.91-4.30]).
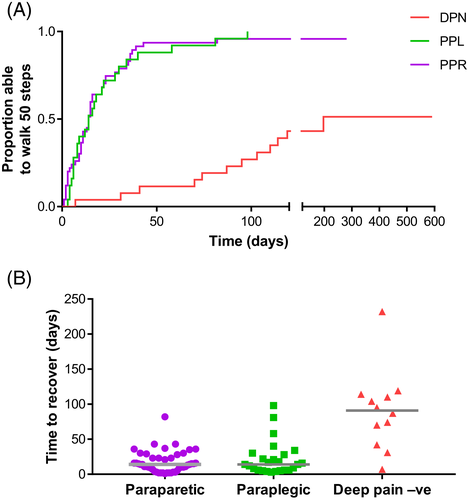
3.3 Relationship of recovery of ambulation with pre- and intraoperative variables
Finally, we used logistic and competing risks regression to explore the possible interaction of various preoperative variables with whether there was recovery and, if so, how rapidly it occurred. We were especially interested in association between: (i) duration of clinical signs; (ii) delay between presentation and commencement of surgery; and, (iii) presurgical anesthetic time or surgical time and diminished recovery, because these variables have previously been associated with delay or failure to recover.6, 17, 35, 36
In univariable logistic regression, neurologic status at presentation, presurgical anesthetic time and neuter status were associated at P < .2 with whether ambulation recovered (whereas sex, weight, duration of inability to ambulate, delay from admission to surgery, and surgery time were not) and so were introduced into a multivariable analysis. Using the Bayesian Information Criterion to guide modeling resulted in retention of only neurologic status at time of presentation (ie, deep pain negative vs positive) and presurgical anesthetic time in the final model—deep pain status: odds ratio (OR) 0.043 (95% confidence interval [CI]: 0.012-0.160; P < .001); presurgical anesthetic time: OR = 0.270 (95% CI: 0.071-1.020; P = .06), see Table 3.
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Deep pain status | 0.043 | 0.116-0.161 | <.001 |
| Presurgery anesthetic time | 0.270 | 0.071-1.030 | .06 |
Similarly, in competing risks regression, the same variables (neurologic status at presentation, presurgical anesthetic time, weight and neuter status) were associated at P < .2 with rapidity of recovery of ambulation in univariable analysis. Postestimation analysis indicated that neurologic status at presentation violated the proportional hazards assumption37 (because its association with outcome varied with time) and so it was also included as a time-varying covariate in the final optimized multivariable model (Table 4). This analysis indicates that at day 0 the “hazard” of recovery is 0.022 in deep pain-negative animals compared to those with intact deep pain, but with every subsequent day the hazard of recovery to walk the 50-step distance from initial deep pain negative status improved by a factor of 1.04 relative to deep pain-positive dogs. Presurgical anesthetic time is also retained in the optimized final model (that minimizes BIC).
| Variable | Subhazard ratio | 95% CI | P value |
|---|---|---|---|
| Deep pain status | 0.022 | 0.004-0.125 | <.001 |
| Presurgery anesthetic time | 0.753 | 0.567-1.001 | .05 |
| Time varying covariate: deep pain status | 1.036 | 1.009-1.063 | .01 |
To explore the association of recovery with presurgical anesthetic time in more detail we compared its duration in association with the 2 imaging modalities; median presurgical anesthetic time was 0.9 hours (IQR: 0.63-1.2) and 2.4 hours (IQR: 2.1-2.7) for dogs undergoing CT and MR imaging respectively. All 16 dogs (of which all but 1 were deep pain positive) that underwent CT imaging alone recovered ambulation by a median of 6 days; in contrast, 67/88 (76%) dogs undergoing MRI recovered ambulation, by a median of 23 days.
4 DISCUSSION
Our data endorse the 50-step walking test as a simple practical test that can be easily and widely applied as a standard outcome for decompressive thoracolumbar spinal surgery in dogs. The test clearly discriminates between the outcomes for dogs with and without deep pain sensation at presentation, provides approximate expected times for recovery and meets owner expectation for “everyday” ambulation. Furthermore, the equation describing the relationship between ulna length and 50-step distance will apply to dogs examined in other clinics no matter their geographic location, although only to dogs weighing <20 kg. An advantage of this measure is that our data demonstrate that, under veterinary guidance, it can be used by owners without specific training, implying that it could be used for large-scale clinical trials or observational studies that aim to compare outcomes following different interventions. Therefore, this measure aligns well with the goals of pragmatic clinical trials, which focus on real-life outcomes of importance for patients (and owners).21
Concerns are sometimes expressed, especially in veterinary medicine, regarding pragmatic outcome measures that are perceived to be “imprecise” (because they are not measured by experts at a specialized center with specialized equipment). However, this concern presents a serious conflict with the need for easily measured and widely applicable outcomes that is demanded by large clinical studies. The perceived imprecision associated with outcomes such as this 50-step test must be weighed against the ability to reliably detect differences in recovery between individual animals and groups of animals. A good pragmatic outcome measure will achieve this goal, even though it may not be able to make fine distinctions between subtly different levels of function. While such distinctions may be important when judging whether an intervention has any detectable effect (which might be a more major preoccupation of preclinical researchers), the goal of pragmatic clinical research is to identify interventions that make a meaningful difference to the lives of treated individuals. Therefore, outcome measures assessed with greater inherent (although not unlimited) variability can even be considered advantageous because they will delineate clinical interventions that have major and clearly recognizable effects in real life. [For instance, it is of little practical relevance to an owner, or even the dog itself, whether a treatment improves fore and hindlimb coordination—an outcome used for a previous explanatory trial in dogs,38 whereas recovery to walk 50-steps is important in everyday life.] On the other hand, a useful pragmatic outcome needs to be measurable, preferably on a numeric scale (so that formal statistical comparisons can easily and reliably be made). Importantly, the 50-step walking test can examine both whether a dog recovers to this criterion and also how long it takes to recover, thereby maximizing the data that can be accrued from dogs in clinical trials. Furthermore, it is a good match for similar pragmatic outcomes used in humans, thereby facilitating translation of intervention results between species.
Although there is a very strong differentiation of outcome (both whether and time taken) between dogs that are deep pain-negative and those in which deep pain sensation is intact, there appears not to be a difference in rapidity of recovery nor whether dogs recover between those presenting as paraplegic or paraparetic. This is surprising at first sight, although previously reported5 and perhaps, on reflection, not wholly unexpected: the clinical ability to distinguish these 2 states is limited, because a dog may or may not show voluntary limb movement in the clinic depending upon motivation. Moreover, the 2 categories may simply represent different stages at which the animals are examined. The data we present here suggest that it is unnecessary to categorize animals as paraplegic or paraparetic in regard to predicting recovery of ambulation or rapidity of recovery.
The 50-step test data presented here enabled reexploration of some aspects of recovery after thoracolumbar disc herniation that have been highlighted previously. First, our data again support the notion that the duration of clinical signs before presentation and the intraclinic delay before commencement of surgery do not have a bearing on whether dogs recover nor how long it takes. Nevertheless, it is important to note that we categorized animals by their status at presentation and so it is possible that in some animals, if it had been possible to repeatedly reexamine them over a prolonged period before then, the severity of their neurologic deficits (notably from deep pain-positive to deep pain-negative) might have changed before presentation to our clinic and such a time-associated change would not be captured in our records. Nevertheless, out of the 75 deep pain-positive dogs included in the recovery analysis, only 1 deteriorated to deep pain negative status within the clinic before surgery, suggesting that the risk that this will occur—even within a period as long as 8 hours—is not high. This result contrasts with another recent study that reported a considerably higher incidence (15 of 122) of such deterioration in neurologic status within their clinic.19 For comparison, in our study 1 dog showed similar deterioration between pre- and postoperative examination, suggesting that the risk of deterioration during surgery is similar to that before surgery.
Our survival analysis also supports the notion that longer anesthetic time—more specifically, “pre-surgical anesthetic time”—might be associated with prolonged recovery. Our data present evidence to suggest that this is likely not to be cause and effect. Dogs undergoing CT scans had considerably shorter presurgical anesthetic duration, a substantially higher proportion of recovery and shorter median time to recover than those undergoing MRI. It is possible that shorter presurgical anesthetic time is linked to superior recovery in those undergoing CT because of a reduced opportunity for episodes of preoperative hypotension or other risks for exacerbating spinal cord injury. However, a more plausible explanation is that the dogs selected for CT were those that were inherently more likely to recover quickly; this notion is supported by the high and low proportions of deep-pain negative dogs in the MRI and CT groups respectively. Therefore, in this study at least, the association of presurgery anesthetic time with slower recovery is quite likely not direct, but instead represents “reverse causality,” in which longer presurgical anesthetic duration is associated with increased severity of presentation and the use of more time-consuming imaging to achieve precise diagnosis of lesion character and location.
Lastly, only 12 of the 29 (41%) deep pain negative dogs recovered to walk 50 steps after surgery, which is a low proportion compared with many other reports on similar cases.28 There are probably 2 main explanations. First, the 50-step distance used here is a more stringent test of recovery of ambulation than that used in other studies, meaning that dogs that have only partially recovered, including those that might be deemed “spinal walkers,”39 will not be included in our count of recoveries. Second, the deep pain-negative cases included here were recruited consecutively without taking into account any perceived negative neurologic exam or imaging findings; dogs were not excluded because they exhibited indicators, such as spinal cord hypointensity,40 that might be considered to suggest poor prognosis.
In conclusion, our data demonstrate that the 50-step walking test can be applied by owners, under veterinary supervision, to produce outcome data that clearly differentiates recovery between deep pain-negative and deep pain-positive dogs, in terms of both whether they recover and also how long it takes. Because of the ability to quantify both these aspects of recovery this test will be useful in future to examine the effectiveness of novel interventions aiming to enhance recovery, especially in dogs with severe spinal cord injuries. The method could be usefully streamlined by development of a smart phone app to aid data recording. Although used here in dogs weighing <20 kg with thoracolumbar injuries the method could readily be adapted for assessment of recovery in larger dogs, more caudally located lumbar disc lesions (ie, L4-S3 segments) and cervical lesions. Lastly, we used this outcome as a means of reexamining previously reported associations between recovery and various pre- and intraoperative factors. This exploratory analysis suggests that, in addition to the predicted association with preoperative neurologic status, time under anesthesia (specifically, presurgical anesthetic time) is also associated with more prolonged recovery. The inference from our data is that this may represent reverse causality, although this supposition requires support from analysis of additional clinical datasets.
ACKNOWLEDGMENT
No funding was received for this study.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




